Five membered heterocycles with a nitrogen atom, such as pyrroles and imidazoles, are important building blocks in a wide number of biologically active compounds [1, 2, 3, 4, 5, 6]. Among them, pyrroles are heterocycles of great importance because of their frequent presence in natural products similar to heme, chlorophyll, vitamin B12, and various cytochrome enzymes [7]. Some recently isolated pyrrole-containing marine natural products have been found to display significant cytotoxicity and function as multidrug resistance (MDR) reversal agents [8]. Many of these biologically active compounds function as chemotherapeutic agents. Also, polysubstituted pyrroles are molecular structures with immense importance in material science [9]. They have also been employed as antioxidative [10], antibacterial [11, 12], ionotropic [13, 14], antitumor [15], anti-inflammatory [16, 17] and antifungal agents [18]. Also, the imidazole system can be found in numerous medically relevant compounds, such as the fungicide Ketoconazole [19] and its family members, the benzodiazepine antagonist Flumazenil [20], the antineoplastic drug Dacarbazine [21], the antibiotic Metronidazole [22], the antiulcerative agent Cimetidine [23], the antihyperthyroid drug Methimazole [24], the prohormone Thyroliberin [25], the muscarinic receptor agonist Pilocarpine [26] and the hypnotic agent Etomidate [27].Our research group reported the synthesis of a series of pyrroles and imidazoles using the reaction of primary amines with either alkyl propiolates or isothiocyanates in the presence of oxalyl chloride 3 under solvent-free conditions in good yields.
All chemicals used in this work were purchased from Fluka (Buchs, Switzerland) and were used without further purification. Melting points were measured on an Electrothermal 9100 apparatus. Elemental analyses for C, H, and N were performed using a Heraeus CHN-O-Rapid analyzer. Mass spectra were recorded on a FINNIGAN-MAT 8430 spectrometer operating at an ionization potential of 70 eV. IR spectra were measured on a Shimadzu IR-460 spectrometer. 1HNMR and 13CNMR spectra were measured with a BRUKER DRX-500 AVANCE spectrometer at 500.1 and 125.8 MHz, respectively, and were obtained for solutions in CDCl3 using TMS as the internal standard or 85% H3PO4 as the external standard.
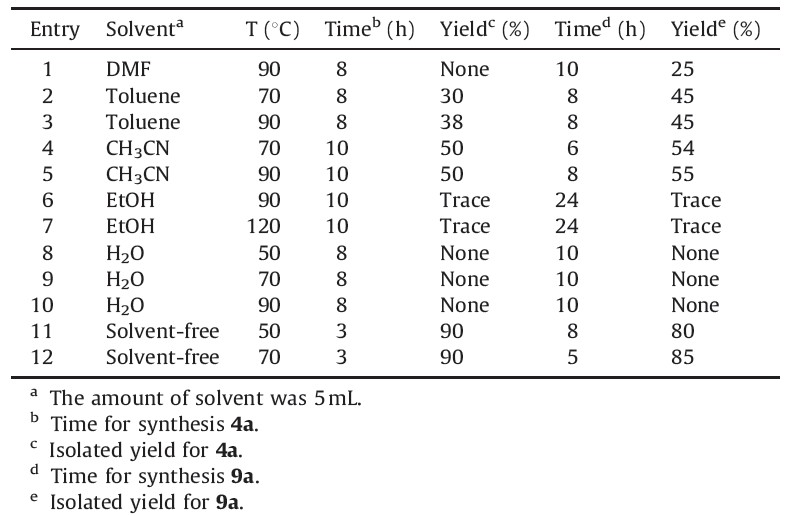
To a mixture of primary amine 1 (2 mmol) and alkyl propiolate 2 (2 mmol) was added oxalyl chloride 3 (2.5 mmol) at 50 ℃. The reaction mixture was then stirred for 3 h. After completion of the reaction [TLC (AcOEt/hexane, 1:6, v/v) monitoring], the reaction mixture was purified by flash column chromatography on silica gel (Merck 230-400 mesh) using n-hexane-EtOAc as eluent to afforded pure compounds 4 (Scheme 1).
|
Download:
|
| Scheme 1.Synthesis of compound 4 using primary amine, alkyl propiolate and oxalyl chloride. | |
To a mixture of primary amine 1 (2 mmol) and arylisothiocyanate 8 (2 mmol) was added oxalyl chloride 3 (2.5 mmol) at 70 ℃. The reaction mixture was then stirred for 5 h. After completion of the reaction [TLC (AcOEt/hexane, 1:4, v/v) monitoring], the reaction mixture was purified by flash column chromatography on silica gel (Merck 230-400 mesh) using nhexane- EtOAc as eluent to afforded pure compounds 9 (Scheme 2).
Synthesis of pyrrole derivatives 4 using the reaction of primary amines 1 and alkyl propiolate 2 in the presence of oxalyl chloride 3 under solvent-free conditions at 50 ℃ in good yields was investigated.
The starting point for our experiments was to optimize the reaction conditions such as solvent and reactions time for the production of 1H-pyrrole and 1H-imidazol derivatives (Table 1). Solvent free condition and 50 ℃ is suitable for synthesis of compound 4a and solvent free condition and 70 ℃ is suitable for synthesis of compound 9a.
| Table 1 Optimization of reaction conditions of compounds 4a and 9a. |

|
Download:
|
| Scheme 2.Synthesis of compound 9 using primary amine, isothiocyanate and oxalyl chloride. | |
|
Download:
|
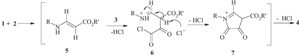
| Scheme 3.Proposed mechanism for the synthesis of compound 4. | |

|
Download:
|
| Scheme 4.Proposed mechanism for the synthesis of compound 9. | |
Under similar conditions, three component reactions between primary amine 1, arylisothiocyanate 8 and oxalyl chloride 3 at 70 ℃ under solvent-free conditions produce 1H-imidazole derivatives 9 in excellent yields (Scheme 2).
The structures of compounds 9 were assigned by IR, 1H NMR, 13C NMR and mass spectral data, and these data were showed in Supporting information. For example, the 1H NMR spectrum of 9a exhibited one singlet for methyl protons at (δ 2.35) and one singlet for NCH2 protons at (δ 5.14) along with signals for an aromatic moiety. Three resonances at 154.3 (C=O), 156.7 (C=O), and 183.6 (C=S) ppm were observed in the 13C NMR spectrum of 9a, which is attributed to the carbonyl and thionyl groups, further confirming the proposed structure. Although we have not established the mechanism of the reaction between the amines and arylisothiocyanate in the presence of oxalyl chloride in an experimental manner, a possible explanation is proposed in Scheme 4. Compound 9 result from the initial addition of the amine to isothiocyanate and subsequent attack of the resulting reactive compound 10 on the oxalyl chloride to yield intermediate 11. Cyclization of the intermediate 11 by elimination of HCl leads to compound 9.
In conclusion, we reported a novel method involving primary amines and alkyl propiolates or isothiocyanate in the presence of oxalyl chloride for the synthesis of 1H-pyrrole or 1H-imidazole derivatives. The advantages of our work are that the reaction is performed under solvent-free conditions without using a catalyst.
Supplementary material related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2013.09.015.
| [1] | (a) G.W. Gribble, Recent developments in indole ring synthesis -methodology and applications, J. Chem. Soc., Perkin Trans. 1 (2000) 1045-1075; (b) A. Nobuyoshi, O. Akihiko, M. Chikara, et al., Synthesis and antitumor activity of duocarmycin derivatives: modification of segment-A of A-ring pyrrole compounds, J. Med. Chem. 42 (1999) 2946-2960; (c) P.S. Baran, J.M. Richter, D.W. Lin, Total synthesis of stephacidin A, Angew. Chem. Int. Ed. 44 (2005) 606-609; (d) M. Torok, M. Abid, S.C. Mhadgut, B. Torok, Organofluorine inhibitors of amyloid fibrillogenesis, Biochemistry 45 (2006) 5377-5383. |
| [2] | K. Ramesh, K. Karnakar, G. Satish, Y.V.D. Nageswar, Novel and efficient supramolecular synthesis of pyrroles in the presence of β-cyclodextrin in water, Chin. Chem. Lett. 23 (2012) 1331-1334. |
| [3] | S.Z. Yuan, J. Liu, L. Xu, A convenient synthesis of pyrroles catalyzed by acidic resin under solvent-free condition, Chin. Chem. Lett. 21 (2010) 664-668. |
| [4] | F. Rostami-Charati, Z. Hossaini, M.A. Khalilzadeh, H. Jafaryana, Solvent-free synthesis of pyrrole derivatives, J. Heterocycl. Chem. 49 (2012) 217-220. |
| [5] | T. Sano, Y. Horiguchi, J. Toda, K. Imafuku, Y. Tsuda, Syntheses of 2-aryl-3-ethoxycarbonyl-Δ2-pyrroline-4,5-diones from condensation of enamino-esters with oxalyl chloride, Chem. Pharm. Bull. 32 (1984) 497-503. |
| [6] | Y. Cheng, H. Yang, M. Wang, D.J. Williams, Annulation reaction of heterocyclic secondary enamines with dicarboxylic acid dichlorides, Tetrahedron 58 (2002) 2821-2830. |
| [7] | R.J. Sundberg, Pyrroles and their benzo derivatives: synthesis, in: A. Katritzky, C.W. Rees, E F.V. Scriven (Eds.), Comprehensive Heterocyclic Chemistry II, vol. 2, Pergamon Press, Oxford, 1996, pp. 119-206. |
| [8] | H. Tao, I. Hwang, D.L. Boger, Multidrug resistance reversal activity of permethyl ningalin B amide derivatives, Bioorg. Med. Chem. Lett. 14 (2004) 5979-5981. |
| [9] | (a) M. Baumgarten, N. Tyutyulkov, Nonclassical conducting polymers: new approaches to organic metals, Chem. Eur. J. 4 (1998) 987-989; (b) A. Deronzier, J.C. Moutet, Functionalized polypyrroles as versatile molecular materials for electrode modification, Curr. Top. Electrochem. 3 (1994) 159-200; (c) G.A. Pagani, Heterocycle-based electric conductors, Heterocycles 37 (1994) 2069-2086. |
| [10] | J. Lehuede, B. Fauconneau, L. Barrier, et al., Synthesis and antioxidant activity of new tetraarylpyrroles, Eur. J. Med. Chem. 34 (1999) 991. |
| [11] | R.W. Burli, D. McMinn, J.A. Kaizerman, et al., DNA binding ligands targeting drugresistant Gram-positive bacteria. Part 1: Internal benzimidazole derivatives, Bioorg. Med. Chem. Lett. 14 (2004) 1253-1257. |
| [12] | R.W. Burli, P. Jones, D. McMinn, et al., DNA binding ligands targeting drugresistant Gram-positive bacteria. Part 2: C-terminal benzimidazoles and derivatives, Bioorg. Med. Chem. Lett. 14 (2004) 1259-1263. |
| [13] | R. Jonas, M. Klockow, I. Lues, et al., Synthesis and biological activities of meribendan and related heterocyclic benzimidazolo-pyridazinones, Eur. J. Med. Chem. 28 (1993) 129-140. |
| [14] | W. von der Saal, J.P. Hoelck, W. Kampe, A. Mertens, B. Mueller-Beckmann, The inotropic activity of linear, tricyclic 5-6-5 fused heterocycles, J. Med. Chem. 32 (1989) 1481-1491. |
| [15] | W.A. Denny, G.W. Rewcastle, B.C. Baguley, Potential antitumor agents. 59: Structure-activity relationships for 2-phenylbenzimidazole-4-carboxamides, a new class of "minimal" DNA-intercalating agents which may not act via topoisomerase II, J. Med. Chem. 33 (1990) 814-819. |
| [16] | E. Toja, D. Selva, P.J. Schiatti, 3-Alkyl-2-aryl-3H-naphth[1,2-d]imidazoles, a novel class of nonacidic antiinflammatory agents, Med. Chem. 27 (1984) 610-616. |
| [17] | V.J. Demopoulos, E.J. Rekka, Isomeric benzoylpyrroleacetic acids: some structural aspects for aldose reductase inhibitory and anti-inflammatory activities, Pharm. Sci. 84 (1995) 79-82. |
| [18] | M. Del Poeta, W.A. Schell, C.C. Dykstra, et al., Structure-in vitro activity relationships of pentamidine analogues and dication-substituted bis-benzimidazoles as new antifungal agents, Antimicrob. Agents Chemother. 42 (1998) 2495-2502. |
| [19] | J. Heeres, L.J.J. Backx, J.H. Mostmanns, J. van Cutsem, Synthesis and antifungal activity of ketoconazole, a new potent orally active broad-spectrum antifungal agent, J. Med. Chem. 22 (1979) 1003-1005. |
| [20] | W. Hunkeler, H. Mohler, L. Pieri, et al., Selective antagonists of benzodiazepines, Nature 290 (1981) 514-516. |
| [21] | Y.F. Shealy, C.A. Krauth, J.A. Montgomery, Coupling reactions of 5-diazoimidazole-4-carboxamide, J. Org. Chem. 27 (1962) 2150-2154. |
| [22] | R.N. Brogden, R.C. Heel, T.M. Speigt, Review of activity, pharmacokinetics and use in anaerobic infection, Drugs 16 (1978) 387-417. |
| [23] | R.W. Brimblecombe, W.A.M. Duncan, G.J. Durant, et al., Vascular reactions to histamine and compound 48/80 in human skin: suppression by a histamine H2-receptor blocking agent, J. Int. Med. Res. 3 (1975) 86-92. |
| [24] | D.S.N. Cooper, 2-(2-Chlorophenyl)-4,5-diphenyl-1H-imidazole, Engl. J. Med. 311 (1984) 1353-1362. |
| [25] | G. Fluoret, Synthesis of pyroglutamylhistidylprolineamide by classical and solid phase methods, J. Med. Chem. 13 (1970) 843-845. |
| [26] | A.J. Mayorga, M.S. Cousins, J.T. Trevitt, et al., Characterization of the muscarinic receptor subtype mediating pilocarpine-induced tremulous jaw movements in rats, Eur. J. Pharmacol. 364 (1999) 7-11. |
| [27] | E.F. Godefroi, P.A.J. Janssen, C.A.M. van der Eycken, A.H.M.T. van Heertum, C.J.E. Niemegeers, A novel type of hypnotic agents, J. Med. Chem. 8 (1965) 220-223. |