b College of Chemistry and Chemical Engineering, Ocean University of China, Qingdao 266100, China
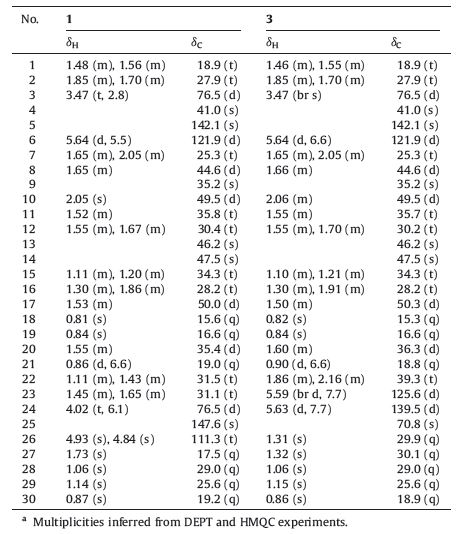
Hibiscus tiliaceus (Malvaceae family),called ‘‘Huang Jin’’ in traditional Chinese medicine and recorded in ‘‘National Chinese Herbal Medicine’’ [1],is a common coastal semi-mangrove plant native to Eastern and Northern Australia,Oceania and South-East Asia and is currently distributed in the margins of mangrove forest near the estuary. The leaves,bark and flowers of H. tiliaceus in folk medicine have long been used as a therapy for the diseases such as fever,cough,phlegm,and dysentery. Its extracts have also showed diverse pharmacological effects, e.g.,anti-inflammation [1],cytotoxicity [2],antioxidant [2],antimutagenic [3], and antidepressant [4]. Apart from our previous work [5],its chemical constituents have not been thoroughly investigated. In our course of searching for new bioactive compounds from the mangrove plants,a recent continuing studies on H. tiliaceus collected in Hainan island of China afforded three tetracyclic triterpenoids (1-3) with the 19(10→9)-abeo-euphane/tircallane carbon skeleton (Fig. 1). Tiliacols A and B (1 and 3) were new compounds. Here,we describe the isolation,structure elucidation and the bioactivity of all isolates.
|
Download:
|
| Fig. 1. Structures of compounds 1-3 and antiquol B. | |
The air dried leaves and branches of H. tiliaceus (11.0 kg) were pulverized and extracted with MeOH (3×20 L,4 d each time) at room temperature. The desalinated extract (336 g) was extracted successively with petroleum ether,EtOAc and n-BuOH. The petroleum ether and EtOAc layers were combined as PE fraction and was further separated into seven subfractions (PE1-PE7) by gradient silica gel column chromatography [petroleum ether/ acetone (20:1,10:1,5:1,3:1,1:1,v/v)]. Compounds 1 (1.8 mg),2 (1.6 mg) and 3 (10.1 mg) were obtained from subfraction PE4 by repeated silica gel column chromatography eluted by ether/ acetone (10:1,v/v),followed by semi-preparative HPLC eluted by MeOH-H2O (85:15,v/v).
The in vitro cytotoxicity of 1-3 was evaluated against the selected P388,HeLa,and K562 tumor cell lines,using the MTT method [6] with 5-fluorouracil (5-FU) as a positive control. The cells were cultured in RPMI-1640 supplemented with 10% FBS under a humidified atmosphere of 5% CO2 and 95% air at 37 ℃. Those cell suspensions (200 μL) at a density of 5 × 104 cells/mL were plated in 96-well microtiter plates and incubated for 24 h at the above conditions. The test compound solution (2 μL in DMSO) at different concentrations in triplicate was added to each well and further incubated for 72 h under the same conditions. 20 mL of the MTT solution (5 mg/mL in IPMI-1640 medium) was then added to each well and incubated for 4 h. The old medium containing MTT (150 μmol/L) was then gently replaced by DMSO and vibrated to dissolve any formazan [1-(4-iodophenyl)-5-(4-nitrophenyl)-3- phenylformazan] crystals formed. The optical density of the solution was measured on a Spectra Max Plus plate reader at 570 nm. The IC50 value of each compound was calculated by Reed and Muench’s method. Compounds with inhibition rate <50% at 50 μmol/L were considered to be inactive in the preliminary test.
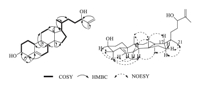
Tiliacol A (1) was obtained as a colorless oil,[α]20 D =24:1 (c 0.075,CHCl3). The HR-ESI-MS spectrum gave a positive quasimolecular ion at m/z 465.3710 ([M+Na]+,calcd. 465.3703), consistent with a molecular formula of C30H50O2,requiring six degrees of unsaturation. The 13C NMR spectrum of compound 1 revealed the presence of 30 C-atoms,which were classified by DEPT experiment into seven Me,ten CH2,and seven CH,and six quaternary C-atoms (Table 1). Its 1H NMR spectrum displayed six characteristic methyl signals for triterpenoid skeleton composed of one doublet methyl at δH0.86 (J = 6.6 Hz,Me-21),six singlet methyls at δH0.81 (Me-18),0.84 (Me-19),0.87 (Me-30), 1.06 (Me-28),1.14 (Me-29) and 1.73 (Me-27),respectively. Further analysis of NMR spectra indicated the presence of one trisubstituted double bond [δH 5.64 (d,1H,J = 5.5 Hz,H-6); δC142.1 (s,C-5) and 121.9 (d,C-6)],and one terminal double bond [δH 4.84 (s) and 4.93 (s),H-26; δC147.6 (s,C-25) and 111.3 (t,C-26)],accounting for two of the six degrees of unsaturation. Thus,compound 1 was assigned as a tetracyclic triterpenoid to fulfill the molecular formula. A comparative analysis of NMR data for 1 with those of antiquol B,euphorbol,and boeticol [7, 8] revealed that they possessed closely similar nuclei apart from the side chain difference. The consecutive 1H-1H COSY starting Me-21 to H-24 together with the key HMBC correlations of H2-26 and Me-27 with C-25 and C-24 [76.5 (d)] (Fig. 2) could establish the side chain of 1 as shown in Fig. 1.
| Table 1. 1HNMR and 13CNMR data (600 and 150 MHz,resp.; CDCl3) of compounds 1 and 3(δin ppm,J in Hz)a |
The stereochemistry of compound 1 was determined by an NOESY experiment in combination with NMR data. The NOESY correlations between H-3 and bothMe-28 and Me-29,between H- 10 and Me-28 and H-8,between H-8 and Me-18,between H-30 and Me-19 and H-17 (Fig. 2),in addition to the highly similar 1D NMR data for the skeletonwith those of antiquol B,suggested that 1 had the same relative configurations in the mother nucleus moiety. The assignment of 20R (Hβ-20) configuration for 1 was evident from the chemical shift value of H3-21 at δH0.86 observed in its 1H NMR spectrum [in 20S (Hα-20) tirucallan-type case,the chemical shift value of Me-21 usually appeared at about δH0.90] [7, 8],in contrast with the co-isolated compound 2 [9]. In the same way,the C-24 configuration was deduced as S according to the observation of the chemical shifts of C-24 and C-26 at δC76.5 and 111.3,respectively [10]. Thus,the structure of compound 1 was unambiguously established as tiliacol A,an epimer of 2 at C-20.
|
Download:
|
| Fig. 2. Key COSY,HMBC,and NOESY correlations of compound 1. | |
The HR-ESI-MS [465.3709 ([M+Na]+,C30H50O2Na+; calcd. 465.3703)] and 1D NMR data indicated that compound 3 [a colorless oil,[α]20 D =15:8 (c 0.65,CHCl3)] was another isomer of 1 and 2. The major difference was found in the side chain moiety where a disubstituted double bond [δH5.59 (br d,1H,J = 7.7 Hz,H- 23) and 5.63 (d,1H,J = 7.7 Hz,H-24); δC125.6 (d,C-23) and 139.5 (d,C-24)] located at C-23 and a hydroxyl group located at C-25 due to the two downfield singlet methyl signals [δH1.31 (3H,Me-26) and 1.32 (3H,Me-27); δC29.9 (q,C-26) and 30.1 (q,C-27)] and a quaternary carbon signal [δC 70.8 (s,C-25)] observed in 3. These data closely resembled those of cucurbita-5,23-diene-3β,25-diol [11]. The Z-geometry of the double bondD23 of 3 was determined by the small coupling constant value (JH-23/H-24 = 7.7 Hz) and comparing the 1H NMR data of H-23 and H-24 with those of kansenonol,11- oxo-kansenonol,kansenol,and 3β,25-dihydroxy-tirucalla-7,23- diene [12, 13]. The structure of 3 was thus determined as (3β, 23Z)-19(10!9)-abeo-8α,9β,10α-tircalla-5,23-dien-3,25-diol,named as tiliacol B.
Three tumor cell lines,P388 (leukemia),HeLa (cervical carcinoma),and K562 (erythroleukemia) were selected to test the cytotoxic activity for the isolates. Against the tested tumor cell lines,compound 2 showed potent activity with IC50 values of 11.2, 11.5,and 13.5 mmol/L,compound 1 showed relatively weak cytotoxic activity with IC50 values of 40.6,19.9,21.7 μmol/L, respectively,whereas the new compound 3 was inactive (inhibition rate < 50% at 50 μmol/L) although it just had a slightly different side chain in structure relative to 1 and 2.
Our present work on H. tiliaceus yielded two new tetracyclic triterpenoids with 19(10→9)-abeo-euphane/tircallane skeleton, (3β,24S)-19(10→9)-abeo-8α,9β,10a-eupha-5,25-dien-3,24-diol (tiliacol A,1) and (3β,23Z)-19(10→9)-abeo-8α,9β,10a-tircalla- 5,23-dien-3,25-diol (tiliacol B,3),and one known analog (2). Peculiarly,the 9(10→9)-abeo-tircallane/euphane type triterpenes isolated from the species had not been reported previously.
This work was supported by the Key International Cooperation Project of CMST (No. 2008DFA31040) and the Special Funds for Young Teachers of Ocean University of China (No.841113070). Special thanks are attributed to Professor J. Li and her research group (Key Laboratory of Marine Drugs, Chinese Ministry of Education,School of Medicine and Pharmacy, Ocean University of China,Qingdao,China) for the tests of cytotoxicity activities.
| [1] | X.P. Zhang, Y.H. Pei, J.Q. Zhang, et al., Research progress in chemical constituents of Hibiscus tillisceus and their pharmacological activities, Drugs Clin. 26 (2011) 434-438. |
| [2] | S.J. Uddin, I.D. Grice, E. Tiralongo1, Cytotoxic effects of bangladeshi medicinal plant extracts, Evid. Based Complem. Altern. Med. 2011 (2011) 1-7. |
| [3] | R.M. Rosa, D.J. Moura, M.I.S. Melecchi, et al., Protective effects of Hibiscus tiliaceus L. methanolic extract to V79cells against cytotoxicity and genotoxicity induced by hydrogen peroxide and tert-butyl-hydroperoxide, Toxicol. In Vitro 21 (2007) 1442-1452. |
| [4] | C. Vanzella, P. Bianchetti, S. Sbaraini, et al., Antidepressant-like effects of methanol extract of Hibiscus tiliaceus flowers in mice, BMC Complem. Altern. Med. 12 (2012) 41-45. |
| [5] | Z.Z. Wang, J. Li, X.L. Tang, G.Q. Li, Triterpenes and steroids from semi-mangrove plant Hibiscus tiliaceus, Chin. J. Nat. Med. 9 (2011) 191-193. |
| [6] | M.C. Alley, D.A. Scudiero, A. Monks, et al., Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay, Cancer Res. 44 (1988) 589-601. |
| [7] | T. Akihisa, E.M.K. Wijeratne, H. Tokuda, et al., Eupha-7,9(11),24-trien-3β-ol ("Antiquol C") and other triterpenes from Euphorbia antiquorumlatex and their inhibitory effects on Epstein-Barr virus activation, J. Nat. Prod. 65 (2002) 158-162. |
| [8] | M.J.U. Ferreira, J.R. Ascenso, O.S. Tavares, Boeticol, a new tetracyclic triterpene from Euphorbia boeitica, J. Nat. Prod. 58 (1995) 275-279. |
| [9] | I.Valente,M.Reis,N.Duarte, etal., JatrophanediterpenesfromEuphorbiamelliferaand theiractivityasP-glycoproteinmodulatorsonmultidrug-resistantmouselymphoma and human colon adenocarcinoma cells, J. Nat. Prod. 75 (2012) 1915-1921. |
| [10] | H.T. Zhong, F. Li, B. Chen, M.K. Wang, Euphane triterpenes from the bark of Broussonetia papyrifera, Helv. Chim. Acta 94 (2011) 2061-2065. |
| [11] | S. Nakano, Y. Fujimoto, Y. Takaishi, et al., Cucurbita-5,23-diene-3b,25-diol from Sicana odorifera, Fitoterapia 75 (2004) 609-611. |
| [12] | L.Y. Wang, N.L. Wang, X.S. Yao, et al., Euphane and tirucallane triterpenes from the roots of Euphorbia kansui and their in vitro effects on the cell division of xenopus, J. Nat. Prod. 66 (2003) 630-633. |
| [13] | X.D. Luo, S.H. Wu, Y.B. Ma, D.G. Wu, Tirucallane triterpenoids from Dysoxylum hainanense, Phytochemistry 54 (2000) 801-805. |