b School of Pharmacy, Guangdong Medical College, Dongguan 523808, China
Tanshinones are the major lipid soluble pharmacological constituents of Danshen,the dried roots of Salvia miltiorrhiza Bunge (SMB),a well known traditional Chinese medicine used for the treatment of cerebrovascular diseases. Among these tanshinones, tanshinone I (TI),tanshinone IIA (TIIA),dihydrotanshinone I (DHTI) and cryptotanshinone (CTS) (Fig. 1) are the major bioactive constituents,and they possess various kinds of pharmacological effects including prevention of angina pectoris,myocardial infarction,coronary insufficiency and coronary death. Additionally, further researches have demonstrated that these pharmacological activities of the four tanshinones are closely associated with their ability to eliminate oxygen free radicals [1, 2, 3]. Hence,there is a theoretical and practical significance in a deep study on pharmacological effects of SMB through investigating the mechanisms of oxygen reduction reaction (ORR) of these tanshinones. In addition,electrochemical and spectroelectrochemical methods are now widely used in researching natural antioxidants,biosensor and pharmaceutical analysis [4, 5, 6],since it has advantages of fast, simple and high sensitivity. Furthermore,spectroelectrochemical method can provide current information for both reactants and intermediate products. Consequently,studying the ORR mechanisms on these tanshinones with the spectroelectrochemical method is the inevitable development trend of SMB research.
|
Download:
|
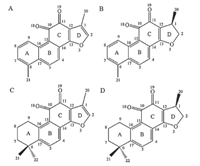
| Fig 1 Structures of tanshinone I (A),dihydrotanshinone I (B),tanshinone IIA (C),and cryptotanshinone (D). | |
On the whole,electrochemical studies of these tanshinones from SMB were mainly focused on dynamics of electrode process, content detection and interaction with DNA [7]; and these studies mostly centered around one fat-soluble active ingredient,without comparing with other ingredients. Furthermore,few experimental data were available to delineate the structure-activity relationships (SARs) of the tanshinones with antioxidant activity. Therefore,the aim of this study was to explore the reduction products,investigate the SARs of the four tanshinones (TI,TIIA, DHTI and CTS) extracted from SMB,and compare their antioxidant activities using the spectroelectrochemical method.
2. ExperimentalTI,TIIA,DHTI,and CTS were of HPLC grades (≥98%) and purchased from Beijing Shanglifang United Chemical Technology Research Institute. Other chemical reagents were of AR grades. All tanshinone solutions were prepared with anhydrous alcohol. The buffer solution was Britton-Robinson (B-R) buffer (Vethanol/ Vwater = 2/3,containing 0.5 mol/L KCl as a supporting electrolyte) which was prepared with phosphoric acid,boracic acid and glacial acetic acid. The pH value of the buffer was 4.10.
Electrochemical measurements were performed on a CHI660C electrochemical workstation (Shanghai Chen Hua Co.,Ltd.) with conventional three-electrode cell. The working,counter,and reference electrodes were a glassy carbon electrode (GCE,Φ 3 mm),a platinum electrode (Φ 1 mm) and an Ag/AgCl electrode (RE-1C) respectively.
UV spectrophotometer (Shanghai Mapuda Instrument Co.,Ltd., model: UV-1800PC) was used to conduct the wavelength scanning. Spectroelectrochemical tests were carried out with a threeelectrode spectroelectrochemical cell (Tianjin Aida Hengsheng Technology Co.,LTD). A platinum (Pt) web electrode (10 mm×5mm) was used as a working electrode; a platinum wire electrode (Φ 0.5mm) as a counter electrode; an Ag/AgCl electrode (Φ 4mm) as a reference electrode. These four tanshinone solutions were diluted to certain concentrations with B-R buffer, and then injected into the spectroelectrochemical cell.
All the potentials in the text were reported with respect to the reference electrode. And all these experiments were carried out at room temperature (ca. 25 ℃). High purity nitrogen (99.999%) was used for solution de-aeration before individual experiments.
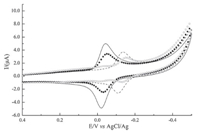
3. Results and discussionFig. 2 showed the CVs recorded with GCE in B-R buffer (pH 4.1). Electrochemical results indicated that all of these tanshinones showed a stable and quasi-reversible electrochemical behavior. The reduction and oxidation peak potentials of TI were similar to those of DHTI,while the oxidation peak current of TI was higher than that of DHTI. Meanwhile,the situations of TIIA and CTS were similar to those of TI and DHTI. The reduction peak potentials of TI, TIIA,DHTI,and CTS were at -0.05 V,-0.07 V,-0.10 V and-0.15 V (vs Ag/AgCl),respectively. These data revealed that the rank of antioxidant activity of these tanshinones,from week to strong,was TI,DHTI,TIIA,and CTS. Besides,the applied potentials for the potentiostatic reduction process were determined based on the results of the voltammetric experiment recorded with Pt web electrode in B-R buffer (pH 4.1).
|
Download:
|
| Fig 2 CVs of tanshinone I (—),dihydrotanshinone I (?),tanshinone IIA (—),and cryptotanshinone (○) (υ = 50mV s-1,pH 4.10). | |
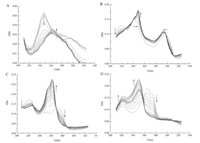
As shown in Fig. 1,none of the compounds have phenolic hydroxyl groups which are easy to be oxidized. Meanwhile,we applied spectroelectrochemical cell in present research to observe the changes of the functional groups of these tanshinones during the potentiostatic reduction process,and the UV spectra were shown in Fig. 3. Our results illustrated that the Soret bands of the four tanshinones were at 245 nm and 270 nm (shoulder band) for TI; 223 nm,250 nm (shoulder band) and 273 nm for TIIA; 218 nm, 239 nm and 288-291 nm for DHTI; 219 nm,263 nm and 272 nm (shoulder band) for CTS,respectively. These results are consistent with the results from previous studies [8, 9].
|
Download:
|
| Fig 3 UV spectra of tanshinone I (A; E =-0.45 V),dihydrotanshinone I (B; E =-0.47 V),tanshinone IIA (C; E =-0.50 V) and cryptotanshinone (D; E =-0.55 V) in constant potential reduction process. All the concentrations of these tanshinones are 9.09 ×10-5 mol/L. | |
For TI,the band at 245 nm progressively decreased,and finally disappeared,while a new band at 270 nm was observed,indicating that the Soret band of TI was red shifted during this process. For TIIA,bands at 230 nm and 273 nm were continuously shrinking and finally vanished while a new band formed at 225 nm; on the other hand,the bands at 250 nm (shoulder band) and 263 nm were rapidly increased but eventually plateaued. These data showed that in this process,the Soret band of TIIA was red shifted as well. For DHTI,the band at 218 nm was continually diminished,and finally vanished. Meanwhile,the band at 239 nm was slightly red shifted,and the band at 288 nm was slightly blue shifted. As for CTS,the bands at 263 nm and 272 nm (shoulder band) were constantly weakening and finally disappeared,while the bands at 219 nm and 245 nm were continually increasing and eventually plateaued. Beside,the band at 245 nm became the maximum band. The experimental data showed that the Soret band of CTS was blue shifted in this process.
From a structural perspective,A,B,C or D rings form a large conjugated system for TI and DHTI,while B,C or D rings form a small conjugated system for TIIA and CTS. It was then reasonable to infer that the carbonyls at positions 10 and 11 in this process were reduced to hydroxyl with a p-π conjugation effect,which caused the density of electron cloud of the conjugated system to rise and the band red-shifted. However,the methyl at position 1 of these tanshinones,which have dihydrofuran ring,is not in the same plane with A,B,C and D rings. Moreover,methyl groups at positions 21 and 22 of these tanshinones,which in A ring does not have double bond,are also not in the same plane with A,B,C and D rings. This may increase the angle strain of the chemical bond in these compounds. From this viewpoint,we inferred that as the carbonyls reduced to hydroxyl at positions 10 and 11,the angle strain of the chemical bond could constantly reinforce,and the band blue-shifted. The increasing angle strain also reduced the oxidation peak current,and the rate of redox reaction slowed as well. In addition,the Soret band of CTS only performed blue shift, indicating that the influence of the increasing angle strain could be stronger than that of the p-p conjugation effect from the hydroxyl.
4. ConclusionThese results obtained from our study reveal that the carbonyl groups at positions 10 and 11 of these four tanshinones are most probably reduced to the corresponding hydroxy groups in the potentiostatic reduction process. It caused the Soret band to redshift. Besides,the antioxidant activities of these tanshinones are strongly influenced by the size of the conjugated system. When the size of the conjugated system is larger,the structure of the compound is stable and difficult to be oxidized. As a result,the antioxidant activity is weaker. In the structure,where A,B and C rings connect through a single double bond,the conjugated system is larger than that where A ring does not have double bond. Therefore,the antioxidant activity of TI and DHTI is weaker than that of TIIA and CTS. In addition,the increasing angle strain in the reduction process caused the Soret band to blue-shift,slowed down the rate of redox reaction and the antioxidant activity was strengthened.
| [1] | D.X. Li, C. Chen, Z.W. Wang, et al., Effect of Salvia miltiorrhiza on free radical level in patients with Left obstructive colonic carcinomas, Clin. J. Med. Off. 36 (2008) 400- 401. |
| [2] | X.L. Du, W. Tian, H.Y. Kong, Regulative effect of Danshen shujin wan on oxygenderived free radical injury in rats with cervical spondylotic myelopathy, Chin. J. Clin. Rehabil. 27 (2006) 32-34. |
| [3] | X. Ouyang, K. Takahashi, K. Komatsu, et al., Protective effect of Salvia miltiorrhiza on angiotensin II-induced hypertrophic responses in neonatal rat cardiol cells, Jpn. J. Pharmacol. 87 (2006) 289-296. |
| [4] | P. Wang, X.F. Fu, J. Li, et al., Preparation of hydrophilic molecularly imprinted polymers for tetracycline antibiotics recognition, Chin. Chem. Lett. 22 (2011) 611- 614. |
| [5] | H.F. Yang, Y.S. Zhang, B. Qiu, et al., The electrochemiluminescent behavior of nickel phthalocyanine (NiTSPc)/H2O2 system on a heated ITO electrode, Chin. Chem. Lett. 23 (2012) 711-714. |
| [6] | D. Millo, Spectroelectrochemical analyses of electroactive microbial biofilms, Biochem. Soc. Trans. 40 (2012) 1284-1290. |
| [7] | W.J. Li, Z.B. Jia, H. Luo, et al., Application of electrochemical methods in natural antioxidants of Salvia miltiorrhiza, Guangdong Chem. Ind. 208 (2010) 9-10. |
| [8] | M.K. Qian, B.J. Yang, W.H. Gu, et al., Studies on the active principles of Dan-shen I, the structure of sodium tanshinone IIA sulfonate and methylene tanshinquinone, Acta Chim. Sin. 36 (1978) 199-206. |
| [9] | D.Y. Kong, X.K. Liu, Structure of dihydroisotanshinone I of Danshen, Acta Pharm. Sin. 19 (1984) 755-759. |




