b Department of Chemistry, Guiyang Medical College, Guiyang 550004, China
Over the past few decades,macrocyclic compounds have become important synthetic targets due to their wide applications in molecular recognition,supramolecular structures,catalysis,and material chemistry [1, 2, 3, 4, 5, 6]. One representative group of macrocycles is the Schiff-base macrocyclic compound family. The Schiff macrocycles are synthesized by the reaction of dicarbonyl compounds with diamines by well understood mechanisms [7, 8]. The macrocyclic systems can be functionalized by inserting appropriate groups in the aliphatic and/or aromatic chains of the formyl- or keto- and amine-precursors. Furthermore,the Schiff bases can be reduced to the related polyamine derivatives by reaction with an appropriate reducing agent. These polyamine compounds are less sensitive to hydrolysis and more flexible, which may greatly influence the ability to discriminate among the different charged or neutral species [9, 10, 11, 12].
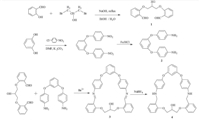
In this paper,the new Schiff-base macrocycle 3 was synthesized by the condensation reaction of 1,3-bis(2'-formylphenoxy)-2- propanol and resorcinol-bis(4-aminophenyl)ether. Furthermore, the macrocycle 3 was reduced to the corresponding saturated macrocycle 4 in order to increase its flexibility (Scheme 1). The alkoxyl chain of compounds 3 and 4 contain a hydroxyl functional group,which may be further functionalized by appropriate synthetic procedures.
|
Download:
|
| Scheme 1. Syntheses of the Schiff-base macrocycle 3 and the macrocycle 4. | |
Precursor dialdehyde 1 [1,3-bis(2'-formylphenoxy)-2-propanol] was prepared using the reported procedure [13]. The solid product was dried under vacuum before analysis. Yield: 37%,mp 116-118 ℃. 1H NMR (400 MHz,CDCl3): δ 10.40 (s,2H,CHO),7.81 (d,2H,ArH),7.57 (t,2H,ArH),7.03-7.18 (m,4H,ArH),4.33 (d,4H, CH2),4.20 (s,1H,CH),3.30 (s,1H,OH).
Precursor diamine 2 [resorcinol-bis(4-aminophenyl) ether] was synthesized using the reported procedure [14]. Resorcinol-bis(4- nitrophenyl)ether was obtained as a pale yellow solid in 65% yield, mp 136-138 ℃. 1H NMR (400 MHz,CDCl3): δ 7.116-7.157 (t,1H, Ar-H),6.849-6.880 (m,4H,ArH),6.649-6.688 (m,4H,ArH),6.529- 6.554 (t,3H,ArH),3.579 (s,2H,NH2),1.646 (s,2H,NH2).
[1,3-Bis(2‘-formylphenoxy)-2-propanol (0.151 g,0.5 mmol)] in methanol (50 mL) was added slowly,with stirring,to resorcinolbis( 4-aminophenyl)ether (0.146 g,0.5 mmol) and BaCl2 (0.105 g, 0.5 mmol) in methanol (100 mL). The mixture was stirred for 12 h at room temperature; the bright yellow precipitate was collected by filtration and washed with ethanol,and dried at room temperature to give 3 as a yellow solid in 75.7% yield,mp 271- 273 ℃. The solid was dissolved in excess methanol at room temperature; slow evaporation of this solution yielded white block-shaped single crystals that proved suitable for X-ray analysis. 1H NMR (400 MHz,CDCl3): δ 8.70 (s,2H,CH=N),7.83- 7.86 (d,2H,ArH),7.39-7.46 (m,3H,ArH),7.169-7.191 (d,4H,ArH), 7.082-7.119 (t,2H,ArH),6.951-7.072 (d,8H,ArH),6.575 (t,1H, ArH),4.519 (t,1H,CH),4.31-4.339 (d,2H,CH2),4.00 (t,1H,OH), 3.485-3.498 (d,2H,CH2); IR (KBr,cm-1): n 2925(CH2),1618 (C=N), 1497,1482,1451 (C=C),1283 (C-O); FABMS (m/z): 579 [M+Na]+; Anal. Calcd. for C35H28N2O5: C 75.52,H 5.07,N 5.03; Found: C 75.50,H 5.10,N 5.09.
NaBH4 (0.3 g,6.7mmol) in ethanol (30 mL) was added dropwise, with stirring,to Schiff-base macrocycle 3 (0.8 g,1.43mmol) in THF (60 mL) and the reaction solution heated at reflux for 12 h. The solution was concentrated to a volume of ca. 10-15 mL in a rotary evaporator whereupon distilled water (30 mL) was added,and reaction solution was extracted with methylene chloride. The retained organic phase was dried with Na2SO4 before removal of solvent in a rotary evaporator. The residue was purified by recrystallization from methylene chloride/ethyl acetate (2/1,v/v) to give pale yellow solid 0.45 g,yield in 56%,mp 96-98 ℃. The solid was dissolved in excess ethanol at room temperature; slow evaporation of this solution yielded white needle-shaped single crystals that proved suitable for X-ray analysis. 1H NMR (500 MHz, CDCl3): δ 7.28-7.32 (m,5H,ArH),6.978 (t,2H,ArH),6.910-6.926 (d, 2H,ArH),6.77-6.82 (m,6H,ArH),6.598-6.616 (d,4H,ArH),6.214 (t, 1H,ArH),4.433 (t,1H,CH),4.254 (t,4H,ArOCH2),4.198 (s,1H,NH), 4.174 (s,1H,NH),4.078 (t,4H,N-CH2); IR (KBr,cm-1): n 3422 (N-H), 3040 (Ar-H),2927 (CH2),1448,1508,1598 (C=C),1245(C-N). FABMS (m/z): 583 [M+Na]+; Anal. Calcd. for C35H32N2O5: C 74.98,H 5.75,N 4.99; Found: C 74.95,H 5.71,N 5.01.
The reaction of 1,3-bis(2’-formylphenoxy)-2-propanol and resorcinol-bis(4-aminophenyl)ether using Ba2+ as template in ethanol solution yields the Schiff base macrocycle 3,isolated as an air-stable yellow solid in 75.7% yield. In order to increase the flexibility of macrocycle 3,the Schiff base macrocycle 3 was reduced to the corresponding saturated macrocycle 4 via NaBH4 in THF ethanol solution,and the pale yellow solid 4 was extracted from the reaction solution in a 56% yield.
The two macrocycles were characterized by elemental analysis, FAB MS,IR,and 1H NMR spectra. The FAB mass spectrum of 3 shows the parent ion peak at m/z 579 [M+Na]+,confirming the [1+1] nature of the Schiff-base compound,and the peak of molecular ion corresponding to the reduced macrocycle 4 at m/z 583 [M+Na]+ is shown in the FAB mass spectrum of 4. The FAB mass spectra and 1H NMR spectra of 3 and 4 can be found in the Supporting information.
The 1H NMR spectrum of 3 in CDCl3 solution is complicated and shows more than the expected number of signals with the signal at δ 8.70 attributed to the CH=N- proton. Comparison of the 1H NMR spectra of the macrocycles 3 and 4 reveals the disappearance of the signal at d 8.70 due to the imino-protons and the appearance of two new singlets at δ 4.174 and 4.198 corresponding to the NHCH2 amine protons of 4 (see Supporting information). Crystallographic data for structures reported in this article have been deposited in the Cambridge Crystallographic Data Centre,CCDC: 934142 for 3, and 934143 for 4.
The IR spectrum of the Schiff-base macrocycle 3 features a strong band at 1618 cm-1 attributable to the azomethine group, while the IR spectrum of the macrocycle 4 features a secondary amine N-H stretching band at ca. 3422 cm-1 and no C=N band at ca. 1618 cm-1. The absorption band due to C=N stretching at 1618 cm-1 for 3 and the absorption band due to N-H stretching at 3422 cm-1 for 4,are also in good agreement with the molecular structures (see below).
The macrocyclic structures of 3 and 4 were determined by single crystal X-ray analysis. Crystal data,structural refinements and selective bond lengths or angles for the two compounds are shown in Tables S1 and S2 (Supporting information).
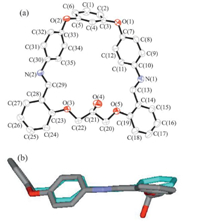
According to X-ray analysis,compound 3 has a [1+1]- condensation 26-membered macrocyclic structure (Fig. 1a). In the resorcinol-bis(4-iminophenyl)ether subunit,the dihedral angles between the two benzene rings located in the two ‘‘arms’’ of the central phenolic-ring (top) are 102° and 94°,respectively. The dihedral angles of the two pairs of benzene rings (lying in the same side chain) bridged by the Schiff base C=N are 1188 and 1298,respectively. The dihedral angle of the two benzene rings linked via the alkyl bridge is 158°,and the macrocycle 3 adopts a folded conformation (Fig. 1b). Bond lengths of the two C=N groups are 0.1264(4) nm and 0.1271(4) nm,respectively (a typical C=N,ca. 0.1269 nm) [15]. The diameter of the macrocycle hole is 0.9378 nm [distance between N(1) and N(2)],and no solvent molecules were found included in the macrocyclic cavity.
|
Download:
|
| Fig. 1. (a) Molecular structure of the compound 3 (probability of ellipsoid is 30%);(b) molecular structure viewed along side chain. Hydrogen atoms have beenomitted for clarity. | |
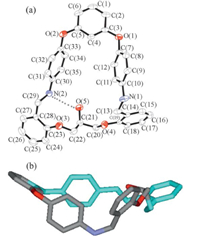
The crystal structure of 4 is shown in Fig. 2a. In the resorcinolbis( 4-iminophenyl)ether subunit,the dihedral angles between the two benzene rings located in the two ‘‘arms’’ of the central phenolic-ring (top) are 111° and 107°,respectively. The dihedral angles of the two pairs of benzene rings (lying in the same side chain) bridged by the Schiff base C=N are 958 and 948,respectively. The dihedral angle of the two benzene rings linked via the alkyl bridge is 129°. This is reinforced by intramolecular O-H ··· N hydrogen-bond interaction (O-H: 0.082 nm,O ··· N: 0.3053 nm,O- H ··· N: 155°). The lengths of the two C-N bands are 0.1438(4) nm and 0.1457(4) nm,respectively,and the bond length of carbon- nitrogen single bond is longer than the typical C=N double bond. The diameter of the macrocycle hole is 0.7231 nm [distance between N(1) and N(2)],and no solvent molecules were found included in the macrocyclic cavity. Unlike the structure of 3,the flexibility of the macrocycle 4 allows it to adopt a twisted and folded conformation (Fig. 2b).
|
Download:
|
| Fig. 2. (a) Molecular structure of the compound 4 (probability of ellipsoid is 30%);(b) molecular structure viewed along side chain. Hydrogen atoms have beenomitted for clarity,the O-H ··· N hydrogen bond is shown as dashed line. | |
Both macrocyclic products 3 and 4 exhibited maximal adsorption at 327 nm and 309 nm,respectively,and no changes were observed in their adsorption spectra when C60 was added to their benzene solution. Attempts to create some transitional metal (Cu2+,Zn2+,Cd2+,Fe3+) complexes with macrocycles 3 or 4 failed to give any positive results.
A 26-membered Schiff-base macrocycle 3 with a hydroxyl functional group was synthesized and macrocycle 3 was further reduced giving macrocyclic compound 4. X-ray crystallographic analysis revealed that the macrocyclic compound 3 has a folded conformation,and the corresponding reduced product 4 adopts a twisted and folded conformation due to its flexible nature.
This work was supported by the National Natural Science Foundation of China (No. 21061003),the International Collaborative Project of Guizhou Province (No. [2009]700104) and the Natural Science Foundation of the Guizhou Province (No. (2012)2151).
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2013.06.014.
| [1] | J. Lisowski, Enantiomeric self-secognition in homo-and heterodinuclear macrocyclic lanthanide(Ⅲ) complexes, Inorg. Chem. 50 (2011) 5567-5576. |
| [2] | C. Liu, M.R. Sawaya, P.N. Cheng, et al., Characteristics of amyloid-related oligomers revealed by crystal structures of macrocyclic b-sheet mimics, J. Am. Chem. Soc. 133 (2011) 6736-6744. |
| [3] | S. Srimurugan, B. Viswanathan, T.K. Varadarajan, et al., Synthesis and crystal structure of [2+2] calixsalens, Org. Biomol. Chem. 4 (2006) 3044-3047. |
| [4] | I. Alfonso, M. Bolte, M. Bru, M.I. Burguete, et al., Supramolecular control for the modular synthesis of pseudopeptidic macrocycles through an anion-templated reaction, J. Am. Chem. Soc. 130 (2008) 6137-6144. |
| [5] | S.O. Kang, J.M. Llinares, D. Powell, et al., New polyamide cryptand for anion binding, J. Am. Chem. Soc. 125 (2003) 10152-10153. |
| [6] | (a) B. Zheng, F. Wang, S. Dong, et al., Supramolecular polymers constructed by crown ether-based molecular recognition, Chem. Soc. Rev. 41 (2012) 1621-1636; |
| [7] | N.E. Borisova, M.D. Reshetova, Y.A. Ustynyuk, Metal-free methods in the synthesis of macrocyclic schiff bases, Chem. Rev. 107 (2007) 46-79. |
| [8] | P.A. Vigato, S. Tamburini, The challenge of cyclic and acyclic schiff bases and related derivatives, Coord. Chem. Rev. 248 (2004) 1717-2128. |
| [9] | J.L. Sessler, E. Katayev, G.D. Pantos, et al., Fine tuning the anion binding properties of 2,6-diamidopyridine dipyrromethane hybrid macrocycles, J. Am. Chem. Soc. 44 (2005) 7386-7390. |
| [10] | P.A. Vigato, S. Tamburini, L. Bertolo, The development of compartmental macrocyclic Schiff bases and related polyamine derivatives, Coord. Chem. Rev. 251 (2007) 1311-1492. |
| [11] | A. Easwaran, C.F. Christopher, C.N. Bruce, et al., Squaraine-derived rotaxanes: sterically protected fluorescent near-IR dyes, J. Am. Chem. Soc. 127 (2005) 3288-3289. |
| [12] | P. Hu, S.Y. Guo, Q.L. Zhang, et al., Synthesis, characterization of novel [1+1] schiff base macrocyclic compounds, Chin. J. Org. Chem. 33 (2013) 325-331. |
| [13] | A.A. Khandar, A.H.Y. Seyed, Synthesis, X-ray crystal structure, and solution properties of nickel(Ⅱ) complexes of new 16-membered mixed-donor macrocyclic Schiff base ligand incorporating a pendant alcohol function, Polyhedron 22 (2003) 1481-1487. |
| [14] | D.M. Liu, Study on the synthesis and characterization of new macrocyclic Schiff base and macrocyclic amide, Master's thesis of Jilin University, 2009, 22-24. |
| [15] | S. Özkar, D. Ülkü, L.T. Yíldírím, et al., Crystal and molecular structure of bis(acetylacetone) ethylenediimine: intramolecular ionic hydrogen bonding in solid state, J. Mol. Struct. 688 (2004) 207-211. |




