b College of Science, Nanjing University of Technology, Nanjing 210009, China;
c College of Food Science and Light Industry, Nanjing University of Technology, Nanjing 210009, China
Glucose is considered not only a nutrient but also a biomarker correlated with certain diseases, such as renal glycosuria, cystic fibrosis, diabetes, human cancer, and so on. As an important regulatory signal, glucose levels also have a great impact on the hormone secretions. The current research of glucose relies mostly on the epidemics of obesity and the increased incidence of diabetes, which represent major threats for human health. At present, the detection of glucose based on glucose oxidase is very popular, but this method has several severe disadvantages, specially the potential instability during use or sterilization [1, 2]. Thus, there is a strong demand for the development of an efficient, cheap and stable glucose detecting strategies.
However, to achieve this goal, we must manipulate the characteristics of the recognized molecular. It has been demonstrated that a tetrahedral boronate ion can form stable complexes with carbohydrates with either 1,2- or 1,3-diol groups. Thus, phenylboronic acid derivatives can specifically recognize carbohydrate and have already been widely employed as promising components for glucose detection [3]. Herein, we used 3- aminophenylboronic acid (APBA) as the affinity selection part, since it is a cheap commercial compound that can stay stable for relatively long period of time.
In this study, we focused on the borate interactions with glucose at different pH ranges reported by gold nanoparticles (AuNPs). Colorimetric assays based on AuNPs have been widely used in a variety of research fields owing to the simple technology and flexible mechanical system. According to the rational surface/ interface design, AuNPs exhibit excellent recognition ability. The high extinction coefficient of AuNPs transduces the binding events into detectable optical signals that can be directly observed by naked eyes [4]. Comparing with the fluorescent methods, colorimetric assays need no tedious fabrication operation or any complicated instruments [5].
HAuCl4⋅3H2O, 3-aminophenylboronic acid (APBA), α-amylase, and all metallic salts used in this study were obtained from Sigma Chemical Company. The glucose was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). All other chemicals were of analytical grade. All solutions used in this study were prepared with ultrapure water (18.2 MΩ/cm) from an ultrapure water system (Sartorius, Germany). An artificial saliva matrix was prepared according to the ISO/TR 10271 standard [6].
AuNPs were prepared by using the usual citrate reduction method [7, 8] with some modifications. Briefly, trisodium citrate (3.2 mL 1 wt%) was rapidly added to a boiling solution of HAuCl4, the color of the solution changed from colorless to wine red after boiling for 10 min with stirring. The resulting AuNPs molar concentration was calculated to be 2.2 nmol/L. APBA (20 μL) was added to the AuNPs to produce a reaction mixture for the further glucose determination. UV-vis spectroscopy analysis was performed to determine the optimal detection conditions by a UV-vis absorption spectrophotometer Lambda 25 (Perkin Elemer). UV-vis extinction spectra of the standard solution of glucose with different concentrations and artificial saliva matrix were recorded. Transmission electron microscopy (TEM) analysis of the resulting AuNPs was obtained using a JEOL, JEM-200CX microscope (Japan).
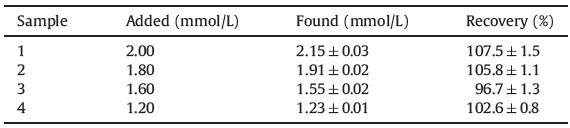
Sensing mechanism employed in this study was shown in Scheme 1. AuNPs can be triggered to aggregate by the electric charge transition after the addition of APBA. The amine group in the APBA plays an important role in this strategy due to its strong binding to the AuNPs interface through both covalent and electrostatic attractions [9, 10]. Also, the amine group lowers the pseudo pKa-value of the boronic acid. The charged forms of APBA can form a stable complex with glucose through reversible covalent binding whereas the neutral forms are highly susceptible to hydrolysis. When the pH-value is higher than the pKa of the APBA, the high density charges caused by the boronic cyclic ester adsorbed around the AuNPs would largely amplify the electrostatic repulsions and keep them from coming close as shown in Scheme 1(A). Here, glucose acts just like a protector, which successfully prevents the formation of bis-bidentate complexes and effectively maintains the AuNPs dispersion [11, 12]. However, as described in Scheme 1(B) when the pH-value of the detection system went down below the pKa of APBA, the presence of positive charges minimized the electrostatic repulsions between the negatively charged boronates, inducing cross-linking of the borate moieties upon bis-bidentate complex formation with glucose. The mechanism illustrates the AuNPs-aggregation involves one glucose molecule bound simultaneously to two boronates and brings the gold nanoparticle closer, eventually causes a color change.
|
Download:
|
| Scheme 1.Detection of glucose at pH-value upon pKa (A), and below pKa (B). The pKa of the APBA is 8.86. | |

|
Download:
|
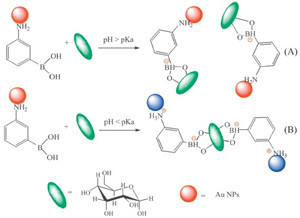
| Fig. 1.TEM images of AuNPs with APBA and glucose addition at pH 9 (A) AuNPs with APBA addition only (B) AuNPs with APBA and glucose addition at pH 6 (C). | |
|
Download:
|
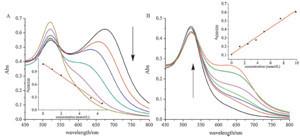
| Fig. 2.UV–vis spectra of solutions of 15 nm AuNPs upon the addition of 30 μmol/L APBA and glucose at pH 9 (A); and AuNPs upon the addition of 10 μmol/L APBA and glucose at pH 6 (B). | |
|
Download:
|
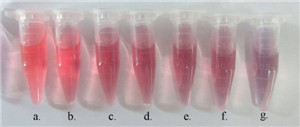
| Fig. 3.Visual color changes of 15 nmAuNPs solutions after addition of 8 μmol/L APBA and different concentrations of glucose in artificial saliva. (a) 0 mmol/L, (b) 0.25 mmol/L, (c) 1.5 mmol/L, (d) 2.75 mmol/L, (e) 4.5 mmol/L, and (f) 6.75 mmol/L. | |
| Table 1 The results of recovery test. |
In this work, we managed to design a novel strategy for the effective detection of glucose that exhibited dual behaviors in response to glucose concentrations depending on the operating pH-value. This work provided a new thought for the development of simple, inexpensive assays thatmay be highly useful for practical glucose monitoring. We anticipate that more improvements can be achieved in such systems for the development of glucose sensors and more information can be gathered for the studies on the interactions between boronic acids and glycoproteins on cell surfaces.
This work was supported by the National Natural Science Foundation of China for Young Scholars (No. 21106064), the National Basic Research Program of China (No. 2012CB725204), the National Science Foundation for Distinguished Young Scholars of China (No. 21225626), the Natural Science Foundation of Jiangsu Province (Nos. BK2012822 and BK20131406).
| [1] | T.S. Li, K. Zhu, S. He, et al., Sensitive detection of glucose based on gold nanoparticles assisted silver mirror reaction, Analyst 136 (2011) 2893-2896. |
| [2] | H. Wei, E. Wang, Fe3O4 magnetic nanoparticles as peroxidase mimetics and their applications in H2O2 and glucose detection, Anal. Chem. 80 (2008) 2250-2254. |
| [3] | Q. Wu, L. Wang, H. Yu, J. Wang, Z. Chen, Organization of glucose-responsive systems and their properties, Chem. Rev. 111 (2011) 7855-7875. |
| [4] | X.R. Yang, J. Xu, X.M. Tang, H.X. Liu, D.B. Tian, A novel electrochemical DNAzyme sensor for the amplified detection of Pb2+ ions, Chem. Commun. 46 (2010) 3107-3109. |
| [5] | X.W. Xu, X.R. Yang, Facile colorimetric detection of glucose based on an organic Fenton reaction, Anal. Methods 3 (2011) 1056-1059. |
| [6] | ISO TR 10271, Dentistry-Determination of Tarnish and Corrosion of Metals and Alloys, first ed., Bern, Switzerland, 1993, pp. 3-8. |
| [7] | J.J. Storhoff, R. Elghanian, R.C. Mucic, C.A. Mirkin, R.L. Letsinger, One-pot colorimetric differentiation of polynucleotides with single base imperfections using gold nanoparticle probes, J. Am. Chem. Soc. 120 (1998) 1959-1964. |
| [8] | J. Wang, Y. Cao, Y.Y. Xu, G.X. Li, Colorimetric multiplexed immunoassay for sequential detection of tumor markers, Biosens. Bioelectron. 25 (2009) 532-536. |
| [9] | H.L. Zhang, S.D. Evans, J.R. Henderson, R.E. Miles, T.H. Shen, Spectroscopic characterization of gold nanoparticles passivated by mercaptopyridine and mercaptopyrimidine derivatives, J. Phys. Chem. B. 107 (2003) 6087-6095. |
| [10] | X.R. Yang, H.X. Liu, J. Xu, et al., A simple and cost-effective sensing strategy of mercury (Ⅱ) based on analyte-inhibited aggregation of gold nanoparticles, Nanotechnology 22 (2011) 275503-275508. |
| [11] | C. Ancla, V. Lapeyre, I. Gosse, B. Catargi, V. Ravaine, Designed glucose-responsive microgels with selective shrinking behavior, Langmuir 27 (2011) 12693-12701. |
| [12] | K. Nakashima, R. Iguchi, S. Shinkai, Diaza-18-crown-6-based saccharide receptor bearing two boronic acids. Possible communication between bound saccharides and metal cations, Ind. Eng. Chem. Res 39 (2000) 3479-3483. |
| [13] | J. Böeseken, The use of boric acid for the determination of the configuration of carbohydrates, Adv. Carbohydr. Chem. 4 (1949) 189-210. |