b Key Laboratory of Monitoring and Management of Crop Diseases and Pest Insects, Ministry of Agriculture, Nanjing Agricultural University, Nanjing 210095, China;
c College of Science, Nanjing Agricultural University, Nanjing 210095, China
As nitrogenous heterocyclic compounds with the pyrrolidine- 2,4-dione moiety, natural tetramic acids often exhibit not only antitumor and antiviral [1, 2], but also herbicidal, insecticidal and fungicidal activity [3, 4, 5]. Using natural tetramic acid as the lead compound, many pyrrolidine-2,4-dione derivatives were designed and synthesized, a considerable part of which was found to present remarkable biological activity [6, 7, 8, 9, 10]. It is noteworthy that when proper groups, such as oxime ethers and Schiff bases, were introduced to the 3-position of the pyrrolidine-2,4-dione, the corresponding tetramic acid derivatives showed better inhibitory activity against phytopathogenic fungi than the naturally occurring tenuazonic acid (also called TeA) in the tetramic acid family [11, 12, 13, 14].
Strobilurin fungicides were derived from another naturally occurring lead compound strobilurin A with a reactive group of β-methoxyacrylate. They have the outstanding characteristics of unique action mechanism, broad spectrum, long duration, high activity and outstanding environmental tolerability [15]. In this study, two reactive groups, namely pyrrolidine-2,4-dione and β-methoxyacrylate, were spliced in a same structure through an oxime ether bridging group or a methylene bridging group. This modification was expected to improve the fungicidal activity.
The melting points of the synthesized compounds were measured on a WRS-1B digital melting point apparatus. IR spectra were recorded on a Bruker Tensor 27 FT-IR spectrometer with a KBr disk. Elemental analyses were determined on an Elementar Vario EL cube analyser. 1H NMR and 13C NMR spectra were collected on a Bruker AV 400 MHz spectrometer with CDCl3 as the solvent and TMS as the internal reference. Mass spectra were recorded on a GC/MS-QP2010 spectrometer using a direct injection technique.
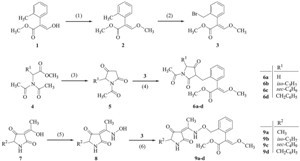
The synthetic routes for the targeted compounds were illustrated in Scheme 1. (E)-Methyl-2-(2-(bromomethyl)phenyl)- 3-methoxyacrylate 3 was prepared by the methylation of the hydroxy group followed by a bromination of the methyl group in (E)-methyl-2-(2-methylphenyl)-3-hydroxyacrylate 1 according to the method reported in the literatures [16, 17]. 1-Acetylpyrrolidine- 2,4-diones 5 were prepared by the cyclization of N,Ndiacetyl- amino acid methyl esters 4 according to the method reported in the literature [18]. Compound 5 (5 mmol) and K2CO3 (10 mmol) were stirred in DMF (50 mL) at room temperature for 30 min, then compound 3 (5 mmol) was added. The mixture was heated at 60 ℃ for 8 h and the reaction was monitored by TLC analysis. After the reaction completed, the mixture was diluted with 300 mL of water and extracted with ethyl acetate. The organic layer was washed with water and saturated NaCl solution sequentially, dried over MgSO4, and concentrated in vacuo. The residue was separated by a silica gel column to afford the targeted compounds 6a-6d.
|
Download:
|
| Scheme 1.Synthetic routes for the compounds 6 and 9. Condition and regent: (1) (CH3)2CO, K2CO3, (CH3)2SO4; (2) CCl4, NBS, AIBN; (3) Na, xylene; (4) DMF, K2CO3; (5) NH2OH·HCl, C2H5OH; (6) DMF, K2CO3. | |
Through the reactions of the key intermediate 3 with compounds 5 and 8 respectively, two series of novel β-methoxyacrylate derivatives 6a-6dand 9a-9dwere prepared in yields of 35-55%. An efficient and operationally simple route was developed to introduce substituted benzyl moieties at the 3- position of pyrrolidine-2,4-dione. By contrast, the compounds 2- (substituted benzyl)-1,3-cyclopentadiones were prepared by a two-step procedure including a Knoevenagel condensation and a hydrogenation reaction [20]. And the compounds 2-(substituted benzyl)-1,3-cyclohexanediones were prepared by an organocatalytic reductive alkylation between an aldehyde and the hantzsch ester [21]. The targeted compounds 9a-9dwere prepared using a traditional method. The heterocyclic pyrrolidine-2,4-dione was attached to the reactive structure of β-methoxyacrylate through an oxime ether group.
The structures of the targeted compounds were confirmed by IR, 1H NMR, 13C NMR, MS and elemental analysis. The IR spectrum showed peaks at 1743-1627 cm-1 assigned to the carbonyl groups and peaks at 3268-3189 cm-1 due to N-H stretching vibration of compounds 9a-9d. Compounds 6a-6dgave peaks at 3367- 3305 cm-1 due to O-H stretching vibration, because these four oily liquids belong to N-acyl tetramic acids and exist in both keto and enol forms [22]. The 1H NMR spectrum of all the compounds showed peaks between δ 3.83 and 4.90 for the protons at the 5- postion of the pyrrolidine, and peaks between δ 3.69 and 3.85 for the protons of the OCH3 group. Compounds 6a-6dshowed peaks at d 1.98-2.06 for the COCH3 protons at the 1-postion of the pyrrolidine. Compounds 9a-9dshowed peaks at δ 5.59-8.19 for the proton of N-H at the 1-postion. The 13C NMR spectrum showed all corresponding peaks except that of PhCH2 at the 3-position of compounds 6a-6d. This phenomenon was also observed in the compounds 2-(substituted benzyl)-1,3-cyclohexanediones [21]. Only compounds 9a and 9c showed [M+] peaks, but other targeted compounds gave matched fragment ion peaks. Moreover, all the target compounds showed satisfactory elemental analysis data. Selected characterization data of the targeted compounds are listed below.
6a: Yellow viscous liquid, yield: 46%; IR (KBr, cm-1): v 3344, 3066, 2942, 2851, 1743, 1627, 1557, 1436, 1263, 1197, 1132; 1H NMR(400 MHz, CDCl3): δ 2.06 (s, 3H, CH3CO), 3.71 (s, 3H, COOCH3), 3.85 (d, 3H, J = 6.8 Hz, OCH3), 4.02-4.08 (m, 2H, NCH2), 5.10 (s, 2H, PhCH2), 6.07 (s, 1H, COCHCO), 7.15-7.18 (m, 1H, PhH), 7.31-7.37 (m, 2H, PhH), 7.40-7.43 (m, 1H, PhH), 7.58 (d, 1H, J = 5.3 Hz, C=CH); 13CNMR(100 MHz, CDCl3): δ 22.45 (CH3CO), 41.43 (NCH2), 51.84 (COOCH3), 62.16 (OCH3), 65.41 (COCHCO), 109.55 (C=CH), 128.11, 128.32, 128.76, 131.24, 132.19, 134.31 (6C, C6H4), 160.71 (C=CH), 168.24, 169.90, 172.06, 187.93 (4C, 4×CO); MS m/z (%): 321(1), 204(4), 190(100), 145(35); Anal. Calcd. for C18H19NO6: C, 62.60; H, 5.55; N, 4.06; Found: C, 62.98; H, 5.46; N, 4.17.
6b: Yellow viscous liquid, yield: 55%; IR (KBr, cm-1): v 3361, 3066, 2964, 2876, 1738, 1634, 1528, 1435, 1258, 1200, 1145; 1H NMR (400 MHz, CDCl3): δ 0.87 (d, 3H, J = 6.9 Hz, CH3CHCH3), 0.93 (d, 3H, J = 6.8 Hz, CH3CHCH3), 2.14-2.18 (m, 1H, CH3CHCH3), 2.04 (s, 3H, CH3CO), 3.72 (s, 3H, COOCH3), 3.84 (s, 3H, OCH3), 4.61 (dd, 1H, J = 8.8, 4.7 Hz, NCH), 5.07 (q, 2H, J = 12.6 Hz, PhCH2), 6.00 (d, 1H, J = 7.8 Hz, COCHCO), 7.16-7.18 (dd, 1H, J = 8.3, 4.8 Hz, PhH), 7.32-7.38 (m, 2H, PhH), 7.39-7.45 (m, 1H, PhH), 7.59 (s, 1H,C=CH); 13C NMR (100 MHz, CDCl3): δ 17.70 (CH3CHCH3), 19.00 (CH3CHCH3), 22.66 (CH3CO), 31.02 (CH3CHCH3), 51.71 (COOCH3), 57.40 (NCH), 62.07 (OCH3), 65.17 (COCHCO), 109.57 (C=CH), 128.04, 128.16, 128.53, 131.22, 132.08, 134.31 (6C, C6H4), 160.59 (C=CH), 167.93, 171.27, 171.93, 187.87 (4C, 4×CO); MS m/z (%): 363(0.5), 205(4), 190(40), 145(19); Anal. Calcd. for C21H25NO6: C, 65.10; H, 6.50; N, 3.62; Found: C, 65.26; H, 6.66; N, 3.57.
6c: Yellow viscous liquid, yield: 45%; IR (KBr, cm-1): v 3367, 3067, 2963, 2878, 1738, 1630, 1537, 1456, 1255, 1205, 1143; 1H NMR (400 MHz, CDCl3): δ 0.82-0.94 (m, 6H, CH3CH2 + CH3CH), 1.07-1.42 (m, 2H, CH3CH2), 1.84-1.99 (m, 1H, CH3CH), 2.03 (d, 3H, J = 4.4 Hz, CH3CO), 3.71 (s, 3H, COOCH3), 3.84 (s, 3H, OCH3), 4.63, 4.74 (dd, dd, 1H, J = 8.7, 5.0, 9.1, 3.8 Hz, NCH), 5.01-5.12 (m, 2H, PhCH2), 6.00 (dd, 1H, J = 30.2, 8.6 Hz, COCHCO), 7.14-7.20 (m, 1H, PhH), 7.31-7.38 (m, 2H, PhH), 7.38-7.44 (m, 1H, PhH), 7.59 (s, 1H, C=CH); 13C NMR (100 MHz, CDCl3): δ 11.72 (CH3CH3), 15.45 (CH3CH), 22.76 (CH3CO), 26.16 (CH3CH3), 37.55 (CH3CH), 51.83 (COOCH3), 55.66 (NCH), 62.16 (OCH3), 65.43 (COCHCO), 109.66 (C=CH), 128.14, 128.26, 128.54, 131.26, 132.02, 134.24(6C, C6H4), 160.59 (C=CH), 168.06, 171.35, 171.91, 187.83 (4C, 4×CO); MS m/z (%): 377(1), 205(5), 190(40), 145(25); Anal. Calcd. for C22H27NO6: C, 65.82; H, 6.78; N, 3.49; Found: C, 65.58; H, 6.86; N, 3.37.
6d: Yellow viscous liquid, yield: 55%; IR (KBr, cm-1): v 3305, 3063, 2948, 2844, 1741, 1703, 1634, 1541, 1495, 1434, 1253, 1130; 1H NMR (400 MHz, CDCl3) d: 1.98 (s, 3H, CH3CO), 3.11 (ddd, 2H, J = 30.4, 13.8, 5.7 Hz, phCH2), 3.69 (s, 3H, COOCH3), 3.82 (s, 3H, OCH3), 4.90 (dt, 1H, J = 7.7, 5.6 Hz, NCH), 5.06 (s, 2H, phCH2), 5.95 (d, 1H, J = 7.6 Hz, COCHCO), 7.00-7.03 (m, 2H, PhH), 7.18 (d, 1H, J = 6.4 Hz, PhH), 7.23-7.26 (m, 3H, PhH), 7.31-7.40(m, 3H, PhH), 7.59 (s, 1H, C=CH); 13C NMR (100 MHz, CDCl3): δ 22.70 (CH3CO), 37.60 (phCH2), 51.75 (COOCH3), 53.53 (NCH), 62.07 (OCH3), 65.39 (COCHCO), 109.67 (C=CH), 127.02, 128.09, 128.27, 128.56, 128.77, 129.32, 131.29, 132.26, 134.27, 136.24 (12C, C6H5 + C6H4), 160.62 (C=CH), 167.94, 170.69, 171.57, 187.95 (4C, 4×CO); MS m/z (%): 411(1), 205(45), 190(62), 145(76); Anal. Calcd. for C25H25NO6: C, 68.95; H, 5.79; N, 3.22; Found: C, 68.78; H, 5.86; N, 3.27.
9a: Yellow viscous liquid, yield: 35%; IR (KBr, cm-1): v 3268, 2923, 2852, 1704, 1633, 1565, 1431, 1256, 1129, 1109; 1H NMR (400 MHz, CDCl3): δ 1.37 (dd, 3H, J = 6.9, 4.5Hz, CH3CH), 2.41 (s, 3H, CH3CNH), 3.69 (s, 3H, COOCH3), 3.82 (s, 3H, OCH3), 3.93-4.02 (m, 1H, CHNH), 4.93 (s, 2H, CH2O), 5.59 (br, 1H,CHNH), 7.22 (d, 1H, J = 7.3 Hz, PhH), 7.36-7.45 (m, 3H, PhH), 7.62 (s, 1H, C=CH), 7.78(br, 1H, NHO); 13C NMR (100 MHz, CDCl3): δ 11.31 (CH3CNH), 17.62 (CH3CH), 51.63 (COOCH3), 53.02 (CHNH), 62.04 (OCH3), 75.93 (CH2O), 96.18 (COCCO), 109.83 (C=CH), 128.10, 128.60, 129.16, 131.41, 132.63, 134.26 (6C, C6H4), 160.22 (C=CH), 160.36 (CNH), 167.81, 172.08, 189.66 (3C, 3×CO); MS m/z (%): 374[M+](1), 205(20), 190(80), 145(60); Anal. Calcd. for C19H22N2O6: C, 60.95; H, 5.92; N, 7.48; Found: C, 60.76; H, 5.86; N, 7.57.
9b: White powder, yield: 50%, mp 127.2-128.5 ℃; IR (KBr, cm-1): v 3189, 2955, 2874, 1707, 1679, 1631, 1567, 1487, 1253, 1128, 1105; 1H NMR (400 MHz, CDCl3): δ 0.86 (dd, 3H, J = 5.8, 2.4 Hz, CH3CHCH3), 1.04 (dd, 3H, J = 6.8, 3.1 Hz, CH3CHCH3), 2.21 (br, 1H, CH3CHCH3), 2.39 (s, 3H, CH3CNH), 3.69 (s, 3H, COOCH3), 3.83 (br, 4H, OCH3 + CHNH), 4.94 (s, 2H, CH2O), 7.23 (d, 1H, J = 6.4 Hz, PhH), 7.35-7.47 (m, 3H, PhH), 7.63 (d, 1H, J = 7.4 Hz, C=CH), 8.19 (br, 1H, CHNH), 10.28(br, 1H, NHO); 13C NMR (100 MHz, CDCl3): δ 11.36 (CH3CNH), 15.75 (CH3CHCH3), 19.40 (CH3CHCH3), 29.88 (CH3CHCH3), 51.77 (COOCH3), 62.07 (OCH3), 62.20 (CHNH), 75.86 (CH2O), 97.91 (COCCO), 110.02 (C=CH), 128.15, 128.60, 129.08, 131.38, 132.51, 134.39 (6C, C6H4), 160.22 (C=CH), 160.43 (CNH), 167.80, 172.49, 187.43 (3C, 3×CO); MS m/z (%): 279(2), 190(65), 161(38), 140(65); Anal. Calcd. for C21H26N2O6: C, 62.67; H, 6.51; N, 6.96; Found: C, 63.26; H, 6.50; N, 6.85.
9c: Yellow viscous liquid, yield: 41%; IR (KBr, cm-1): v 3207, 2960, 2868, 1709, 1679, 1634, 1568, 1431, 1257, 1130, 1110; 1H NMR (400 MHz, CDCl3): δ 0.76-1.01 (m, 6H, CH3CH2 + CH3CH), 1.23-1.48(m, 2H, CH3CH2), 1.92-1.98 (m, 1H, CH3CH), 2.39 (d, 3H, J = 3.9 Hz, CH3CNH), 3.69 (s, 3H, COOCH3), 3.82 (s, 3H, OCH3), 3.88- 3.95 (m, 1H, CHNH), 4.94 (s, 2H, CH2O), 7.22 (d, 1H, J = 7.4 Hz, PhH), 7.34-7.40 (m, 2H, PhH), 7.40-7.46 (m, 1H, PhH), 7.62 (s, 1H, C=CH), 8.06 (br, 1H, CHNH), 11.48 (br, 1H, NHO); 13C NMR (100 MHz, CDCl3): δ 11.20 (CH3CNH), 11.81 (CH3CH2), 15.52 (CH3CH), 26.68 (CH3CH2), 36.72 (CH3CH), 51.59 (COOCH3), 60.62 (CHNH), 61.91 (OCH3), 75.66 (CH2O), 98.24 (COCCO), 109.86 (C=CH), 127.95, 128.39, 128.99, 131.33, 132.53, 134.59 (6C, C6H4), 160.04 (CNH), 160.29 (C=CH), 167.76, 172.73, 187.13 (3C, 3×CO); MS m/z (%): 416(M+, 1), 205(23), 190(60), 140(100); Anal. Calcd. for C22H28N2O6: C, 63.45; H, 6.78; N, 6.73; Found: C, 63.56; H, 6.86; N, 6.57.
9d: Yellow viscous liquid, yield: 41%; IR (KBr, cm-1): v 3245, 2923, 2843, 1704, 1633, 1567, 1431, 1363, 1256, 1130, 1110; 1H NMR (400 MHz, CDCl3): δ 2.35 (d, 3H, J = 2.8 Hz, CH3CNH), 2.66- 2.82 (m, 1H, phCH2), 3.24 (dd, 1H, J = 13.9, 3.6 Hz, phCH2), 3.66- 3.71 (m, 3H, COOCH3), 3.81 (d, 3H, J = 8.4 Hz, OCH3), 4.05-4.14 (m, 1H, CHNH), 4.92 (s, 2H, CH2O), 5.76 (br, 1H, CHNH), 7.19-7.47 (m, 9H, PhH), 7.62 (s, 1H, C=CH), 7.71 (m, 1H, NHO); 13C NMR (100 MHz, CDCl3): δ 11.35 (CH3CNH), 38.39 (phCH2), 51.80 (COOCH3), 59.01 (CHNH), 62.08 (OCH3), 76.31 (CH2O), 96.37 (COCCO), 109.89 (C=CH), 126.84, 128.17, 128.57, 128.85, 129.34, 129.93, 131.54, 132.81, 133.95, 136.86 (12C, C6H5 + C6H4), 160.43 (C=CH), 161.75 (CNH), 167.86, 172.10, 189.37 (3C, 3×CO); MS m/z (%): 230(29), 190(53), 161(32), 139(100); Anal. Calcd. for C25H26N2O6: C, 66.66; H, 5.82; N, 6.22; Found: C, 66.55; H, 5.96; N, 6.27.
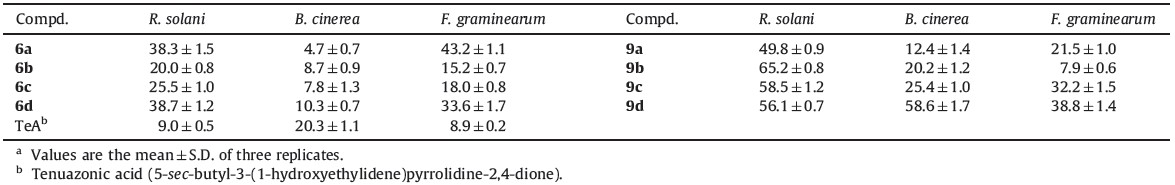
The targeted compounds were evaluated for fungicidal activity against R. solani, B. cinerea and F. graminearum at the concentration of 100 mg/L according to the method reported in the literature [13]. The results were summarized in Table 1. These compounds showed fungicidal activity at different degree. Most compounds exhibited good activity against R. solani, among them the inhibitory rates of compounds 9b, 9c and 9d reached 65.2%, 58.5% and 56.1%, respectively, obviously superior to that of the lead compound TeA, which demonstrated an inhibitory rate of only 9.0%. As a whole the oxime ether derivatives 9a-9dshowed better bioactivity than the benzyl derivatives 6a-6d. Compound 9d with a benzyl group at the 5-position of pyrrolidine-2,4-dione exhibited better bioactivity than other compounds 9a-9c.
| Table 1 Oligonucleotides designed in the present study.a |
In summary, two series of β-methoxyacrylate derivatives containing the pyrrolidine-2,4-dione moiety were designed and synthesized. A preliminary analysis of the structure-activity relationship revealed that the fungicidal activity of compounds 9a-9d with an oxime ether bridging group were better than that of compounds 6a-6dwith a methylene bridging group, especially against R. solani and B. cinerea. Introducing the oxime ether groups at the 3-position and a benzyl group at the 5-position of tetramic acid was helpful to improve the bioactivity of strobilurin derivatives containing the pyrrolidine-2,4-dione moiety.
This work was financially supported by the National Key Technologies R & D Program of China (No. 2011BAE06B04), Science & Technology Pillar Program of Jiangsu Province (No. BE2012371), the National Natural Science Foundation of China (No. 31171889), 863 Program of China (No. 2011AA10A206), and the Fundamental Research Funds for the Central Universities of China (No. KYZ201223).
| [1] | A. Mary, P. Krishna, K.K. Gupta, Inhibition of mouse skin tumor promotion by tenuazonic acid, Cancer Lett. 61 (1991) 21-25. |
| [2] | S.B. Singh, D.L. Zink, M.A. Goetz, et al., Equisetin and a novel opposite stereochemical homolog phomasetin, two fungal metabolites as inhibitors of HIV-1 integrase, Tetrahedron Lett. 39 (1998) 2243-2246. |
| [3] | S.G. Chen, C.Y. Yin, X.B. Dai, et al., Action of tenuazonic acid, a natural phytotoxin, on photosystem II of spinach, Environ. Exp. Bot. 62 (2008) 279-289. |
| [4] | M. Cole, G.N. Rolinson, Microbial metabolites with insecticidal properties, Appl. Microbiol. 24 (1972) 660-662. |
| [5] | J.Y. Li, G. Strobel, J. Harper, et al., Cryptocin, a potent tetramic acid antimycotic from the endophytic fungus Cryptosporiopsis cf Quercina, Org. Lett. 2 (2000) 767-770. |
| [6] | B.F. Han, Q.M. Shi, X.F. Wang, et al., Synthesis and bioactivity of novel 3-(1-hydroxyethylidene)-5-substituted-pyrrolidine-2, 4-dione derivatives, Chin. Chem. Lett. 23 (2012) 1023-1026. |
| [7] | T.F. Si, F.G. Meng, X.F. Wang, et al., Synthesis and herbicidal activities of (Z,E)-1-[1-(2, 4-dioxopyrrolidine-3-ylidene)ethyl]-4-alkylsemicarbazide derivatives, Chin. J. Org. Chem. 31 (2011) 521-527. |
| [8] | X.Q. Zheng, B.F. Han, X.F. Wang, et al., Synthesis and bioactivity of novel (Z,E)-1-(substituted phenyl)-3-[α-(alkyloxyimino)benzylidene]pyrrolidine-2, 4-dione derivatives, Heterocycl. Commun. 17 (2011) 73-78. |
| [9] | Y.Q. Zhu, X.K. Si, X.M. Zou, et al., Synthesis and herbicidal evaluation of novel 3-(hydroxymethylene)pyrrolidine-2, 4-dione, Chin. J. Org. Chem. 27 (2007) 385-390. |
| [10] | Y.Q. Zhu, X.M. Zou, F.Z. Hu, et al., Synthesis and herbicidal evaluation of novel 3-[(a-hydroxy-substituted)benzylidene]pyrrolidine-2, 4-diones, J. Agric. Food Chem. 53 (2005) 9566-9570. |
| [11] | X.F. Wang, T.F. Si, Q.B. Li, et al., Synthesis, characterization and biological activity of novel (5-RS,6-S)-5-sec-butyl-3-(1-substituted-amino)ethylidene-1H-pyrrolidine-2,4-diones, ARKIVOC ii (2010) 31-48. |
| [12] | Z.Y. Zhu, Q.M. Shi, B.F. Han, et al., Synthesis, characterization and biological activities of novel (E)-3-(1-(alkyloxyamino)ethylidene)-1-alkylpyrrolidine-2,4-dione derivatives, Bull. Korean Chem. Soc. 31 (2010) 2467-2472. |
| [13] | Z.Y. Zhu, X.F. Wang, F.G. Meng, et al., Synthesis, characterization, and biological activities of novel (Z)-3-((E)-1-(alkyloxyimino)ethyl)-5-arylidene-4-hydroxypyrroline-2-one derivatives, J. Heterocyclic Chem. 47 (2010) 1328-1334. |
| [14] | X.J. Zhu, L. Huang, X.F. Wang, et al., Synthesis and biological activities of 3-(1'-alkyloxyiminoethyl)-4-hydroxypyrroline-2-one derivatives, Chin. J. Org. Chem. 29 (2009) 1784-1789. |
| [15] | X.J. Yan, S.H. Jin, F.H. Chen, et al., Advance in research of the target of action of strobilurin fungicides, Chin. J. Pestic. Sci. 8 (2006) 299-305. |
| [16] | G.F. Yang, P.L. Zhao, W. Huang, et al., A strobilurin fungicide, preparation method and application, CN Patent no. 101,268,780A (2008). |
| [17] | K.P. Yu, Z.F. Li, J.P. Zhao, et al., Preparing method of fungicide intermediate (E)-methyl-2-(2-bromomethyl)phenyl-3-methoxyacrylate, CN Patent no. 101,381, 305A (2009). |
| [18] | M. Petroliagi, O. Igglessi-Markopoulou, Synthesis and enantiomeric excess measurements of optically active N-acetyl tetramic acids, Tetrahedron: Asymmetry 10 (1999) 1873-1875. |
| [19] | H. Dai, J.B. Liu, W.K. Miao, et al., Synthesis and biological activities of novel pyrazole oxime ether derivatives containing pyridyl ring, Chin. J. Org. Chem. 31 (2011) 1662-1667. |
| [20] | J.W.J. Kennedy, S. Vietrich, H. Weinmann, et al., Synthesis of 7a-substituted Hajos-Wiechert ketone analogues, J. Org. Chem. 73 (2008) 5151-5154. |
| [21] | D.B. Ramachary, M. Kishor, Organocatalytic sequential one-pot double cascade asymmetric synthesis of Wieland-Miescher ketone analogues from a Knoevenagel/hydrogenation/Robinson annulation sequence: scope and applications of organocatalytic biomimetic reductions, J. Org. Chem. 72 (2007) 5056-5068. |
| [22] | Y.C. Jeong, M.G. Moloney, Tetramic acids as scaffolds: synthesis, tautomeric and antibacterial behaviour, Synlett 15 (2009) 2487-2491. |