In recent years,the sensing of anions has emerged as a vigorous research field of supramolecular chemistry [1, 2, 3, 4]. In particular, great effort has been devoted to the recognition of the fluoride ion, because it is highly relevant to environmental and health issues [5, 6, 7, 8, 9, 10, 11, 12, 13, 14]. Several successful examples have been reported [15, 16, 17, 18, 19, 20],e.g., the receptors based on the chemical affinity between fluoride and silicon can exhibit high selectivity for F- [21, 22, 23],but the rational design of F- receptors with remarkable fluorescent or colorimetric response is still a great challenge. One of the design strategies of fluoride receptors is based on the formation of a hydrogen bond between the fluoride ion and the active N-H group of an organic chromophore,which will result in a detectable change of spectral characteristicsof the chromophore upon the recognition of fluoride ion. Among the various organic chromophores investigated as fluoride receptors,indole- and carbazole-based chromogenicsensing molecules are superior hydrogen bond donors than pyrrole and more prone to deprotonation which has been well documented [24, 25]. In our earlier works,we have designed and synthesized several colorimetric F- receptors with bi-indole and indolocarbazole as the recognition sites [26, 27]. However,these receptors can only work well in pure organic solvents,since their performances would be decreased in the presence of small amounts of water, because water as a protic solvent can compete for the formation of a hydrogen bond with F-. Therefore,the development of water tolerant F- receptors is highly desirable for their practical application.
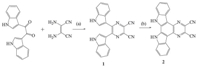
Herein,we report the design and synthesis of two receptors containing bi-indole (1) and indolocarbazole-NH (2) moieties as the recognition sites for fluoride sensing (Scheme 1). Compounds 1 and 2 exhibit remarkable color changes in the presence of F- both in aqueous DMSO medium and pure DMSO. In addition,2 as an efficient colorimetric sensor,can also distinguish AcO- and F- in DMSO.
|
Download:
|
| Scheme 1. The procedure for the synthesis of 1 and 2. (a) Methanol,sulfamic acid and (b) CF3COOH,DDQ. | |
All the starting materials for synthesis were commercially available and used as received. All the solvents used for titration measurements were purified by standard procedures. In the titration experiments,all the anions were added in the form of tetrabutylammonium (TBA) salts,which were purchased from Alfa Aesar or Aladdin,stored in a vacuum desiccator containing selfindicating silica and fully dried before used. DMSO was dried with CaH2 and then distilled under reduced pressure. Structural characterizations were carried out in DMSO-d6 at 25 ℃ with Varian Unity Plus 400 MHz NMR spectrometer (Varian,USA). High resolution mass spectra (HRMS) were recorded on an IonSpec 7.0T FT-ICR mass spectrometer (IonSpec,USA). UV/vis absorption spectra were measured with a Hitachi U-3010 UV-vis spectrophotometer (Hitachi,Japan). Fluorescence spectra were recorded at room temperature on a Varian Cary Eclipse fluorescence spectrometer (Varian,USA).
Synthesis of 1: Under N2 atmosphere,bi-indole (420 mg, 1.4 mmol),diaminomaleonitrile (240 mg,2.2 mmol) and sulfamic acid (1200 mg,12.0 mmol) were refluxed in 30 mL of methanol for 3 h. The residual methanol was removed by vacuum distillation, then the resulting precipitate was washed by saturated aq. NaHCO3 and dried in vacuo to afford a yellow solid. The solid was subjected to chromatography over silica gel (ethyl acetate:petroleum ether, 1:1) to give 1 as yellow powder in about 80% yield. 1H NMR (400 MHz,DMSO-d6,TMS): δ 7.10 (t,2H,J = 7.3 Hz),7.20 (t,2H, J = 7.5 Hz),7.48 (d,2H,J = 8.0 Hz),7.75 (d,2H,J = 8.2 Hz),7.81 (d, 2H,J = 2.4 Hz),11.85 (s,2H); ESI-MS (m/z): 359.2 [M-H]-,394.6 [M+Cl]-,754.5 [2M+Cl]-; HRMS (ESI) (m/z): 359.1047 [M-H]-, calcd. for C22H12N6 360.3709.
Synthesis of 2: Compound 1 (100 mg,0.28mmol) and 2,3- dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) (75 mg,0.33mmol) were dissolved in neat trifluoroacetic acid (10 mL) and heated at reflux for 4 h. Then trifluroacetic acid was removed by vacuum distillation,and the solid was washed repeatedly with saturated aq. NaHCO3 and dried in vacuo to afford 2 as brown-red solid (95 mg, about 95%). 1H NMR (400 MHz,DMSO-d6,TMS): δ 7.50 (t,2H, J = 7.5 Hz) 7.58 (t,2H,J = 7.5 Hz),7.94 (d,2H,J = 8.1 Hz),8.73 (d,2H, J = 7.9 Hz),12.40 (s,2H); ESI-MS (m/z): 357.5 [M-H]-,392.9 [M+Cl]-,750.9 [2M+Cl]-; HRMS (ESI) (m/z) for: 357.0896 [M-H]-, calcd. for C22H10N6 358.3550.
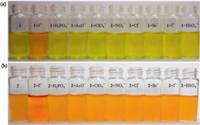
Since compounds 1 and 2 were designed and synthesized as potential receptors of fluoride,their UV-vis spectra were investigated to confirm the specific interactions toward fluoride anions. The UV-vis spectra of 1 and 2 upon the addition of F-, H2PO4-,AcO-,ClO4- ,NO3-,Cl-,Br -,I - and HSO4- as tetrabutylammonium salts (n-Bu4N+) are shown in Fig. 1. As 6 equiv. of F- was added to the solution of 1 (DMSO:H2O,95:5),the absorption at 505 and 403 nm progressively increased,while the response at 350 nm obviously reduced. Upon the addition of 6 equiv. of H2PO4- or AcO -,the UV/vis spectra exhibited little change,whereas other anions (ClO4- ,NO3 -,Cl -,Br- ,I - and HSO4- ) had almost no effect on the absorption spectra of 1.

|
Download:
|
| Fig. 1. (a) The changes in UV/vis spectra of 1 in DMSO:H2O = 95:5 (v/v) upon addition of 6 equiv. of respective anions (as a n-Bu4N+ salt). Other anions are ClO4- ,NO3 -,Cl -,Br -,I - and HSO4- . 1 = 5 × 10- 5 mol/L. (b) The changes in UV/vis spectra of 2 in DMSO:H2O = 90:10 (v/v) upon addition of 6 equiv. of respective anions (as a n-bu4N+ salt). Other anions are H2PO4-,ClO4 -,NO3- ,Cl- ,Br -,I - and HSO4- . 2 = 2.5 × 10- 5 mol/L. | |
Compound 2,which has a more rigid skeleton than 1,showed a different sensing behavior. Upon the addition of 9 equiv. of F- to 2 (DMSO:H2O = 90:10),the absorption at 303 nm and 355 nm reduced accompanied with the obvious enhancement at 321 nm and 391 nm,and a new band appeared at 505 nm. The spectra of 2 changed little with the addition of AcO- ,and no detectable changes were observed upon the addition of other anions (H2PO4 -,ClO4 -,NO3 -,Cl -,Br -,I - and HSO4 -).
|
Download:
|
| Fig. 2. (a) Color changes induced by the addition of anions (5 equiv.) to a DMSO:H2O = 95:5 (v/v) solution of 1. (b) Color changes induced by the addition of anions (5 equiv.) to a DMSO:H2O = 90:10 (v/v) solution of 2. 1 = 2 = 5 × 10-5 mol/L. | |
The fluorescence spectra of 1 and 2 with different amounts of F-
are displayed in Fig. 3. Significant fluorescence quenching was
observed with the addition of F- from 0 to 20 equiv. in aqueous
DMSO medium,and the fluorescence quenching effect of F- could
be explained with a photoinduced electron transfer (PET)
mechanism. The fitting of the fluorescence titration data shows
that 1 recognizes F- in a 1:2 stoichiometry with the logarithm of
the binding constant (log K) of 7.52 (based on the emission change
at 590 nm),while 2 binds with F- with the stoichiometry of 1:2 and
a log K of 8.99 (based on the emission change at 607 nm) [28, 29, 30].
The difference between the binding constants might be attributed
to the configurations of the receptors. As 2 contains indolocarbazole-
NH moieties with rigid recognition sites,configuration
inversion could not occur at the mono-indole moieties during
the hydrogen binding formation. Compared with 2,bi-indole
moieties in 1 may undergo a configuration inversion in order to
adapt to the specific anion shape spaces. Since the configuration
transformation is an energy consuming process,it might decrease
the affinity of 1 and F-.

|
Download:
|
| Fig. 3. (a) Fluorescence (λex = 420 nm) titration of 1 with F- (as a n-Bu4N+ salt) in DMSO:H2O = 95:5 (v/v) (equiv. F from 0 to 20). (b) Fluorescence (λex = 440 nm) titration of 2 with F- (as a n-Bu4N+ salt) in DMSO:H2O = 90:10 (v/v) (equiv. F- from 0 to 20). The excitation and emission slit widths were 10 nm,5 nm. 1 = 2 = 5 × 10-5 mol/L. | |
To understand the binding modes of 1 and 2 with F -,1H NMR titration experiments were carried out in DMSO-d6. When 0.2 equiv. of F- was added to the solution of 1,the signal of the indole-NH proton (δ 12.40) shifted downfield and broadened (see Fig. 4a),which indicates that a weak hydrogen bond is formed between this proton and F- . Further increase of the amount of F - (0.4 equiv.) made the signal of indole-NH proton disappear, probably due to the partial deprotonation. Similarly,the addition of 0.1 equiv. of F- to the solution of 2 also obliterated the indole-NH proton signal of 2 (see Fig. 4b). Unfortunately,the proton peaks of FHF- ion were not observed in these titration processes.

|
Download:
|
| Fig. 4. 1H NMR (400 MHz) spectra of 1 (a) and 2 (b) in DMSO-d6 with addition of TBAF. 1 = 2 = 5.0 × 10-3 mol/L. | |
In addition,we examined the analytical signal for the reaction system in DMSO. Upon addition of 2 equiv. F-,the color of the solution changes from light yellow to henna for 1 and from deep yellow to reddish orange for 2 (see Fig. 5) in pure DMSO. It is worth noting that when 2 equiv. AcO- were added,the yellowish brown solution of 2 in pure DMSO turned to deep orange. Therefore,2 can also operate as an efficient colorimetric sensor for visual detection of AcO- and F-.

|
Download:
|
| Fig. 5. (a) Color changes induced by the addition of F- (2 equiv.) to a DMSO solution of 1. (b) Color changes induced by the addition of F- and AcO- (2 equiv.) to a DMSO solution of 2. 1 = 2 = 5 × 10-5 mol/L. | |
In conclusion,we have successfully synthesized two compounds 1 and 2 based on indole moieties,which display highly selective response to F- both in aqueous DMSO medium and pure DMSO with obvious color change. These two receptors could be used as colorimetric sensors for the visual detection of F-. In addition,2 can be employed as an efficient colorimetric receptor for distinguishing AcO- and F- in pure DMSO. Acknowledgments
We thank the financial supports from NSF of China (Nos. 21031002 21202088,and 51073079),the China Postdoctoral Science Foundation (No. 2012M520570),and the Fundamental Research Funds for the Central Universities.
| [1] | A.N. Swinburne, M.J. Pateron, A. Beeby, et al., A quinolinium-derived turn-off fluorescent anion sensor, Org. Biomol. Chem. 8 (2010) 1010-1016. |
| [2] | J.L. Sessler, P.A. Gale, W.S. Cho, Anion Receptor Chemistry, RSC, Cambridge, 2006. |
| [3] | R. Sakai, S. Okade, E.B. Barasa, et al., Efficient colorimetric anion detection based on positive allosteric system of urea-functionalized poly(phenylacetylene) receptor, Macromolecules 43 (2010) 7406-7411. |
| [4] | A. Aldrey, C. Núñez, V. García, et al., Anion sensing properties of new colorimetric chemosensors based on macrocyclic ligands bearing three nitrophenylurea groups, Tetrahedron 66 (2010) 9223-9230. |
| [5] | M.A. Holland, L.M. Kozlowski, Clinical features and management of cyanide poisoning, Clin. Pharm. 5 (1986) 737-741. |
| [6] | R.J. Carton, Review of the 2006 United States national research council report: fluoride in drinking water, Fluoride 39 (2006) 163-172. |
| [7] | EPA National Primary Drinking Water Standards 2003, see http://www.epa.gov/safewater/contaminants/index.html for more information. |
| [8] | S.Y. Kim, J. Park, M. Koh, Fluorescent probe for detection of fluoride in water and bioimaging in A549 human lung carcinoma cells, Chem. Commun. (2009) 4735-4737. |
| [9] | G. Liu, J.B. Zhou, Y. Cheng, et al., Hierarchically porous calcined lithium/aluminum layered double hydroxides: facile synthesis and enhanced adsorption towards fluoride in water, J. Mater. Chem. 21 (2011) 19353-19361. |
| [10] | M. Park, D. Jang, S.Y. Kim, A chemodosimetric gelation system showing fluorescence and sol-to-gel transition for fluoride anions in aqueous media, New J. Chem. 36 (2012) 1145-1148. |
| [11] | W. Lu, H. Jiang, F.Y. Hu, et al., A novel chemosensor based on Fe(Ⅲ)-complexation for selective recognition and rapid detection of fluoride anions in aqueous media, Tetrahedron 67 (2011) 7909-7912. |
| [12] | A.K. Atta, I.H. Ahh, A.Y. Hong, et al., Fluoride indicator that functions in mixed aqueous media: hydrogen bonding effects, Tetrahedron Lett. 53 (2012) 575-578. |
| [13] | V. Kumar, M.P. Kaushik, A.K. Srivastava, et al., Thiourea based novel chromogenic sensor for selective detection of fluoride and cyanide anions in organic and aqueous media, Anal. Chim. Acta 663 (2010) 77-84. |
| [14] | J. Isaad, A.E. Achari, Biosourced 3-formyl chromenyl-azo dye as Michael acceptor type of chemodosimeter for cyanide in aqueous environment, Tetrahedron 67 (2011) 5678-5685. |
| [15] | Z.H. Lin, Y.G. Zhao, C.Y. Duan, et al., A highly selective chromo-and fluorogenic dual responding fluoride sensor: naked-eye detection of F-ion in natural water via a test paper, Dalton Trans. 30 (2006) 3678-3684. |
| [16] | S.H. Mashraqui, S.S. Ghorpade, S. Tripathi, et al., A new indole incorporated chemosensor exhibiting selective colorimetric and fluorescence ratiometric signaling of fluoride, Tetrahedron Lett. 53 (2012) 765-768. |
| [17] | Y. Qu, S.Y. Qu, L. Yang, A red-emission diketopyrrolopyrrole-based fluoride ion chemosensor with high contrast ratio working in a dual mode: solvent-dependent ratiometric and "turn on" pathways, Sens. Actuators B 173 (2012) 225-233. |
| [18] | M. Dong, Y. Peng, Y.M. Dong, et al., A selective, colorimetric, and fluorescent chemodosimeter for relay recognition of fluoride and cyanide anions based on 1,10-binaphthyl scaffold, Org. Lett. 14 (2012) 130-133. |
| [19] | L.Z. Gai, H.C. Chen, B. Zhou, et al., Ratiometric fluorescence chemodosimeters for fluoride anion based on pyrene excimer/monomer transformation, Chem. Commun. 48 (2012) 10721-10723. |
| [20] | A. Mallick, U.K. Roy, B. Haldar, et al., A newly developed highly selective ratiometric fluoride ion sensor: spectroscopic, NMR and density functional studies, Analyst 137 (2012) 1247-1251. |
| [21] | Y.M. Li, X.L. Zhang, B.C. Zhu, et al., A highly selective colorimetric and "offon-off" fluorescent probe for fluoride ion, Anal. Sci. 26 (2010) 1077-1080. |
| [22] | B.C. Zhu, F. Yuan, R.X. Li, et al., A highly selective colorimetric and ratiometric fluorescent chemodosimeter for imaging fluoride ion in living cells, Chem. Commun. 47 (2011) 7098-7100. |
| [23] | J.R.H. Feng, D.H. Hu, et al., A rapid aqueous fluoride ion sensor with dual output modes, Angew. Chem. 122 (2010) 5035-5038. |
| [24] | M.J. Chmielewski, L. Zhao, A. Brown, et al., Sulfate anion templation of a neutral pseudorotaxane assembly using an indolocarbazole threading component, Chem. Commun. (2008) 3154-3156. |
| [25] | C. Caltagirone, J.R. Hiscock, M.B. Hursthouse, et al., 1,3-DⅡndolylureas and 1,3-dⅡndolylthioureas: anion complexation studies in solution and the solid state, Chem. Eur. J. 14 (2008) 10236-10243. |
| [26] | X.M. Liu, Q. Zhao, W.C. Song, et al., New highly selective colorimetric and ratiometric anion receptor for detecting fluoride ions, Chem. Eur. J. 18 (2012) 2806-2811. |
| [27] | X.M. Liu, Y.P. Li, W.C. Song, et al., A new ion pair receptor fulfilling a dual function as a chromogenic molecular switch for F- and ratiometric selective recognition of HSO4-, Talanta 97 (2012) 111-117. |
| [28] | Q.G. Wang, Y.S. Xie, Y.B. Ding, et al., Colorimetric fluoride sensors based on deprotonation of pyrrole-hemiquinone compounds, Chem. Commun. 46 (2010) 3669-3671. |
| [29] | X. Peng, J. Du, J. Fan, et al., A selective fluorescent sensor for imaging Cd2+ in living cells, J. Am. Chem. Soc. 129 (2007) 1500-1501. |
| [30] | F. Han, Y.H. Bao, Z.G. Yang, et al., Simple bisthiocarbonohydrazones as sensitive, selective, colorimetric, and switch-on fluorescent chemosensors for fluoride anions, Chem. Eur. J. 13 (2007) 2880-2892. |




