The desymmetrization of meso cyclic anhydrides via the addition of carbon-based nucleophiles represents a well-established and powerful synthetic tool in asymmetric synthesis because it allows for the construction of multiple stereogenic centers in one symmetry-breaking operation [1]. Over the past two decades, the asymmetric opening of anhydrides with carbon-based nucleophiles has been extensively investigated because of the great potential for the application of this technique in the asymmetric synthesis of important chiral intermediates and optically active fine chemicals (Scheme 1) [2].

|
Download:
|
| Scheme 1. Asymmetric anhydride openings via carbon-based nucleophiles. | |
The ability of transition-metal complexes to mediate carbon- carbon bond-forming reactions makes them invaluable tools in organic synthesis [4]. Rovis et al. [5] provided a detailed description of the investigations involved in the asymmetric alkylation of anhydrides catalyzed by transition-metals. Nickel-, palladium- and rhodium-based catalysts have all been successfully applied to the desymmetrization of prochiral cyclic anhydrides.
Non-stereoselective methods for the desymmetrization of cyclic anhydrides were initially studied by Rovis [5], providing a platform for the subsequent development of the asymmetric anhydride alkylation reaction. The 2,2'-bipyridyl (bipy) nickel complex was found to be highly effective in promoting the alkylation of succinic anhydrides, whereas the use of the (2- diphenylphosphino)ethylpyridine (pyphos)-derived nickel complex was successfully used to facilitate the alkylation of glutaric anhydrides to give the corresponding keto acids in high yields [5a,c]. Interestingly, the use of nickel allowed for the introduction of chiral ligands to provide enantioselectivity over the bond-forming event. As shown in Scheme 2, the use of the phosphino-oxazoline ligand (i-PrPHOX, 1) afforded an active catalyst for the alkylation of cyclohexane-1,2-dicarboxylic anhydride (2) leading to the formation of the keto acid product in 85% yield and 79% ee [5a].

|
Download:
|
| Scheme 2. Ni-catalyzed asymmetric alkylation of 2. | |
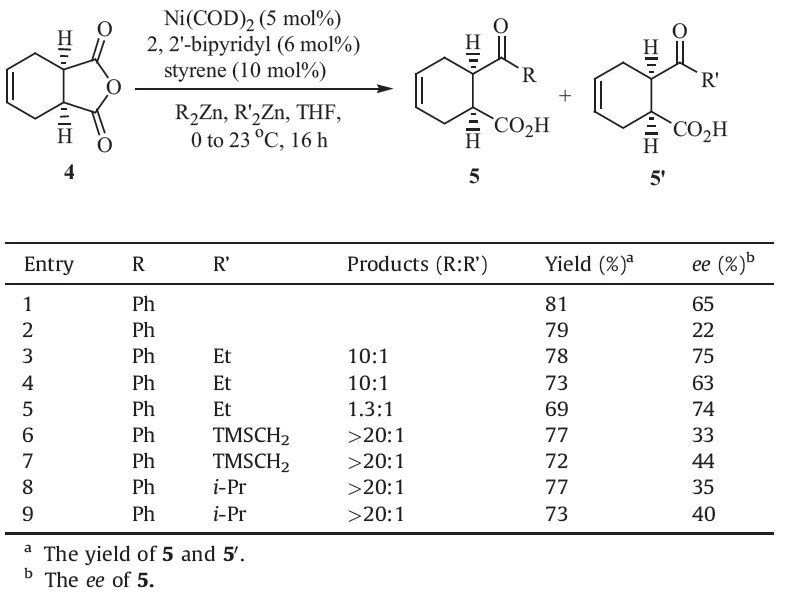
| Table 1 Ni-catalyzed asymmetric opening of 4. |

|
Download:
|
| Scheme 3. The proposed mechanistic pathways. | |
An improved protocol for the enantioselective desymmetrization of cyclic anhydrides using palladium-based catalysts was also reported (Scheme 4) [8]. The enantioselective ring-opening of cyclic anhydrides was initially investigated using (S)-2,2'-bis(diphenylphosphino)- 1,1'-binaphthyl [(S)-BINAP] as the ligand and Pd(OAc2 as the palladium source, with the corresponding keto acid product obtained in 67% yield and 77% ee. The enantioselectivity of the transformation was improved by biphenyl ligands 6 and 7. However, other changes of the electronic nature of the aromatic phosphine portion of the ligand failed to increase the enantioselectivity.

|
Download:
|
| Scheme 4. Ligand screen for the asymmetric arylation of 4. | |

|
Download:
|
| Scheme 5. Pd-catalyzed asymmetric arylation of 4. | |
| Table 2 Pd-catalyzed asymmetric arylation of succinic anhydrides. |
| Table 3 Pd-catalyzed asymmetric alkylation of 4. |
Although the diorganozinc reagents have been used to provide excellent results in both nickel- and palladium-catalyzed methodologies, the commercial availability of the diorganozinc reagents is quite limited in comparison. For this reason, research efforts in this area have been shifted toward the use of zinc nucleophiles generated in situ. Pleasingly, a procedure of enantioselective desymmetrization of anhydrides using organozinc nucleophiles prepared in situ has been successfully developed using rhodium catalysis. Unfortunately, the use of zinc nucleophiles formed in situ was incompatible with the palladiumcatalyzed methodology [9].
In 2007, Rovis et al. [10] reported the successful asymmetric alkylation of cis-2,3-dimethylsuccinic anhydride (16) with 3,4,5- trimethoxyphenylzinc triflate in the presence of Taddol-PNMe2 18 to give the desired product in 85% yield and 87% ee. The 3,4,5- trimethoxyphenylzinc triflate reagent involved in this reaction was generated from the corresponding aryl bromide by lithiumhalogen exchange and subsequent reaction with zinc triflate. The reaction was found to be tolerant of a wide range of nucleophiles (Table 4), providing the corresponding keto acids in good yields (74-88%) with high enantioselectivity (80-88% ee). Furthermore, the newly developed methodology was also successfully applied to the total synthesis of three eupomatilone lignans [11].
| Table 4 Rh-catalyzed asymmetric alkylation of 16. |
| Table 5 Rh-catalyzed asymmetric arylation of succinic anhydrides. |
Although nickel- and palladium-based catalysts are largely ineffective for the addition of alkyl nucleophiles to glutaric anhydrides, Rovis et al. [12] developed a rhodium-catalyzed enantioselective alkylation reaction for the desymmetrization of meso glutaric anhydrides. An initial period of reaction optimization revealed that the alkylation of anhydride 25 with tertbutylPHOX 29 provided the best results in the presence of [Rh(nbd)Cl]2 at 25 ℃ (Table 6).
| Table 6 Catalyst and ligand optimization of alkylation of 25. |

|
Download:
|
| Scheme 6. Rh-catalyzed asymmetric alkylation. | |

|
Download:
|
| Scheme 7. Rh-catalyzed asymmetric alkylation of glutaric anhydrides. | |

|
Download:
|
| Scheme 8. Rh-catalyzed asymmetric arylation of 4. | |

|
Download:
|
| Scheme 9. Rh-catalyzed asymmetric opening of 25. | |
The first desymmetrization of cyclic anhydrides using carbon nucleophiles was reported by Real et al. [3] with chiral Grignard reagents being employed as the carbon-based nucleophiles. The addition of (-)-ephedrine-derived Grignard reagents 39a to anhydride 41 produced a solution of the salts 42a and 42b, which were further converted to 43 and 44 (66% ee) in 56% overall yield by the addition of NaBH4 followed by acid hydrolysis. Under the same conditions, the use of the (+)-pseudoephedrine-derived Grignard reagent 39b provided aldehyde 44 (>99.4% de) in 65% overall yield and 99% ee. The reaction with the compound 39c under the same conditions provided product 44 in 64% overall yield and 99.2% ee (Scheme 10).

|
Download:
|
| Scheme 10. Asymmetric arylation of 41 via Grignard reagents. | |

|
Download:
|
| Scheme 11. Asymmetric arylation of 45 via Grignard reagents. | |
| Table 7 Asymmetric openings of anhydrides via Grignard reagents |

|
Download:
|
| Scheme 12. Asymmetric opening of 53 via Grignard reagents. | |
In 2007, Harada [16] reported the enantioselective ringopening reaction of cyclic anhydrides with methallylstannane in the presence of an oxazaborolidine, which acts as a Lewis acid catalyst [17]. As shown in Scheme 14, the treatment of anhydride 55 with 2 equivalent of methallylstannane (56) in the presence of OXB 57a (30 mol%) at room temperature in CH2Cl2 provided the desired alkylation product 58. Subsequent treatment with base led to the sequential fragmentation and epimerization of the product, followed by acidic work-up and esterification to give the keto ester 60 in 91% yield and 16% ee (Scheme 13).

|
Download:
|
| Scheme 13. Asymmetric opening of 55 via methallylstannane. | |
| Table 8 Asymmetric opening of anhydrides via methallylstannane. |

|
Download:
|
| Scheme 14. Asymmetric opening of 62 via methallylstannane. | |
A number of different approaches and protocols have been developed over the past two decades for the highly enantioselective ring-opening of cyclic anhydrides for asymmetric C-C bondforming reactions. Herein, we have reviewed developments in the enantioselective desymmetrization of cyclic anhydrides with carbon-based nucleophiles. Although good yields and high enantioselectivity have been achieved using Grignard reagents and organozinc-based nucleophiles, the successful alkylation of anhydrides with high enantioselectivity using carbon nucleophiles remains challenging. The key challenges of this approach are the accessibility of the reagents and catalysts, as well as the ease of product purification. Furthermore, the discovery of new catalysts and chiral ligands that could allow for significant expansion in the current scope of the substrates and nucleophiles amenable to this approach represents an even greater challenge. To meet these challenges, considerable efforts are required to develop a broader mechanistic understanding of the reasons behind the success of the currently efficient catalytic systems, and to allow for the continuous exploration of a large range of catalysts.
| [1] | (a) S.R. Magnuson, Two-directional synthesis and its use in natural product synthesis, Tetrahedron 51 (1995) 2167-2213; (b) P. Magnus, I.K. Sebhat, Synthesis of the antitumor alkaloid (+)-pancratistatin using the β-azidonation reaction via a prochiral 4-arylcyclohexanone derivative, J. Am. Chem. Soc. 120 (1998) 5341-5342; (c) J.D. White, J. Kim, N.E. Drapela, Enantiospecific synthesis of (+)-byssochlamic acid, a nonadride from the ascomycete byssochlamys fulva, J. Am. Chem. Soc. 122 (2000) 8665-8671; (d) E. Garcia-Urdiales, I. Alfonso, V. Gotor, Enantioselective enzymatic desymmetrizations in organic synthesis, Chem. Rev. 105 (2005) 313-354. |
| [2] | (a) B.M. Trost, D.L. van Vranken, Asymmetric transition metal-catalyzed allylic alkylations, Chem. Rev. 96 (1996) 395-422; (b) A.C. Spivey, B.I. Andrews, Catalysis of the asymmetric desymmetrization of cyclic anhydrides by nucleophilic ring-opening with alcohols, Angew. Chem. Int. Ed. 40 (2001) 3131-3134; (c) Y. Chen, P. McDaid, L. Deng, Asymmetric alcoholysis of cyclic anhydrides, Chem. Rev. 103 (2003) 2965-2983; (d) C. Bolm, I. Atodiresei, I. Schiffers, M. Kanai, M. Shibasaki, Asymmetric alcoholysis of meso-anhydrides mediated by alkaloids, Org. Synth. 82 (2005) 120-125; (e) I. Atodiresei, I. Schiffers, C. Bolm, Stereoselective anhydride openings, Chem. Rev. 107 (2007) 5683-5712. |
| [3] | S.D. Real, D.R. Kronenthal, H.Y. Wu, A novel and highly efficient desymmetrization of a meso-anhydride by a chiral grignard reagent, Tetrahedron Lett. 34 (1993) 8063-8066. |
| [4] | F. Diederich, P.T. Stang, Metal-Catalyzed Cross-coupling Reactions, Wiley-VCH, Weinheim, 1998. |
| [5] | (a) E.A. Bercot, T. Rovis, A mild and efficient catalytic alkylative monofunctionalization of cyclic anhydrides, J. Am. Chem. Soc. 124 (2002) 174-175; (b) E.M. O'Brien, E.A. Bercot, T. Rovis, Decarbonylative cross-coupling of cyclic anhydrides: introducing stereochemistry at an sp3 carbon in the cross-coupling event, J. Am. Chem. Soc. 125 (2003) 10498-10499; (c) E.A. Bercot, T. Rovis, Highly efficient nickel-catalyzed cross-coupling of succinic and glutaric anhydrides with organozinc reagents, J. Am. Chem. Soc. 127 (2005) 247-254; (d) R.L. Rogers, J.L. Moore, T. Rovis, Alkene-directed regioselective nickel-catalyzed cross-coupling of cyclic anhydrides with diorganozinc reagents, Angew. Chem. Int. Ed. 46 (2007) 9301-9304. |
| [6] | (a) P. Jones, C.K. Reddy, P. Knocel, Conjugate Michael additions with mixed diorganozincs, Tetrahedron 54 (1998) 1471-1490; (b) C. Bolm, N. Hermanns, J.P. Hildebrand, K. Muñiz, Asymmetric catalytic phenyl transfer to aldehydes: enantioselective synthesis of diarylmethanols, Angew. Chem. Int. Ed. 39 (2000) 3465-3467; (c) A.E. Jensen, P. Knochel, Nickel-catalyzed cross-coupling between functionalized primary or secondary alkylzinc halides and primary alkyl halides, J. Org. Chem. 67 (2002) 79-85; (d) D. Soorukram, P. Knochel, Enantioselective synthesis ofα-Ionone derivatives using an anti SN20 substitution of functionalized zinc organometallics, Org. Lett. 6 (2004) 2409-2411. |
| [7] | J.B. Johnson, R.T. Yu, P. Fink, E.A. Bercot, T. Rovis, Selective substituent transfer from mixed zinc reagents in Ni-catalyzed anhydride alkylation, Org. Lett. 8 (2006) 4307-4310. |
| [8] | E.A. Bercot, T. Rovis, A palladium-catalyzed enantioselective alkylative desymmetrization of meso-succinic anhydrides, J. Am. Chem. Soc. 126 (2004) 10248-10249. |
| [9] | J.G. Kim, P.J. Walsh, From aryl bromides to enantioenriched benzylic alcohols in a single flask: catalytic asymmetric arylation of aldehydes, Angew. Chem. Int. Ed. 45 (2006) 4175-4178. |
| [10] | J.B. Johnson, E.A. Bercot, C.M. Williams, T. Rovis, A concise synthesis of eupomatilones 4, 6, and 7 by rhodium-catalyzed enantioselective desymmetrization of cyclic meso anhydrides with organozinc reagents generated in situ, Angew. Chem. Int. Ed. 46 (2007) 4514-4518. |
| [11] | (a) A.R. Carroll, W.C. Taylor, Constituents of Eupomatia species. XII. Isolation of constituents of the tubers and aerial parts of Eupomatia bennettii and determination of the structures of new alkaloids from the aerial parts of E. bennettii and minor alkaloids of E. laurina, Aust. J. Chem. 44 (1991) 1615-1626; (b) A.R. Carroll, W.C. Taylor, Constituents of Eupomatia species. XIV. The structures of eupomatilone-1, -2, -3, -4, -5, -6 and -7 isolated from Eupomatia bennettii, Aust. J. Chem. 44 (1991) 1705-1714. |
| [12] | M.J. Cook, T. Rovis, Rhodium-catalyzed enantioselective desymmetrization of meso-3,5-dimethyl glutaric anhydride: a general strategy to syn-deoxypolypropionate synthons, J. Am. Chem. Soc. 129 (2007) 9302-9303. |
| [13] | J.B. Johnson, M.J. Cook, T. Rovis, Ligand differentiated complementary Rh-catalyst systems for the enantioselective desymmetrization of meso-cyclic anhydrides, Tetrahedron 65 (2009) 3202-3210. |
| [14] | R. Shintani, G.C. Fu, Highly enantioselective desymmetrization of anhydrides by carbon nucleophiles: reactions of Grignard reagents in the presence of (-)-sparteine, Angew. Chem. Int. Ed. 41 (2002) 1057-1059. |
| [15] | M.J. Dearden, M.J. McGrath, P. O'Brien, Evaluation of (+)-sparteine-like diamines for asymmetric synthesis, J. Org. Chem. 69 (2004) 5789-5792. |
| [16] | J. Suzuki, T. Harada, Oxazaborolidinone-catalyzed alkylative ring-opening reaction of cyclic anhydrides with methallylstannane, Synthesis 15 (2006) 2483-2488. |
| [17] | (a) D. Seebach, R.E. Marti, T. Hintermann, Polymer-and dendrimer-bound Ti-TADDOLates in catalytic (and stoichiometric) enantioselective reactions. Are pentacoordinate cationic Ti complexes the catalytically active species? Helv. Chim. Acta 79 (1996) 1710-1740; (b) D. Seebach, G. Jaeschke, K. Gottewald, et al., Resolution of racemic carboxylic acid derivatives by Ti-TADDOLate mediated esterification reactions -a general method for the preparation of enantiopure compounds, Tetrahedron 53 (1997) 7539-7556; (c) G. Jaeschke, D. Seebach, Highly enantioselective ring opening of cyclic meso anhydrides to isopropyl hemiesters with Ti-TADDOLates: an alternative to hydrolytic enzymes? J. Org. Chem. 63 (1998) 1190-1197. |