b State Key Laboratory of the Discovery and Development of Novel Pesticide, Shenyang Research Institute of Chemical Industry Co., Ltd., Shenyang 110021, China
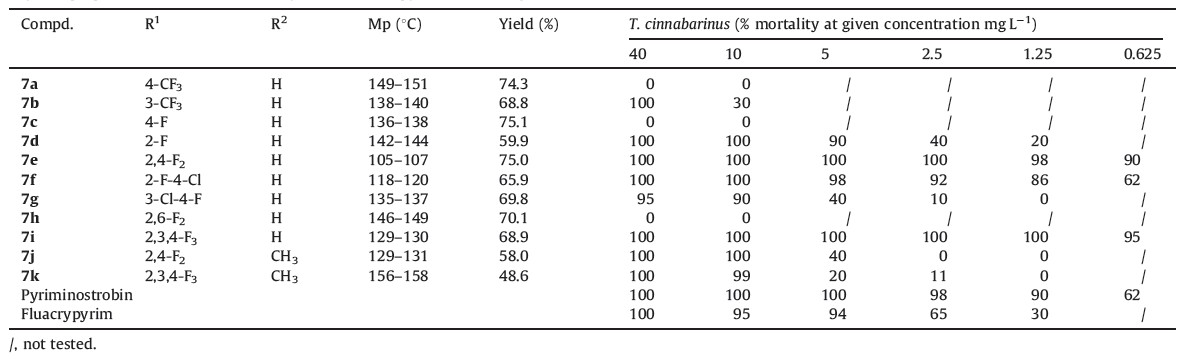
Pyriminostrobin (SYP-11277), a novel acaricide, was reported in our previous work [1, 2]. The discovery of pyriminostrobin was outlined in Fig. 1, in which the lead compound was altered by the intermediate derivatization method [3, 4, 5], and then optimized to synthesize pyriminostrobin. There has been increasing interest in the introduction of fluorine or appropriate fluorinated functional groups into organic compounds in recent years [6, 7, 8, 9, 10, 11, 12]. Incorporation of one or several fluorine atoms into organic molecules can enhance their biological potency due to the intrinsic properties of the fluorine atom. These include having the highest electronegativity, a small atomic radius, high thermal stability and lipophilicity, which could provide easier absorption and transportation of organic molecules within biological systems [12]. Thus, substitution of fluorine into a potential drug molecule is an important strategy in drug and agrochemical development [10].

|
Download:
|
| Fig. 1.The discovery of pyriminostrobin. | |
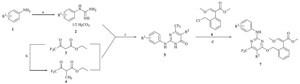
The general synthetic scheme for representative compounds 7a-k is shown in Scheme 1. Reaction yields were not optimized, and each new compound was identified and verified by 1H NMR, MS and elemental analyses. The strobilurin derivatives containing pyrimidine moieties have been reported in previous works [1, 14]. Compounds 7a-k were synthesized by the same synthetic methods. Substituted phenyl guanidines (2) were prepared from substituted anilines (1) and cyanamide under acidic conditions. Ethyl 4,4,4-trifluoro-2-methyl-3-oxobutanoate was obtained from ethyl 4,4,4-trifluoro-3-oxobutanoate following procedures in the literature [15].
|
Download:
|
| Scheme 1.Synthetic route of title compounds 7: (a) NH2CN, HCl, 85 ℃; (b) CH3Br, NaH, DMF; (c) toluene, reflux; and (d) K2CO3, DMF, 80 ℃. | |
General procedure for the synthesis of intermediate pyrimidin- 4(3H)-one (5): Substituted phenyl guanidine carbonates (2, 0.01 mol) and β-keto esters (3 or 4, 0.02 mol) were added to toluene (30 mL), and the mixture was heated to reflux with a Dean Stark trap until all water was azeotropically removed. The reaction solution was cooled and then filtered. The solid was washed with toluene and dried as intermediate 5.
Synthesis of (E)-methyl 2-(2-(((2-((2,4-difluorophenyl)amino)-6- (trifluoromethyl)pyrimidin-4-yl)oxy)methyl)phenyl)-3-methoxyacrylate (7e): A mixture of 2-((2,4-difluorophenyl)amino)-6-(trifluoromethyl) pyrimidin-4(3H)-one (5e) (0.35 g, 1.2 mmol), (E)- methyl 2-(2-(chloromethyl)phenyl)-3-methoxyacrylate (6) (0.30g, 1.25mmol) and K2CO3 (0.33 g, 2.4 mmol) inDMF(15mL)was stirred and heated at 80 ℃ for 8 h. The reaction mixture was then cooled, diluted with water (100 mL), and extracted with ethyl acetate (3 × 25 mL). The organic layerwas separated, washed with saturated brine, dried over MgSO4, and filtered. The filtratewas evaporated, and the crude productwas purified via silica gel column chromatography using a 1:10 (v/v) mixture of ethyl acetate and petroleum ether (boiling point range: 60-90 ℃) as the eluent to obtain 0.44 g of white crystals. 1H NMR (300 MHz, CDCl3):Δ8.28 (s, 1H, Ph'-NH-Py), 7.59 (s, 1H, C=CH), 7.48-6.90 (m, 7h, Ph'-3H + Ph-4H), 6.54 (s, 1H, Py-5-H), 5.31 (s, 2H, PhCH2O), 3.80 (s, 3H, COOCH3), 3.70 (s, 3H,=C-OCH3); Mol. wt: 495.40. LC-MS: m/z 494.2 [M-H]-, 496.3 [M+H]+ , Anal.calcd. (%) for C23H18F5N3O4: C, 55.76;H, 3.66;N, 8.48; Found: C, 55.78; H, 3.66; N, 8.52.
7a: White solid. 1H NMR (300 MHz, CDCl3):Δ7.86 (s, 1H, Ph'- NH-Py), 7.78 (d, 2H, Ph'-2,6-2H, J = 9.0 Hz), 7.62 (s, 1H, C=CH), 7.57 (d, 2H, Ph'-3,5-2H, J = 9.0 Hz), 7.48 (m, 1H, Ph-6-H), 7.35 (m, 2H, Ph-3,5-2H), 7.20 (m, 1H, Ph-4-H), 6.57 (s, 1H, Py-5-H), 5.35 (s, 2H, PhCH2O), 3.81 (s, 3H, COOCH3), 3.74 (s, 3H, =C-OCH3); Mol. wt: 527.42. LC-MS: m/z 526.5 [M-H]-, 528.3 [M+H]+, Anal. calcd. (%) for C24H19F6N3O4: C, 54.65; H, 3.63; N, 7.97; Found: C, 54.69; H, 3.66; N, 7.98.
7b: White solid. 1H NMR (300 MHz, CDCl3):Δ8.06 (br s, 1H, Ph'- NH-Py), 7.81-7.18 (m, 9H, Ph'-4H + Ph-4H + C=CH), 6.53 (s, 1H, Py-5-H), 5.30 (s, 2H, PhCH2O), 3.80 (s, 3H, COOCH3), 3.73 (s, 3H, =C-OCH3); Mol. wt: 527.42. LC-MS: m/z 526.5 [M-H]-, 528.3 [M+H]+, Anal. calcd. (%) for C24H19F6N3O4: C, 54.65; H, 3.63; N, 7.97; Found: C, 54.66; H, 3.65; N, 7.98.
7c:White solid. 1H NMR (300MHz, CDCl3):Δ8.40 (s, 1H, Ph'- NH-Py), 7.58 (s, 1H, C=CH), 7.55 (m, 2H, Ph'-2,6-2H), 7.50 (m, 1H, Ph-6-H), 7.37 (m, 2H, Ph-3,5-2H), 7.31 (m, 2H, Ph'-3,5-2H), 7.20 (m, H, Ph-4-H), 7.00 (s, 1H, Py-5-H), 5.40 (s, 2H, PhCH2O), 3.80 (s, 3H, COOCH3), 3.68 (s, 3H, =C-OCH3); Mol. wt: 477.41. LC-MS: m/z 476.5 [M-H]-, 478.4 [M+H]+, Anal. calcd. (%) for C23H19F4N3O4: C, 57.86; H, 4.01; N, 8.80; Found: C, 57.79; H, 4.00; N, 8.82.
7d: White solid. 1H NMR (300 MHz, CDCl3):Δ7.82 (s, 1H, Ph'- NH-Py), 7.61 (s, 1H, C=CH), 7.48-7.06 (m, 7h, Ph'-3H + Ph-4H), 6.53 (s, 1H, Py-5-H), 5.34 (s, 2H, PhCH2O), 3.82 (s, 3H, COOCH3), 3.73 (s, 3H, =C-OCH3); Mol. wt: 477.41. LC-MS: m/z 476.5 [M-H]-, 478.4 [M+H]+, Anal. calcd. (%) for C23H19F4N3O4: C, 57.86;H, 4.01; N, 8.80; Found: C, 57.80; H, 4.02; N, 8.82.
7f: White solid. 1H NMR (300 MHz, CDCl3):Δ8.37 (s, 1H, Ph'- NH-Py), 7.59 (s, 1H, C=CH), 7.50-7.12 (m, 7h, Ph'-3H + Ph-4H), 6.56 (s, 1H, Py-5-H), 5.33 (s, 2H, PhCH2O), 3.80 (s, 3H, COOCH3), 3.70 (s, 3H, =C-OCH3); Mol. wt: 511.85. LC-MS: m/z 510.7 [M-H]-, 512.8 [M+H]+, Anal. calcd. (%) for C23H18F4N3O4: C, 53.97; H, 3.54; N, 8.21; Found: C, 53.91; H, 3.55; N, 8.21.
7g: White solid. 1H NMR (300 MHz, CDCl3):Δ8.06 (s, 1H, Ph'- NH-Py), 7.81-7.18 (m, 9H, Ph'-4H + Ph-4H + C=CH), 6.53 (s, 1H, Py-5-H), 5.30 (s, 2H, PhCH2O), 3.80 (s, 3H, COOCH3), 3.73 (s, 3H, =C-OCH3); Mol. wt: 511.85. LC-MS: m/z 510.7 [M-H]-, 512.8 [M+H]+, Anal. calcd. (%) for C23H18-ClF4N3O4: C, 53.97; H, 3.54; N, 8.21; Found: C, 53.92; H, 3.50; N, 8.18.
7h: White solid. 1H NMR (300 MHz, CDCl3):Δ7.54 (s, 1H, C=CH), 7.00-7.33 (m, 7h, Ph'-3H + Ph-4H), 6.71 (br s, 1H, Ph'-NHPy), 6.48 (s, 1H, Py-5-H), 5.16 (s, 2H, PhCH2O), 3.75 (s, 3H, COOCH3), 3.67 (s, 3H, =C-OCH3); Mol. wt: 495.40. LC-MS: m/z 494.2 [M-H]-, 496.3 [M+H]+, Anal. calcd. (%) for C23H18F5N3O4: C, 55.76; H, 3.66; N, 8.48; Found: C, 55.78; H, 3.68; N, 8.50.
7i: White solid. 1H NMR (300 MHz, CDCl3):Δ8.06 (br s, 1H, Ph'- NH-Py), 7.59 (s, 1H, C=CH), 7.48-6.98 (m, 6H, Ph'-2H + Ph-4H), 6.57 (s, 1H, Py-5-H), 5.31 (s, 2H, PhCH2O), 3.81 (s, 3H, COOCH3), 3.71 (s, 3H, =C-OCH3); Mol. wt: 513.39. LC-MS: m/z 512.4 [M-H]-, 514.7 [M+H]+, Anal. calcd. (%) for C23H17f6N3O4: C, 53.81; H, 3.34; N, 8.18; Found: C, 53.78; H, 3.40; N, 8.12.
7j: White solid. 1H NMR (300 MHz, CDCl3):Δ8.40 (br s, 1H, Ph'- NH-Ph), 7.44-6.82 (m, 7h, Ph'-3H + Ph-4H), 6.03 (br s, 1H, NH), 5.51 (s, 2H, PhCH2O), 3.67 (s, 3H,=N-OCH3), 2.93 (d, J = 4.8 Hz, 3H, NHCH3), 2.24 (s, 3H, CH3); Mol. wt: 509.43. LC-MS: m/z 509.2 [M-H]-, 511.4 [M+H]+, Anal. calcd. (%) for C24H20F5N3O4: C, 56.58; H, 3.96; N, 8.25; Found: C, 56.60; H, 3.99; N, 8.21.
7k: White solid. 1H NMR (300 MHz, CDCl3):Δ8.20 (br s, 1H, Ph'- NH-Py), 7.40-6.91 (m, 6H, Ph'-2H + Ph-4H), 6.05 (br s, H, NH), 5.49 (s, 2H, PhCH2O), 3.68 (s, 3H, =N-OCH3), 2.94 (d, J = 4.8 Hz, 3H, NHCH3), 2.25 (s, 3H, CH3); Mol. wt: 527.42. LC-MS: m/z 526.3 [M-H]-, 528.5 [M+H]+, Anal. calcd. (%) for C24H19F6N3O4: C, 54.65; H, 3.63; N, 7.97; Found: C, 54.66; H, 3.65; N, 7.94.
As shown in Scheme 1, the target compounds were readily synthesized. Table 1 summarized the physical characteristics, yields, and acaricidal activities of all strobilurin-pyrimidine analogs. Two fluorines were introduced into pyriminostrobin to replace the chlorines to produce 7e, a more potent acaricidal compound with 90% mortality against Tetranychus cinnabarinus at 0.625 mg L-1.
| Table 1 Physical properties and acaricidal activity of strobilurin-pyrimidine analogs 7 |
|
Download:
|
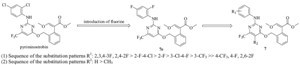
| Fig. 2.Optimizations of compound 7e. | |
| Table 2 Acaricidal activity of 7e and 7i against T. cinnabarinus. |
In order to introduce fluorine into pyriminostrobin to improve its bio-activity, a series of strobilurin-pyrimidine derivatives (7a-k) were synthesized. The highly active compound 7e was discovered by using fluorine to replace chlorine of pyriminostrobin, after which the 2,4-2F-phenylamine moiety was modified into various fluorine substitution patterns (7a-d, 7f-k). Compounds 7e and 7i were shown to be more potent than pyriminostrobin against T. cinnabarinus at 0.625 mg L-1.
We are grateful to the Outstanding Young Scholarship from the National Natural Science Foundation of China (No. 30825043), NSFC (No. 21172202), and the Outstanding Scholar Foundation of Henan Province (No. 094100510019) for financial support. This project was also supported by the National Key Basic Research Program of China (973 Program) (Nos. 2010CB126105 and 2012CB724501) and the National Key Technology Support Program during the 12th Five-Year Plan Period (Nos. 2011BAE06B00, 2011BAE06B01, 2011BAE06B02, 2011BAE06B03 and 2011BAE06B05).
| [1] | B.S. Chai, C.L. Liu, H.C. Li, et al., The discovery of SYP-10913 and SYP-11277: novel strobilurin acaricides, Pest Manag. Sci. 67 (2011) 1141-1146. |
| [2] | B.S. Chai, C.L. Liu, H. Zhang, et al., The discovery of acaricide SYP-11277, Agrochemical (in Chinese) 5 (2011) 325-326. |
| [3] | C.L. Liu, New agrochemical candidates from intermediate derivatization method based on bioisosteric replacement, in: J.K. Wang (Ed.), Frontiers of Modern Chemical Engineering, Metallurgy, and Material Technologies -7th Academic Conference of Chemical, Metallurgy and Material Engineering Department, CAE, Chemical Industry Press Publishers, Beijing, (2009), pp. 86-94. |
| [4] | C.L. Liu, Innovation of pesticide discovery methods and candidate compounds, High-Technol. Ind. (Chinese) 9 (2008) 79-81. |
| [5] | C.L. Liu, New approach for agrochemical discovery and application (1) — intermediate derivatization method, Agrochemical (in Chinese) 1 (2011) 20-22. |
| [6] | W.R. Dolbier, Fluorine chemistry at the millennium, J. Fluorine Chem. 126 (2005) 157-163. |
| [7] | M.A. El-Sharief, Z. Moussa, A.M. El-Sharief, Synthesis, characterization, and derivatization of some novel types of fluorinated mono-and bis-imidazolidineiminothiones with antitumor, antiviral, antibacterial, and antifungal activities, J. Fluorine Chem. 132 (2011) 596-611. |
| [8] | A.P. Liu, X.G. Wang, X.M. Ou, et al., Synthesis and fungicidal activities of novel bis(trifluoromethyl)phenyl-based strobilurins, J. Agric. Food Chem. 56 (2008) 6562-6566. |
| [9] | A.P. Liu, X.G. Wang, C. Chen, et al., The discovery of HNPC-A3066: a novel strobilurin acaricide, Pest Manag. Sci. 65 (2008) 229-234. |
| [10] | H.M. Faidallah, K.A. Khan, A.M. Asiri, Synthesis and biological evaluation of new 3, 5-di(trifluoromethyl)-1,2,4-triazolesulfonylurea and thiourea derivatives as antidiabetic and antimicrobial agents, J. Fluorine Chem. 132 (2011) 870-877. |
| [11] | S. Li, C. Cui, M.Y. Wang, et al., Synthesis and fungicidal activity of new fluorinecontainingmandelic acid amide compounds, J. Fluorine Chem. 137 (2012) 108-112. |
| [12] | A. Saeed, U. Shaheen, A. Hameed, F. Kazmi, Synthesis and antimicrobial activity of some novel 2-(substituted fluorobenzoylimino)-3-(substituted fluorophenyl)-4-methyl-1,3-thiazolines, J. Fluorine Chem. 131 (2010) 333-339. |
| [13] | H.C. Li, B.S. Chai, Z.N. Li, J.C. Yang, C.L. Liu, Synthesis and fungicidal activity of novel strobilurin analogues containing substituted N-phenylpyrimidin-2-amines, Chin. Chem. Lett. 20 (2009) 1287-1290. |
| [14] | B.S. Chai, C.L. Liu, H.C. Li, et al., Design, synthesis and acaricidal activity of novel strobilurin derivatives containing pyrimidine moieties, Pest Manag. Sci. 66 (2010) 1208-1214. |
| [15] | K. Harada, H. Kubo, Y. Tomigahara, S. Inoue, A. Kojima, et al., Coumarins as novel 17β-hydroxysteroid dehydrogenase type 3 inhibitors for potential treatment of prostate cancer, Bioorg. Med. Chem. Lett. 20 (2010) 272-275. |
| [16] | C.D.S. Tomlin, Fluacrypyrim, in: The Pesticide Manual, 14th ed., British Crop Protection Council, Alton, Hants, (2006), pp. 471-472. |