b Key Laboratory of Mesoscopic Chemistry of MOE, Center for Multimolecular Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093, China
Polypseudorotaxanes constructed by incorporating multiple pseudorotaxane units into the polymeric framework assisted by noncovalent interactions have attracted tremendous interest in the past years due to their potential applications in biotechnology, stimuli-responsive materials,and life sciences [1, 2, 3, 4, 5, 6]. So far,many kinds of macrocyclic hosts such as cyclodextrin [7, 8],crown ether [9, 10],cucurbituril [11],calixarene [12],and cyclophane [13] have been utilized for the fabrication of supramolecular polypseudorotaxanes. In order to enrich the supramolecular polypseudorotaxanes family,great efforts have been made to explore new macrocyclic hosts and guests. Pillar[5]arenes with unique symmetrical pillar architectures and π-electron rich cavities are a new type of macrocyclic molecules,which were first reported in 2008 [14, 15, 16, 17, 18, 19]. The unique rigid structure renders them outstanding candidates to bind various guests selectively. Recently,the host- guest chemistry of these macrocyclic hosts has been widely investigated [20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30]. Hitherto,various pillar[5]arene-based polypseudorotaxanes constructed by noncovalent interactions have been reported. For example,Huang et al. reported two kinds of on quadruple C-H···π interactions and the pillar[5]arene/imidazolium cation recognition motif,respectively [31, 32]. Stoddart et al. synthesized a viologen-modified pillar[5]arene that exhibits self-complexation in dilute dichloromethane solutions and could form supramolecular daisy chain polymers and eventually form organogels as the concentration increased gradually [33]. Recently, our group has developed pillar[5]arene-based supramolecular polypseudorotaxanes and dynamic polyrotaxanes interlocked by a quadruple hydrogen bonding ureidopyrimidinone (UPy) motif [34, 35].
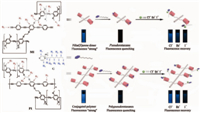
Conjugated polymers that are composed of a large number of repeating conjugated units have received significant attention owning to their superior electronic and optical properties [36, 37]. In the past few years,a variety of biosensors and chemosensors have been designed based on their fluorescence-responsive property [38, 39, 40, 41, 42]. Recently,exploiting novel fluorescent sensors by utilizing supramolecular host-guest interactions in conjunction with π-conjugated structures has become one of the most challenging tasks for chemists. In 2006,Harada et al. reported a water-soluble cyclodextrin-grafted poly(phenylene ethynylene), which exhibited a fluorescence color change or quenching property depending on the types of guests [43]. In 2012,Huang et al. prepared a supramolecular cross-linked conjugated polymer network that could serve as a multiple fluorescent sensor [44]. More recently,our group has reported the synthesis of a pillar[5]arene-modified conjugated host polymer and the construction of novel side-chain polypseudorotaxanes via the host- guest interactions between the pillar[5]arene units and noctylpyrazinium hexafluorophosphate salt (G) (Scheme 1) [45], in which these polypseudorotaxanes showed very weak fluorescence compared with their conjugated host polymer due to the efficient electron transfer from the conjugated backbone to the noctylpyrazinium cation guest,and the fluorescence recovery of the conjugated host polymer was observed upon the addition of Cl- to the solution of the polypseudorotaxanes due to the collapse of the polypseudorotaxanes. Since Cl- could destroy the host-guest interactions between the pillar[5]arene-modified conjugated polymer host and the n-octylpyrazinium cation guest,which resulted in the transition of the side-chain polypseudorotaxanes into linear conjugated host polymer following by the fluorescence recovery of the conjugated host polymer,further investigations of a series of other different anions,F-,Cl-,Br-,I-,CH3COO-,SO42-, and H2PO4- on the pseudorotaxane (formed between pillar[5]arene dimer (M1) and G) and its polypseudorotaxanes (formed between pillar[5]arene-based conjugated polymer (P1) and G) systems were herein carried out (Scheme 1). The results suggest that Cl-, Br-,and I- (tetrabutylammonium salts) could dramatically affect the complexation between the pillar[5]arene-modified conjugated host polymer and G. The binding ability of Cl-,Br-,and I- with G decreases in the order of Cl- > Br- > I-,which also led to the recovery of the fluorescence intensity of the pseudorotaxane and polypseudorotaxane host in this order. The present study will provide new insight into the understanding of the novel side-chain pseudorotaxane and polypseudorotaxane systems and therefore will broaden their applications as highly efficient and selective anion-responsive fluorescent sensors.
|
Download:
|
| Scheme 1. Cartoon representation of the formation of pillar[5]arene-modified pseudorotaxane and polypseudorotaxanes system and their disassembly induced by Cl-,Br-,and I- (tetrabutylammonium salts). | |
The pillar[5]arene dimer (M1),pillar[5]arene-based conjugated polymer (P1) and the guest molecule n-octylpyrazinium hexafluorophosphate (G) were synthesized according to the previously reported methods [41]. Data for the pillar[5]arene dimer (M1): 1HNMR (300 MHz,CDCl3,298 K): δ 7.41-7.34 (m,8H,phenyl protons),7.15 (s,2H,central phenyl protons),6.80-6.72 (m, 20H,phenyl protons from pillar[5]arene),4.33 (t,4H,J = 4.6 Hz, protons from OCH2 linked to phenyl),4.17 (t,4H,J = 4.5 Hz,protons from OCH2 linked to pillar[5]arene),3.78-3.74 (m,20H,methylene bridge protons of pillar[5]arene),3.64-3.53 (m,54H,methoxy protons of pillar[5]arene),3.18 (s,2H,protons of alkyne). LRESIMS (m/z): 1906.75 [M + Na]+,HRESIMS (m/z): calcd. for [M + Na]+ C118H114O22Na,1906.7729,found 1906.7733. Data for the pillar[5]arene-based conjugated polymer (P1): 1H NMR (300 MHz,CDCl3,298 K): δ 7.46-7.37 (m,8H,phenyl protons), 7.17 (s,2H,central phenyl protons),6.81-6.74 (m,20H,phenyl protons from pillar[5]arene),4.34 (brs,4H,protons from OCH2 linked to phenyl),4.19 (brs,4H,protons from OCH2 linked to pillar[5]arene),3.77 (s,20H,methylene bridge protons of pillar[5]arene),3.64-3.54 (m,54H,methoxy protons of pillar[5]- arene). GPC (THF,40 ℃,polystyrene standards as calibrant): Mw = 24,900; Mn = 15,800; PDI = 1.58 (degree of polymerization [DP] ≈ 8). Data for the guest molecule n-octylpyrazinium hexafluorophosphate (G): 1H NMR (300 MHz,CDCl3,298 K): d 9.40 (s, 2H,pyrazine protons),8.68 (s,2H,pyrazine protons),4.66 (m,2H, CH2CH2(CH2)5CH3),2.03 (m,2H,CH2CH2(CH2)5CH3),1.31 (m,10H, CH2CH2(CH2)5CH3),0.88 (t,3H,J = 5.8 Hz,CH2CH2(CH2)5CH3). LRESIMS (m/z): 193.10 [M - PF6]+.
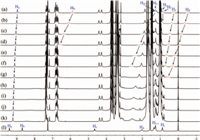
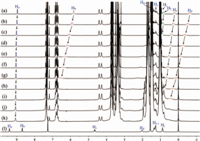
According to our previous report,the addition of chloride anion could switch off the complexation between M1 and G by forming an intimate ion pair with the n-octylpyrazinium cation moiety in G in chloroform [41]. To further investigate the influence of other anions,namely Br- and I-,to the pseudorotaxane formed between M1 and G,1H NMR titration experiments were carried out by adding different concentrations of tetrabutylammonium bromide (TBABr) and tetrabutylammonium iodide (TBAI) to the obtained pseudorotaxane in chloroform-d. As shown in Figs. 1 and 2,the disassembly processes of the obtained pseudorotaxane occurred upon the addition of TBABr and TBAI,respectively,as manifested by the gradual downfield shifts of the proton signals corresponding to the aromatic protons in the pyrazinium ion (Ha and Hb) and the methylene protons (Hc,Hd,He and Hf) in the complexed G. These results suggest that both Br- and I- can form intimate ion pairs with the n-octylpyrazinium cation in chloroform,and the newly formed ion pairs show weak complexing ability with the pillar[5]arene moieties of M1.
|
Download:
|
| Fig. 1. 1H NMR spectra (300 MHz,CDCl3,298 K) of M1 (5.0 mmol/L) and G (8.0 mmol/L) upon addition of TBABr (mmol/L): (a) 0.0,(b) 2.0,(c) 6.0,(d) 10.0,(e) 20.0,(f) 30.0,(g) 50.0,(h) 150.0,(i) 250.0,(j) 300.0,(k) 400.0; (l) the 1H NMR spectrum (300 MHz,CDCl3,298 K) of G (8.0 mmol/L). | |
|
Download:
|
| Fig. 2. 1H NMR spectra (300 MHz,CDCl3,298 K) of M1 (5.0 mmol/L) and G (8.0 mmol/L) upon addition of TBAI (mmol/L): (a) 0.0,(b) 2.0,(c) 6.0,(d) 10.0,(e) 20.0,(f) 30.0,(g) 50.0,(h) 150.0,(i) 250.0,(j) 350.0,(k) 400.0; (l) the 1H NMR spectrum (300 MHz,CDCl3,298 K) of G (8.0 mmol/L). | |
Fig. 3 shows the 1H NMR spectra of the pseudorotaxane formed between M1 and G upon the addition of different halide anions (Cl -,Br- ,and I- ),in which the proton signal derived from the pyrazinium aromatic proton (Ha) shifted downfield,and the chemical shift changes (Δδ) of Ha increase in the order of I- < Br- < Cl- . The differences in chemical shift change were caused by the different ion-pairing binding affinity of three halide anions to the n-octylpyrazinium cation moiety in G in chloroform. Among these three halide anions,the size of C- is the smallest and it possesses the highest charge density,thus Cl- shows the strongest binding affinity with the n-octylpyrazinium cation in chloroform. On the other hand,compared with Cl - and Br- ,the size of I - is bigger and it has lower charge density,so I - forms a relatively loose ion pair with the n-octylpyrazinium cation. Therefore,the chemical shift change of Ha was larger for Cl - than that for Br- and I-,respectively.

|
Download:
|
| Fig. 3. Partial 1H NMR spectra (300 MHz,CDCl3,298 K) of (a) M1 (5.0 mmol/L) and G (8.0 mmol/L),(b) M1 (5.0 mmol/L) and G (8.0 mmol/L) + TBAI (400.0 mmol/L),(c) M1 (5.0 mmol/L) and G (8.0 mmol/L) + TBABr (400.0 mmol/L),(d) M1 (5.0 mmol/L) and G (8.0 mmol/L) + TBACl (400.0 mmol/L),and (e) G (8.0 mmol/L). | |
Our previous work has reported that the polypseudorotaxanes formed between P1 and G showed very weak fluorescence due to the efficient electron transfer from the conjugated backbone of P1 to the n-octylpyrazinium cation moiety in G,whereas the fluorescence recovery of the conjugated polymer occurred upon the addition of chloride as its tetrabutylammonium form (TBACl) to the polypseudorotaxanes in chloroform (Fig. 4D),and similarly, after adding TBACl to the pseudorotaxane formed between M1 and G,its fluorescence recovery could also be observed (Fig. 4A). In order to further study the responsive properties of the polypseudorotaxane system and the pseudorotaxane system to other anions,namely Br- and I-,the fluorescence spectra of the pseudorotaxane ([M1] = 10 μmol/L,[G] = 230 μmol/L) and the polypseudorotaxanes ([P1,RU] = 10 μmol/L,[G] = 116 μmol/L) in the presence of different concentrations of TBABr and TBAI were recorded,respectively. As shown in Fig. 4B and E,both of the fluorescence intensity at 415 nm (the pseudorotaxane system) and 445 nm (the polypseudorotaxane system) enhanced gradually with the increased amount of TBABr. Similarly,such a phenomenon can also be detected by adding TBAI to the pseudorotaxane system and the polypseudorotaxane system,respectively (Fig. 4C and F).

|
Download:
|
| Fig. 4. Fluorescence spectra of the pseudorotaxane ([M1] = 10 μmol/L,[G] = 230 μmol/L) in chloroform in the presence of different concentrations of (A) TBACl (0,120,210,300,480,and 840 μmol/L),(B) TBABr (0,210,480,and 840 mmol/L) and (C) TBAI (0,210,and 840 μmol/L),λex = 369 nm. Fluorescence spectra of the polypseudorotaxane ([P1,RU] = 10 mmol/L,[G] = 116 μmol/L) in chloroform in the presence of different concentrations of (D) TBACl (0,90,180,360,and 630μ μmol/L),(E) TBABr (0,90,360,and630 μmol/L),and (F) TBAI (0,90,360,and 630 μmol/L),λex = 408 nm. | |
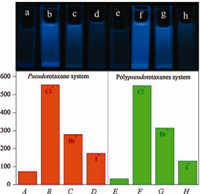
Fig. 5 illustrates the fluorescence intensity at 415 nm of the pseudorotaxane ([M1] = 10 μmol/L,[G] = 230 μmol/L) with a constant concentration (840 μmol/L) of TBACl,TBABr and TBAI (Fig. 5A-D) and the fluorescence intensity at 445 nm of the polypseudorotaxanes ([P1,RU] = 10 μmol/L,[G] = 116 μmol/L) with a constant concentration (630 μmol/L) of TBACl,TBABr and TBAI (Fig. 5E-H). It is observed that the fluorescence intensity of the pseudorotaxane and the polypseudorotaxane systems recovers more completely in the presence of TBACl,and the fluorescence recovery of the two systems increases in the order of TBAI,TBABr, and TBACl (I- ﹤ Br- ﹤ Cl-). A possible reason for the fluorescence recovery is that after adding different halide anions to the pseudorotaxane and polypseudorotaxane systems,intimate ion pairs could be formed between the halide anions and the noctylpyrazinium cation,which would decrease the electron transfer from the conjugated backbone of M1 and P1 to the noctylpyrazinium cation in G. Compared with Br- and I-,Cl- has stronger binding affinity to the n-octylpyrazinium cation and could form a tighter ion pair,thus both pseudorotaxane and the polypseudorotaxane systems show stronger emission after the addition of TBACl. On the other hand,I- has lower binding affinity to the n-octylpyrazinium cation and forms a looser ion pair, therefore both pseudorotaxane and the polypseudorotaxane systems show weaker emission after adding TBAI. Moreover,as shown in the inset of Fig. 5,the differences in fluorescence enhancement of the pseudorotaxane and polypseudorotaxane systems upon the addition of different halide anions can be easily distinguished by naked eyes when illuminating these solutions with UV light (365 nm).
|
Download:
|
| Fig. 5. The fluorescence intensity of the pseudorotaxane ([M1] = 10 μmol/L, [G] = 230 μmol/L) and the polypseudorotaxanes ([P1,RU]=10μmol/L,[G] = 116 μmol/L) upon addition of different halide anions (tetrabutylammonium salts) in chloroform,listed from left to right: (A) M1 (10 μmol/L) and G (230 μmol/L),(B) M1 (10 μmol/L) and G (230 μmol/L) + TBACl (840 μmol/L),(C) M1 (10 μmol/L) and G (230 μmol/L) + TBABr (840 μmol/L),(D) M1 (10 μmol/L) and G (230 μmol/L) + TBAI (840 μmol/L),(E) P1 ([RU] = 10 μmol/L) and G (116 μmol/L),(F) P1 ([RU] = 10 μmol/ L) and G (116 μmol/L) + TBACl (630 μmol/L),(G) P1 ([RU] = 10 μmol/L) and G (116 μmol/L) + TBABr (630 μmol/L),and(H)P1([RU] = 10 mmol/L) and G(116 μmol/ L) + TBAI (630 μmol/L); the fluorescence intensity was monitored at 415 nm for the pseudorotaxane and at 445 nmfor the polypseudorotaxanes. Inset: photographs of the solution of (a) A,(b) B,(c) C,(d) D,(e) E,(f) F,(g) G,and (h) H in chloroform under UV light (365 nm) illumination. | |
In order to further investigated the effect of other anions besides the above halogen ions (Cl -,Br -,and I- ) to the pseudorotaxane and polypseudorotaxane systems,F -,CH3COO -,SO42 -,and H2PO4- were then investigated as their tetrabutylammonium salts. However,adding these ions to the solution of G only led to its decomposition to pyrazine. 4. Conclusion
In summary,we have demonstrated that a disassembly process of a pillar[5]arene-based pseudorotaxane and its polypseudorotaxanes occurred upon the addition of Cl -,Br -,and I - (tetrabutylammonium salts),respectively,leading to the fluorescence recovery of the pseudorotaxane and polypseudorotaxane systems. The fluorescence enhancement of the two systems increases in the order of I - ﹤ Br -﹤ Cl- ,and the differences in fluorescence intensity could be easily distinguished by naked eyes under UV light illumination. Such fluorescence enhancement differences could be attributed to the different ion-pairing binding affinity of Cl -,Br -,and I - to the n-octylpyrazinium cation,which lead to the different degree of the electron transfer from the conjugated backbone to the n-octylpyrazinium cation in G. Therefore,the pseudorotaxane and its polypseudorotaxanes formed between pillar[5]arene moieties and n-octylpyrazinium cations could be served as novel fluorescent sensors for the selective detection of halogen ions. Acknowledgments
We gratefully thank the financial support of the National Natural Science Foundation of China (No. 21202083),Natural Science Foundation of Jiangsu (Nos. BK2011055,BK2011551) and the China Postdoctoral Science Foundation (No. 2012M511717).
| [1] | G. Wenz, B.H. Han, A. Müller, Cyclodextrin rotaxanes and polyrotaxanes, Chem. Rev. 106 (2006) 782-817. |
| [2] | A. Harada, A. Hashidzume, H. Yamaguchi, Y. Takashima, Polymeric rotaxanes, Chem. Rev. 109 (2009) 5974-6023. |
| [3] | L. Fang, M.A. Olson, D. Benítez, et al., Mechanically bonded macromolecules, Chem. Soc. Rev. 39 (2010) 17-29. |
| [4] | Y. Liu, Y.L. Zhao, H.Y. Zhang, et al., Supramolecular polypseudorotaxane with conjugated polyazomethine prepared directly from two inclusion complexes of b-cyclodextrin with tolidine and phthaldehyde, Macromolecules 37 (2004) 6362-6369. |
| [5] | P.B. Wan, Y.G. Jiang, Y.P. Wang, Z.Q. Wang, X. Zhang, Tuning surface wettability through photocontrolled reversible molecular shuttle, Chem. Commun. (2008) 5710-5712. |
| [6] | H. Li, Y.W. Yang, Gold nanoparticles functionalized with supramolecular macrocycles, Chin. Chem. Lett. 24 (2013) 545-552. |
| [7] | D. Taura, S.J. Li, A. Hashidzume, A. Harada, Formation of side-chain heteropolypseudorotaxane composed of a-and b-cyclodextrins with a water-soluble polymer bearing two recognition sites, Macromolecules 43 (2010) 1706-1713. |
| [8] | Y.W. Yang, Y. Chen, Y. Liu, Linear polypseudorotaxanes possessing many metal centers constructed from inclusion complexes of a-, b-, and g-cyclodextrins with 4,40-dipyridine, Inorg. Chem. 45 (2006) 3014-3022. |
| [9] | F. Wang, B. Zheng, K.L. Zhu, et al., Formation of linear main-chain polypseudorotaxanes with supramolecular polymer backbones via two self-sorting host-guest recognition motifs, Chem. Commun. (2009) 4375-4377. |
| [10] | S.L. Li, T.X. Xiao, B.J. Hu, et al., Formation of polypseudorotaxane networks by cross-linking the quadruple hydrogen bonded linear supramolecular polymers via bisparaquat molecules, Chem. Commun. 47 (2011) 10755-10757. |
| [11] | T. Ooya, D. Inoue, H.S. Choi, et al., pH-responsive movement of cucurbit[7]uril in a diblock polypseudorotaxane containing dimethyl b-cyclodextrin and cucurbit[7]uril, Org. Lett. 8 (2006) 3159-3162. |
| [12] | C. Talotta, C. Gaeta, T. Pierro, P. Neri, Sequence stereoisomerism in calixarenebased pseudo[3]rotaxanes, Org. Lett. 13 (2011) 2098-2101. |
| [13] | B.M. Rambo, H.Y. Gong, M. Oh, J.L. Sessler, The "texas-sized" molecular box: a versatile building block for the construction of anion-directed mechanically interlocked structures, Acc. Chem. Res. 45 (2012) 1390-1401. |
| [14] | T. Ogoshi, S. Kanai, S. Fujinami, T.A. Yamagishi, Y. Nakamoto, para-Bridged symmetrical pillar[5]arenes: their lewis acid catalyzed synthesis and host-guest property, J. Am. Chem. Soc. 130 (2008) 5022-5023. |
| [15] | D.R. Cao, Y.H. Kou, J.Q. Liang, et al., A facile and efficient preparation of pillararenes and a pillarquinone, Angew. Chem. Int. Ed. 48 (2009) 9721-9723. |
| [16] | P.J. Cragg, K. Sharma, Pillar[5]arenes: fascinating cyclophanes with a bright future, Chem. Soc. Rev. 41 (2011) 597-607. |
| [17] | T. Ogoshi, Synthesis of novel pillar-shaped cavitands "pillar[5]arenes" and their application for supramolecular materials, J. Incl. Phenom. Macrocycl. Chem. 72 (2012) 247-262. |
| [18] | M. Xue, Y. Yang, X.D. Chi, Z.B. Zhang, F.H. Huang, Pillararenes, a new class of macrocycles for supramolecular chemistry, Acc. Chem. Res. 45 (2012) 1294-1308. |
| [19] | Y. Chen, H.Q. Tao, Y.H. Kou, H. Meier, J.L. Fu, D.R. Cao, Synthesis of pillar[7]arene, Chin. Chem. Lett. 23 (2012) 509-511. |
| [20] | C.J. Li, Q.Q. Xu, J. Li, F.N. Yao, X.S. Jia, Complex interactions of pillar[5]arene with paraquatsandbis(pyridinium) derivatives,Org. Biomol.Chem.8 (2010)1568-1576. |
| [21] | C.J. Li, L. Zhao, J. Li, et al., Self-assembly of [2]pseudorotaxanes based on pillar[5]-arene and bis(imidazolium) cations, Chem. Commun. 46 (2010) 9016-9018. |
| [22] | C.J. Li, S.H. Chen, J. Li, et al., Novel neutral guest recognition and interpenetrated complex formation from pillar[5]arenes, Chem. Commun. 47 (2011) 11294-11296. |
| [23] | X.Y. Shu, S.H. Chen, J. Li, et al., Highly effective binding of neutral dinitriles by simple pillar[5]arenes, Chem. Commun. 48 (2012) 2967-2969. |
| [24] | X.Y. Shu, J.Z. Fan, J. Li, et al., Complexation of neutral 1,4-dihalobutanes with simple pillar[5]arenes that is dominated by dispersion forces, Org. Biomol. Chem. 10 (2012) 3393-3397. |
| [25] | C.J. Li, J.W. Ma, L. Zhao, et al., Molecular selective binding of basic amino acids by a water-soluble pillar[5]arene, Chem. Commun. 49 (2013) 1924-1926. |
| [26] | C.Y. Han, G.C. Yu, B. Zheng, F.H. Huang, Complexation between pillar[5]arenes and a secondary ammonium salt, Org. Lett. 14 (2012) 1712-1715. |
| [27] | B.Y. Xia, J.M. He, Z. Abliz, Y.H. Yu, F.H. Huang, Synthesis of a pillar[5]arene dimer by co-oligomerization and its complexation with n-octyltrimethyl ammonium hexafluorophosphate, Tetrahedron Lett. 52 (2011) 4433-4436. |
| [28] | Q.P. Duan, W. Xia, X.Y. Hu, et al., Novel [2]pseudorotaxanes constructed by selfassembly of bis-urea-functionalized pillar[5]arene and linear alkyl dicarboxylates, Chem. Commun. 48 (2012) 8532-8534. |
| [29] | L.Z. Liu, D.R. Cao, Y. Jin, et al., Efficient synthesis of copillar[5]arenes and their host-guest properties with dibromoalkanes, Org. Biomol. Chem. 9 (2011) 7007-7010. |
| [30] | X.S. Hu, H.M. Deng, J. Li, X.S. Jia, C.J. Li, Selective binding of unsaturated aliphatic hydrocarbons by a pillar[5]arene, Chin. Chem. Lett. 24 (2013) 707-709. |
| [31] | Z.B. Zhang, Y. Luo, J.H. Chen, et al., Formation of linear supramolecular polymers that is driven by C-H×p interactions in solution and in the solid state, Angew. Chem. Int. Ed. 50 (2011) 1397-1401. |
| [32] | B.Y. Xia, B. Zheng, C.Y. Han, et al., A novel pH-responsive supramolecular polymer constructed by pillar[5]arene-based host-guest interactions, Polym. Chem. 4 (2013) 2019-2024. |
| [33] | N.L. Strutt, H.C. Zhang, M.A. Giesener, J.Y. Lei, J.F. Stoddart, A self-complexing and self-assembling pillar[5]arene, Chem. Commun. 48 (2012) 1647-1649. |
| [34] | X.Y. Hu, P.Y. Zhang, X. Wu, et al., Pillar[5]arene-based supramolecular polypseudorotaxanes constructed from quadruple hydrogen bonding, Polym. Chem. 3 (2012) 3060-3063. |
| [35] | X.Y. Hu, X. Wu, Q.P. Duan, et al., Novel pillar[5]arene-based dynamic polyrotaxanes interlocked by the quadruple hydrogen bonding ureidopyrimidinone motif, Org. Lett. 14 (2012) 4826-4829. |
| [36] | S.W. Thomas, G.D. Joly, T.M. Swager, Chemical sensors based on amplifying fluorescent conjugated polymers, Chem. Rev. 107 (2007) 1339-1386. |
| [37] | A. Facchetti, p-Conjugated polymers for organic electronics and photovoltaic cell applications, Chem. Mater. 23 (2011) 733-758. |
| [38] | A. Bajaj, O.R. Miranda, R. Phillips, et al., Array-based sensing of normal, cancerous, and metastatic cells using conjugated fluorescent polymers, J. Am. Chem. Soc. 132 (2010) 1018-1022. |
| [39] | X.L. Feng, L.B. Liu, S. Wang, D.B. Zhu, Water-soluble fluorescent conjugated polymers and their interactions with biomacromolecules for sensitive biosensors, Chem. Soc. Rev. 39 (2010) 2411-2419. |
| [40] | H.A. Ho, A. Najari, M. Leclerc, Optical detection of DNA and proteins moth cationic polythiophenes, Acc. Chem. Res. 41 (2008) 168-178. |
| [41] | B. Liu, G.C. Bazan, Synthesis of cationic conjugated polymers for use in label-free DNA microarrays, Nat. Protoc. 1 (2006) 1698-1702. |
| [42] | L. Wang, L.L. Li, H.L. Ma, H. Wang, Recent advances in biocompatible supramolecular assemblies for biomolecular detection and delivery, Chin. Chem. Lett. 24 (2013) 351-358. |
| [43] | T. Ogoshi, Y. Takashima, H. Yamaguchi, A. Harada, Cyclodextrin-grafted poly (phenylene ethynylene) with chemically-responsive properties, Chem. Commun. (2006) 3702-3704. |
| [44] | X.F. Ji, Y. Yao, J.Y. Li, X.Z. Yan, F.H. Huang, A supramolecular cross-linked conjugated polymer network for multiple fluorescent sensing, J. Am. Chem. Soc. 135 (2013) 74-77. |
| [45] | S. Sun, X.Y. Hu, D.Z. Chen, et al., Pillar[5]arene-based side-chain polypseudorotaxanes as an anion-responsive fluorescent sensor, Polym. Chem. 4 (2013) 2224-2229. |




