1. Introduction
Highly substituted furans play an important role in organic chemistry,not only as the key structural units in many natural products,common subunits in pharmaceuticals [1, 2, 3, 4, 5, 6, 7] and flavors [8] but also as useful building blocks in synthetic chemistry [9, 10, 11, 12, 13]. They have also found utilities as synthetic intermediates or synthons for numerous functional groups,inter alia,carboxylic acids,α-ketoesters,and aromatics [14]. For this reason,the efficient syntheses of highly substituted furans continue to attract the interest of synthetic chemists [15, 16]. Accordingly,many strategies have been developed for the preparation of furans [17, 18, 19, 20]. The above-mentioned methodologies suffer from certain limitations such as long synthetic routes,use of costly metal catalysts and highly volatile solvents,etc. Recently Narayana et al.and Ramesh et al.developed a methodology for the synthesis of furan-2(5H)-ones via a reaction of aromatic amines,aldehydes and dialkyl acetylenedicarboxylate. Narayana et al.described the synthesis of 2(5H)-furanones in the presence of β-cyclodextrin [21]. Ramesh and Nagarajan reported the preparation of 2(5H)-furanones via a three-component reaction of a series of aldehydes (aromatic,heterocyclic),dialkylacetylenedicarboxylates and 9-alkyl-9H-carbazol-3-amines in the presence of KOH [22]. The synthesis of 2(5H)-furanones promoted by SnCl2 has also been reported by Nagarapu et al.[23].
We have recently reported the synthesis of 2(5H)-furanones in the presence of acetic acid [24]. In continuation of our research work on the synthesis of furan derivatives [25, 26, 27, 28, 29, 30, 31, 32, 33],herein we describe a novel use of tetra-n-butylammonium bisulfate in a multi-component reaction of aldehyde,dialkyl acetylenedicarboxylates and aniline derivatives in generating furan-2(5H)-one derivatives under mild conditions (Scheme 1).

|
Download:
|
| Scheme 1. Synthesis of furan-2(5H)-one derivatives. | |
Melting points and IR spectra of all compounds were measured on an Electrothermal 9100 apparatus and a JASCO FTIR 460 Plus spectrometer,respectively. The 1H NMR and 13C NMR spectra were recorded on Bruker DRX-250 and 400 Avance instruments with CDCl3 as a solvent. Elemental analyses were performed using a Heraeus CHN-O-Rapid analyzer. Mass spectra were recorded on an Agilent Technology (HP) spectrometer operating at an ionization potential of 70 eV. All reagents and solvents obtained from Fluka and Merck were used without further purification.
General procedures for synthesis of furan-2(5H)-ones: A mixture of aldehyde (1 mmol),amine (1 mmol),dialkylacetylenedicarboxylate (1 mmol) and tetra-n-butylammonium bisulphate was stirred in ethanol at room temperature. After completion of the reaction (monitored by TLC),the reaction mixture was filtrated and washed with ethanol (10 mL×3) to obtain a pure product.Physical and chemical data of chosen products are demonstrated below.
Methyl 2,5-dihydro-5-oxo-2-phenyl-4-(phenylamino)furan-3-carboxylate (4a): White solid; 0.284 g (92%); mp 195-196℃; IR (KBr,cm-1):ν 3260,3208,1702,1661;1H NMR (400 MHz,CDCl3):δ 3.77 (s,3 H,OCH3),5.76 (s,1H,benzylic),7.13 (t,1H,J= 7.3 Hz), 7.24-7.31 (m,7H),7.52 (d,2H,J= 8 Hz),8.90 (br,NH,1H);13C NMR (100 MHz,CDCl3):δ 165.3 and 162.7 (ester CO),156.3,136.1,134.9, 129.0,128.7,128.6,127.4,125.9,122.3,112.8 (aromatic C),61.6 (methoxy C),52.1 (benzylic C); MS (positive mode,m/z (%)): 57 (100),97 (75),152 (24),213 (51),240 (39),250 (33),309 (M+,44); Anal. calcd. for C18H15NO4: C 69.89,H 4.89,N 4.53. Found: C 70.08, H 4.97,N 4.60.
Methyl 4-(p-tolylamino)-2,5-dihydro-5-oxo-2-phenylfuran-3-carboxylate (4b): White solid; 0.287 g (89%); mp 173-175℃; IR (KBr,cm-1):ν 3228,2950,1706,1677,1513;1H NMR (400 MHz, CDCl3):δ 2.27 (s,3H,CH3),3.76 (s,3H,OCH3),5.72 (s,1H,benzylic), 7.09 (d,2H,J= 8 Hz),7.22-7.270 (m,5H,aromatic),7.34 (d,2H, J= 8.4 Hz),8.86 (br,1H,NH);13C NMR (100 MHz,CDCl3):δ 165.3 and 162.8 (CO of ester),156.4,135.8,135.0,133.5,129.6,128.6, 128.5,127.5,122.4,112.6 (C of aromatic),61.3 (C of methoxy),52.0 (benzylic C),20.95 (C of methyl); MS (m/z(%)): 130 (96),131 (21), 133 (19),158 (39),189 (34),263 (14),264 (33),265 (12),291 (24), 323 (M+,100); Anal. calcd. for C19H17NO4: C 70.58,H 5.30,N 4.33. Found: C 70.77,H 5.38,N 4.35.
Ethyl 2-(4-cyanophenyl)-2,5-dihydro-5-oxo-4-(phenylamino)-furan-3-carboxylate (4e): White solid; 0.324 g (93%); mp 188- 189℃; IR (KBr,cm-1):ν 3293 (NH),2977,2225 (CN),1731,1684, 1666,1500;1H NMR (400 MHz,CDCl3): δ 1.23 (t,3H,J= 7.2 Hz, CH3),4.24 (q,2H,J= 7.2 Hz,CH2),5.82 (s,1H,benzylic),7.17 (t,1H, J= 7.2 Hz),7.32-7.47 (m,6H,aromatic),7.59 (d,2H,J= 8 Hz),9.03 (br,1H,NH);13C NMR (100 MHz,CDCl3): δ 164.6,162.5 (CO of ester),156.89,140.8,135.7,132.5,129.2,128.3,126.3,122.1,118.1, 112.6 (aromatic C),112.2 (C of CN),61.6 (methoxy),60.8 (benzylic), 14.02 (CH3 of ethoxy). MS (m/z(%)): 93 (17),119 (9),155 (70),183 (29),228 (13),275 (59),302 (14),348 (M+,100); Anal. calcd. for C20H16N2O4: C 68.96,H 4.63,N 8.04. Found: C 69.10,H 4.69,N 8.11.
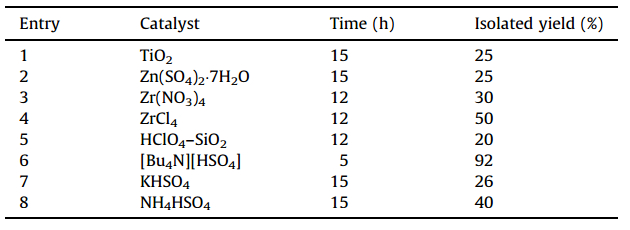
3. Results and discussionWe have discovered a one-pot three-component condensation reaction (MCR) for the synthesis of organic compounds using tetra-n-butylammonium bisulfate as a catalyst at room temperature in a single step. As previously mentioned,products can be easily separated and purified by simple filtration. Benzaldehyde,aniline, and dimethyl acetylenedicarboxylate were taken as model compounds for the optimization of the reaction conditions. For this purpose,the reaction was initially carried out in ethanol using different catalysts (Table 1). As can be seen,[Bu4N][HSO4] was found to be the most effective catalyst for the reaction at room temperature.
| Table 1 Optimization of catalyst for the synthesis of furan-2(5H)-ones from the reaction between benzaldehyde,aniline and dimethyl acetylenedicarboxylate at room temperature. |
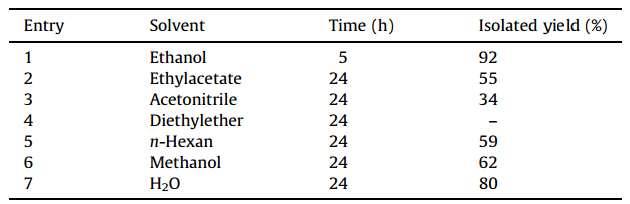
In this work,various solvents were tested to optimize the conditions for the synthesis of compound 4a. Ethanol was found to be the best solvent,in which the product was obtained in 92% yield (Table 2). Here,[Bu4N][HSO4] and ethanol were finally selected as the suitable catalyst and the solvent,respectively.
| Table 2 Optimization of solvent for the synthesis of furan-2(5H)-ones from the reaction of benzaldehyde,aniline and dimethyl acetylenedicarboxylate in the presence of [Bu4N][HSO4] as an optimized catalyst. |
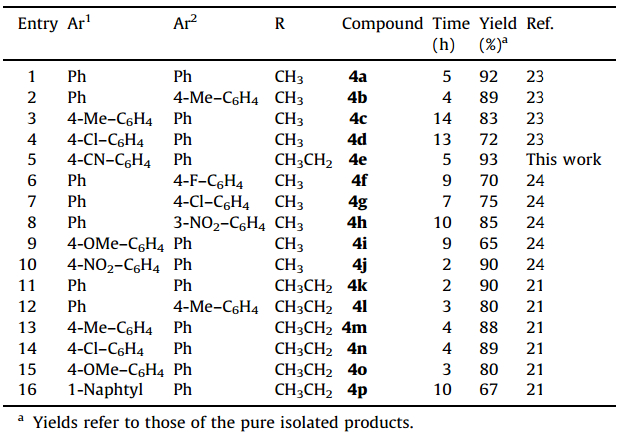
Under the optimized reaction conditions,the generality of the reaction was fully investigated with different aldehydes,anilines and dialkyl acetylenedicarboxylate to produce furan-2(5H)-one derivatives. The results are summarized in Table 3. These results show the effects of electron-withdrawing and electron-donating groups on the time required and the yield of the reactions. Benzaldehydes with electron-withdrawing groups react with aniline more efficiently than the benzaldehydes substituted with electron-donating groups. In our work,aliphatic aldehydes and amines such as propanal and 1-buthyl amine did not work well under the reaction conditions.
| Table 3 Synthesis of furan-2(5H)-one derivatives. |
The structures of the new compounds in Table 3 were established on the basis of IR,1H NMR,13C NMR,MS and elemental analysis. The mass spectrum of ethyl 2-(4-cyanophenyl)-2,5-dihydro-5-oxo-4-(phenylamino)furan-3-carboxylate (Table 3,entry 5) displayed the molecular ion peak at m/z 348,which is consistent with the proposed structure. The 1H NMR spectrum of this product,exhibited a triplet at δ 1.23 (J= 7.2 Hz) for the methyl protons and a quartet for the methylene protons of the carboxylate group at δ 4.24 and one sharp singlet arising from the benzylic proton at δ 5.82. The aromatic protons of the product were observed at δ 7.17-7.59. A broad singlet for the NH group at δ 9.03 indicated an intramolecular hydrogen bond formed with the vicinal carbonyl group. The 13C NMR spectrum of this product showed 16 distinct resonances in agreement with the proposed structure. The IR spectrum contained one sharp peak at 3293 cm-1 for the NH group in the product.
A proposed mechanism for the formation of 4 is shown in Scheme 2.

|
Download:
|
| Scheme 2. The speculative proposed mechanism for the formation of furan-2(5H)-one derivatives. | |
In conclusion we have identified an efficient and simple one-pot reaction for the synthesis of furanone derivatives using tetra-n-butylammonium bisulfate as an economic catalyst in ethanol. This methodology has several advantages such as simplified workup procedures,mild conditions,and the use of green solvents.
AcknowledgmentWe gratefully acknowledge financial support from the Research Council of the University of Sistan and Baluchestan.
| [1] | F.A.Dean, Naturally Occurring Oxygen Ring Compounds, Butterworth, London, 1963. |
| [2] | K. Nakanishi, T. Goto, S. Ito, S. Natori, S. Nozoe (Eds.), Natural Products Chemistry, vols. 1-3, Kodansha, Tokyo, 1974. |
| [3] | F.M. Dean, Recent advances in furan chemistry. Part Ⅱ, in: A.R. Katritzky (Ed.), Advances in Heterocyclic Chemistry, vol. 31, Academic Press,NewYork, 1983, p.237. |
| [4] | M.V. Sargent, F.M. Dean, Furans and their benzo derivatives: (ii) reactivity, in: C.W. Bird, G.W.H. Cheeseman (Eds.), Comprehensive Heterocyclic Chemistry, vol. 3, Pergamon Press, Oxford, 1984, p. 599. |
| [5] | B.H. Lipshutz, Five-membered heteroaromatic rings as intermediates in organic synthesis, Chem. Rev. 86 (1986) 795-819. |
| [6] | I. Bock, H. Bornowski, A. Ranft, H. Theis, New aspects in the synthesis of mono-and dialkylfurans, Tetrahedron 46 (1990) 1199-1210. |
| [7] | D.S. Mortensen, A.L. Rodriguez, K.E. Carlson, et al., Synthesis and biological evaluation of a novel series of furans: ligands selective for estrogen receptor a, J. Med. Chem. 44 (2001) 3838-3848. |
| [8] | M. Naim, I. Zuker, U. Zehavi, R.L. Rouseff, Inhibition by thiol compounds of offflavor formation in stored orange juice 2. Effect of L-cysteine and N-acetyl-Lcysteine on p-vinylguaiacol formation, J. Agric. Food Chem. 41 (1993) 1359-1361. |
| [9] | R. Benassi, Furans and their benzo derivatives: structure, in: A.R. Katritzky, C.W. Rees, E.F.V. Scriven (Eds.), Comprehensive Heterocyclic Chemistry Ⅱ, vol. 2, Pergamon Press, Oxford, 1996, p. 259. |
| [10] | H. Heaney, J.S. Ahn, Furans and their benzo derivatives: reactivity, in: A.R. Katritzky, C.W. Rees, E.F.V. Scriven (Eds.), Comprehensive Heterocyclic Chemistry Ⅱ, vol. 29, Pergamon Press, Oxford, 1996, p. 297. |
| [11] | W. Friedrichsen, Furans and their benzo derivatives: synthesis, in: A.R. Katritzky, C.W. Rees, E.F.V. Scriven (Eds.), Comprehensive Heterocyclic Chemistry Ⅱ, vol. 2, Pergamon Press, Oxford, 1996, p. 351. |
| [12] | B.A. Keay, P.W. Dibble, Furans and their benzo derivatives: applications, in: A.R. Katritzky, C.W. Rees, E.F.V. Scriven (Eds.), Comprehensive Heterocyclic Chemistry Ⅱ, vol. 2, Pergamon Press, Oxford, 1996, p. 395. |
| [13] | B.M. Trost, I. Fleming, Comprehensive Organic Synthesis, Pergamon Press, Oxford, 1991. |
| [14] | A.I. Meyers, Heterocycles in Organic Synthesis, Wiley-Interscience, New York, 1974. |
| [15] | X.L. Hou, Z. Yang, H.N.C. Wong, Five-membered ring systems: furans and benzofurans, in: G.W. Gribble, T.L. Gilchrist (Eds.), The Structure, Reactions, Synthesis, and Uses of Heterocyclic Compounds, Progress in Heterocyclic Chemistry, vol. 14, Pergamon Press, Oxford, 2002, p. 139. |
| [16] | X.L. Hou, H.Y. Cheung, T.Y. Hon, et al., Regioselective syntheses of substituted furans, Tetrahedron 54 (1998) 1955-2020. |
| [17] | R. Cao, C. Liu, L. Liu, A convenient synthesis of 2(5H)-furanose, Org. Prep. Proc. Int. 28 (1996) 215-216. |
| [18] | C. Fu, S. Ma, Efficient preparation of 4-iodofuran-2(5H)-ones by iodolactonisation of 2,3-allenoates with I2, Eur. J. Org. Chem. 2005 (2005) 3942-3945. |
| [19] | (a) G.J. Hollingworth, G. Perkins, J.B. Sweeney, Preparation and palladium-catalysed cross-coupling reactions of 3-and 4-tributylstannylfuran-2(5H)-ones, J. Chem. Soc. Perkin Trans. 1 (1996) 1913-1917; (b) R. Mabon, A.M.E. Richecoeur, J.B. Sweeney, Preparation and reactions of 3,4-bis(tributylstannyl)-2(5H)-furanone, J. Org. Chem. 64 (1999) 328-329; (c) R. Mabon, A.M.E. Richecoeur, J.B. Sweeney, Preparation and reactions of 3,4-bisstannyl-2(5H)furanones, Tetrahedron 58 (2002) 9117-9129. |
| [20] | M. Bassetti, A. D'Annibale, A. Fanfoni, F. Minissi, Synthesis of a,b-unsaturated 4, 5-disubstituted g-lactones via ring-closing metathesis catalyzed by the firstgeneration Grubbs' catalyst, Org. Lett. 7 (2005) 1805-1808. |
| [21] | S. Narayana Murthy, B. Madhav, A. Vijay Kumar, K. Rama Rao, Y.V.D. Nageswar, Facile and efficient synthesis of 3,4,5-substituted furan-2(5H)-ones by using bcyclodextrin as reusable catalyst, Tetrahedron 65 (2009) 5251-5256. |
| [22] | R. Subburethinam, N. Rajagopal, Efficient one-pot multicomponent synthesis of (carbazolylamino)furan-2(5H)-one and carbazolyltetrahydropyrimidine derivatives, Synthesis 20 (2011) 3307-3317. |
| [23] | L. Nagarapu, U. Nikhil Kumar, P. Upendra, R. Bantu, Simple, convenient method for the synthesis of substituted furan-2(5H)-one derivatives using Tin(Ⅱ) chloride, Synth. Commun. 42 (2012) 2139-2148. |
| [24] | R. Doostmohammadi, M.T. Maghsoodlou, N. Hazeri, S.M. Habibi-Khorassani, Acetic acid as an efficient catalyst for the one-pot preparation of 3,4,5-substituted furan-2(5H)-ones, Res. Chem. Intermed. (2013), http://dx.doi.org/10.1007/s11164-012-0922-1 (This article is being published). |
| [25] | M.T. Maghsoodlou, N. Hazeri, S.M. Habibi-Khorassani, G. Marandi, M. Nassiri, g-Spiroiminolactone synthesis by reaction of acetylenic esters and a-dicarbonyl compounds in the presence of aryl isocyanide, Synth. Commun. 35 (2005) 2771-2777. |
| [26] | M.T. Maghsoodlou, N. Hazeri, S.M. Habibi-Khorassani, et al., Reaction of alkyl and aryl Isocyanides with floren-9-ones in the presence of acetylenic esters: preparation of g-spiroiminolactones, Synth. Commun. 35 (2005) 2569-2574. |
| [27] | M.T. Maghsoodlou, N. Hazeri, S.M. Habibi-Khorassani, G. Marandi, M. Nassiri, 1, 8-Diazafloren-9-one with alkyl and aryl isocyanides in the presence of acetylenic esters: a facile synthesis of g-spiroiminolactones, J. Heterocycl Chem. 43 (2006) 481-484. |
| [28] | N. Hazeri, M.T. Maghsoodlou, R. Heydari, et al., g-Dispiro-iminolactone synthesis by three component reaction between alkyl isocyanides and acetylenic esters with a-dicarbonyl compounds, ARKIVOC xiii (2007) 34-40. |
| [29] | N. Hazeri, M.T. Maghsoodlou, R. Heydari, et al., Synthesis of novel 2-pyridylsubstituted 2,5-dihydro-2-imino-and 2-amino-furan derivatives via a three component condensation of alkyl isocyanides and acetylenic esters with di-(2-pyridyl) ketone or 2-pyridinecarboxaldehyde, ARKIVOC i (2007) 173-179. |
| [30] | F. Rostami-Charati, M.T. Maghsoodlou, S.M. Habibi Khorassani, R. Heydari, M. Makha, Green diastereoselective synthesis of highly functionalised trifluoromethylated c-lactone phosphonate esters bearing a thioester or ketothiophene, Tetrahedron Lett. 49 (2008) 343-347. |
| [31] | M.T. Maghsoodlou, N. Hazeri, S.M. Habibi-Khorassani, et al., Diastereoselective synthesis of g-ispiroiminolactone bearing naphthalene or bipyridine pendant groups, J. Heterocycl. Chem. 46 (2009) 843-848. |
| [32] | M.T. Maghsoodlou, S.M. Habibi-Khorassani, A. Moradi, et al., One-pot threecomponent synthesis of functionalized spirolactones by means of reaction between aromatic ketones, dimethyl acetylenedicarboxylate, and N-heterocycles, Tetrahedron 67 (2011) 8492-8495. |
| [33] | M.T. Maghsoodlou, G. Marandi, N. Hazeri, S.M. Habibi-Khorassani, A.A. Mirzaei, Synthesis of 5-aryl-1,3-dimethyl-6-(alkyl-or aryl-amino)furo[2,3-d] pyrimidine derivatives by reaction between isocyanides and pyridinecarbaldehydes in the presence of 1,3-dimethylbarbituric acid,Mol. Divers. 15 (2011) 227-231. |