bModern Research Center for Traditional Chinese Medicine, Beijing University of Chinese Medicine, Beijing 100029, China
Artemisia (Compositae) species are widespread throughout China and receive much attention due to their remarkable biological activities and structural diversities [1, 2, 3, 4]. Artemisia vestita Wall. is one of the members of Compositae family with a distribution in most western areas of China. It is commonly known as ‘‘Maolianhao’’ (alias: Wannianpeng),a folk medicine used to treat various inflammatory diseases in Tibet. Previously,only one phytochemical investigation was conducted,which revealed the presence of flavonoids,coumarins,and sesquiterpene lactones [5].
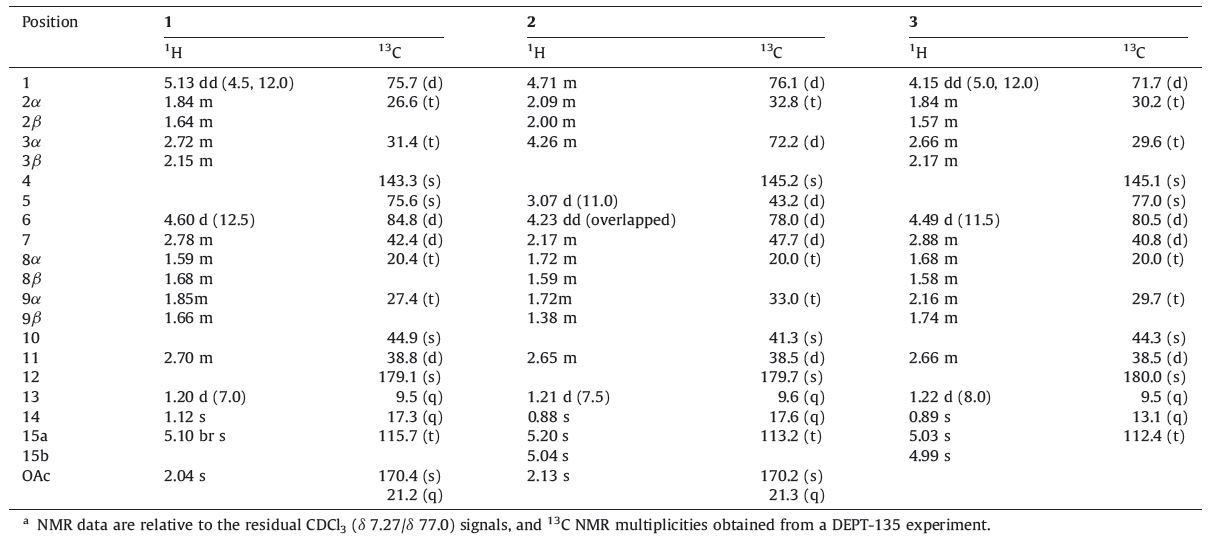
Great efforts from our group have been devoted to the phytochemical screenings of Artemisia species,resulting in the discovery of a number of structurally intriguing sesquiterpenoid dimers and sesquiterpenoids [6, 7, 8]. In this study,we focused on the isolation of the sesquiterpene constituents from the chloroform extracts of A. vestita,leading to the isolation of three new sesquiterpenes (1-3) along with three known 11-epimeric lactones (4-6) (Fig. 1). Their planar structures and absolute stereochemistry were established by analysis of 1D NMR and 2D NMR,MS and single-crystal X-ray diffraction (with copper radiation).
|
Download:
|
| Fig. 1. Chemical structures of compounds 1-6. | |
In addition,an inhibition assay on lipopolysaccharide (LPS)- induced NO production was performed for the new compounds. The results showed that 1-3 exhibit a moderate inhibitory effect against NO production induced by LPS in BV-2 microglial cells. We report herein the isolation,structural elucidation,and biological evaluation of these compounds.
Structurally,1-3 are of considerable interest,as they all possess a 13β-methyl in the molecule. Although a large number of sesquiterpenoids have been discovered from the genus Artemisia, those possessing 13β-methyl are very rare,as the 13α-methyl eudesmane sesquiterpenes are the overwhelming majority in nature.
The aerial parts of A. vestita were collected in Yunnan Province in September 2010. The plant material was authenticated by one of the authors (P.-F. Tu). A voucher specimen (No. 20100905) is deposited at the herbarium of the Peking University Modern Research Center for Traditional Chinese Medicine.
The dried aerial parts (45 kg) of A. vestitawere extractedwith 95% aqueous ethanol (360 L × 3 h × 3) at room temperature. The concentrated extracts (3.8 kg) were suspended in H2O (15 L) and extracted with petroleum ether (PE) and chloroform successively. The CHCl3 extracts (450 g) were subjected to a silica gel column eluted with a step-wise gradient of PE/EtOAc (1:0,8:1,3:1,1:1,and 0:1,v/v) to produce 10 fractions (F1-F10),monitored by TLC analysis. F7 (23 g) was chromatographed on a column of silica gel, eluting successively with a gradient of n-hexane/acetone (10:1,9:2, 8:3,6:5,and 5:5,v/v),to give three subfractions (F7a-F7c). F7b (4.3 g) was chromatographed on a reversed-phase C18 silica gel column eluted with MeOH/H2O (30:70,50:50 and 70:30,v/v) to yield four subfractions (F7b1-F7b4). F7b2 (120 mg) was purified repeatedly by using semi-preparative HPLC (40% MeCN/H2O, Thermo Sientific BDS-C18 column,250 mm× 10mm,5 mm; flow rate: 2.5 mL/min) to afford 1 (5.4 mg,tR = 22 min). F7b3was purified repeatedly by using semi-preparative HPLC (35% MeCN/H2O, Thermo Sientific BDS-C18 column,250 mm× 10mm,5 mm; flow rate: 2.5 mL/min) to afford compound 2(6.2 mg,tR = 15 min). Finally,compound 3 (4.7 mg,tR = 13 min) was purified repeatedly by using semi-preparative HPLC (32% MeCN/H2O,Thermo Sientific BDS-C18 column,250 mm× 10mm,5 μm; flow rate: 2.5 mL/min) from F7b4.
BV-2 cells were obtained from Peking Union Medical College, Cell Bank (Beijing,China) and grown in DMEM medium supplemented with 10% (v/v) FBS,penicillin (100 U/mL),and streptomycin (100ug/mL) in a humidified incubator containing 95% air and 5% CO2 at 37 ℃. During the logarithmic growth phase,BV- 2 cells (5×104 cells/well) were seeded in 48-well plates with DMEM medium supplemented with 10% (v/v) FBS. After 24 h of incubation,the cells were treated with different concentrations of tested compounds,and stimulated for 24 h with 1 μg/mL of LPS (Escherichia coli 0111:B4,Sigma,MO,USA). A control group was treated with LPS only. Accumulation of NO in the culture media was determined using a commercial assay kit,according to the manufacturer’s instruction using sodium nitrite as a standard. The production of NO was determined by detecting cell culture supernatants for nitrite,a major stable derivative of NO,by the Griess reagent [9]. Briefly,cell culture supernatants (100 μL) were treated with 100 μL of the Griess reagent in a 48-well plate. After incubation for 10 min at room temperature,the optical density was measured at 540 nm using a microplate reader. Sodium nitrite was used as a standard in the assay. The IC50 values,defined as the sample concentration resulting in a 50% inhibition of NO production,were generated by GraphPad Prism 5 software (GraphPad Software,Inc.,San Diego,CA). Quercetin was used as a positive control with an IC50 value of 10.01 μmol/L. In addition, cells viability was also evaluated by the MTT assay [10].
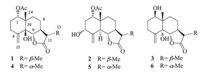
Artemivestinolide A (1) [11] was obtained as colorless crystal. The molecular formula of 1 was assigned as C17H24O5 by HRESIMS (m/z 331.1516 [M+Na]+,calcd. 331.1521) with six degrees of unsaturation. The IR spectrum of 1 showed the characteristic absorption bands for hydroxy groups (3478 cm-1),carbonyl groups (1766 and 1726 cm-1) and double bonds (1641 cm-1). The 1H NMR data of 1 (Table 1) exhibited the appearance of one secondary methyl at δH 1.20 (d, J = 7.0 Hz,H-13),two tertiary methyl groups at δH 1.12 (s,H-14) and 2.04 (s,H-17),two olefinic proton signals at dH 5.10 (br s,H2-15) and two oxymethine protons at δH 5.13 (dd,J = 4.5,12.0 Hz,H-1) and δH 4.60 (d,J = 12.5 Hz,H-6). The 1H NMRand 13C NMRspectra of 1 were compared with those of the known compound 4 [12] and showed a close similarity to those of 4,except for one methyl (C-13) shifting from δC 12.5 to δC 9.5 in the 13C NMR spectrum,suggesting a b configuration in compound 1 instead of an a in compound 4,which was supported by NOESY cross peaks of H-6/H3-13,H3-13/H-8b and H3-14/H-8β (Fig. 2).
| Table 1 1H NMR and 13C NMR data (500/125 MHz,CDCl3) for compounds 1-3,δ in ppm and J in Hz.a |
|
Download:
|
| Fig. 2. Key NOESY correlations of compounds 1-3. | |
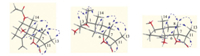
Fortunately,a single crystal of 1 was obtained from MeOH-H2O (10:1),and an X-ray diffraction experiment was performed with Cu Kα radiation,which unambiguously determined the b-orientation of the 13-methyl group (Fig. 3). Thus,the absolute stereochemistry (1S,5S,6S,7S,10S,11R) of 1 was determined.
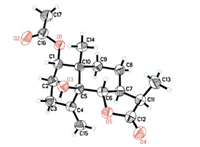
|
Download:
|
| Fig. 3. X-ray ORTEP drawing of 1 by Cu Kα radiation. | |
The molecular formula of compound 2 [13] was established as C17H24O5 using its HRESIMS data giving an m/z of 331.1526 for [M+Na]+. Analysis of its 1H NMR and 13C NMR,HSQC,HMBC,and 1H-1H COSY data (Table 1) showed that 2 has almost the same structure with the known compound 5 [14],except for the stereochemistry of C-11,NOESY correlations of H-6/H3-13,H3-13/ H-8β/H3-14 were observed,which strongly suggested a β-orientation (Fig. 2),indicating that C-13 is located above the rings,and this assignment was confirmed by the 13C NMR signal for this carbon at δC 9.60. The absolute stereochemistry of 2 was therefore determined as (1S,3R,5S,6S,7S,10R,11R).
Artemivestinolide C (3) [15] has a molecular formula of C15H22O4 determined by HRESIMS at m/z 289.1429 for [M+Na]+ (calcd 289.1416,C15H22O4Na). The overall appearance of 1H and 13C NMR spectra of 3 is highly similar to those of 6 [12]. A careful comparison of their 1H and 13C NMR data revealed that the only difference in the 13C NMR was a signal shifted from δC 12.5 to δC 9.50,implying the different configuration of the carbon 11 that the methyl (C-13) is attached to. The NOESY cross-peaks of H-6/H3-13, H-6/H3-14 unambiguously established that these protons were on the same face of the molecule,indicating a β-orientation of the C- 13 methyl group (Fig. 2). Thus,the absolute stereochemistry of 3 was determined as (1R,5S,6S,7S,10R,11R).
In the CD spectra,compounds 1 and 2 displayed negative cotton effects at around 200 nm caused by π-π* transition in double bond (C-4/C-5),and compound 3 showed positive cotton effect at about 200 nm. It is highly possible that the direction of cotton effects depend on the absolute configuration of C-5,a chiral center near the chromophore,for in compounds 1 and 2 both have a 5S configuration but C-5 is an R configuration in compound 3.
1H NMR,13C NMR and CD spectra of compounds 1-3 are supplied in Supporting information.
In addition,compounds 1-3 exhibited weak negative cotton effects at about 220 nm,possibly attributed to the absorptions of lactone rings,further confirmed the 7S configuration [16].
The isolated compounds were assayed for their inhibitory effects on the production of NO in LPS-stimulated BV-2 microglial cells using the Griess assay [9],with quercetin as a positive control (Table 2). Compounds 1-3 exhibited a moderate inhibition with IC50 values of 47.39 μmol/L,51.87 μmol/L and 65.59 μmol/L, respectively.
| Table 2 Inhibitory effects of compounds 1-3 against LPS-induced NO production in BV-2 microglial cells (n = 3,mean±SD). |
From the aerial parts of A. vestita,three new eudesmane sesquiterpenoids featuring a rare 13β-methyl,named artemivestinolides A-C (1-3),were isolated. The structure of artemivestinolide A (1) was confirmed by X-ray crystallographic analysis. The NO production and cell growth inhibitory activity were assayed using BV-2 microglial cells in vitro. Artemivestinolides A-C (1-3) were moderately active against NO production induced by LPS in BV-2 microglial cells.
This work was financially supported by the grants from the National Natural Science Foundation of China (No. 30973629) and National Key Technology R&D Program ‘‘New Drug Innovation’’ of China (Nos. 2012ZX09301002-002-002,2012ZX09304-005).
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2013.05.020.
| [1] | B.M. Fraga, Natural sesquiterpenoids, Nat. Prod. Rep. 27(2010) 1681-1708. |
| [2] | B.M. Fraga, Natural sesquiterpenoids, Nat. Prod. Rep. 28(2011) 1580-1610. |
| [3] | B.M. Fraga, Natural sesquiterpenoids, Nat. Prod. Rep. 29(2012) 1334-1366. |
| [4] | Z.J. Zhan, Y.M. Ying, L.F. Ma, et al., Natural disesquiterpenoids, Nat. Prod. Rep. 28(2011) 594-629. |
| [5] | Y.J. Bai, Y. Li, Y.P. Shi, Chemical constituents of Artemisia vestita, Chin. Pharm. J. 32(1997) 462-465. |
| [6] | J. Wen, H.M. Shi, P.F. Tu, Dimeric guaianolides and sesquiterpenoids from Artemisia anomala, J. Nat. Prod. 73(2010) 67-70. |
| [7] | K. Zan, X.Y. Chai, P.F. Tu, Artanomadimers A-F: six new dimeric guaianolides from Artemisia anomala, Tetrahedron 68(2012) 5060-5065. |
| [8] | S. Wang, J. Li, P.F. Tu, NO inhibitory guaianolide-derived terpenoids from Artemisia argyi, Fitoterapia 85(2013) 169-175. |
| [9] | L.C. Green, D.A. Wagner, J. Glogowski, et al., Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids, Anal. Biochem. 126(1982) 131-138. |
| [10] | J.M. Sargent, C.G. Taylor, Appraisal of the MTT assay as a rapid of chemosensitivity in acute myeloid leukaemia, Br. J. Cancer 60(1989) 206-210. |
| [11] | Artemivestinolide A(1): colorless blocks, mp 169-171℃; [α]D10-0.67(c 0.18, MeOH); UV(MeOH) λmax(logε): 202(0.47) nm; IR(KBr, cm-1): ν 3478, 2957, 2875, 1766, 1726, 1641, 1458, 1378,1261, 1190, 1041, 960, 799; CD λ(MeOH)(nm(Δε)) 200.4(-48.59), 220.8(-10.62); HR-ESI-MS:m/zobsd 331.1516 [M+Na]+(m/zcalcd [C17H24O5]+331.1521). Crystal data of artemivestinolide A(1): C17H24O5, M=308.36 g mol-1, colorless blocks, size 0.05 mm×0.07 mm×0.10 mm, orthorhombic, space group P21, a=7. 3325(15)Å, b=8.5129(17)Å, c=26.654(5)Å, β=90.0(2)°, V=1663.8(6)Å3, T=296(2) K, Z=4, d=1.2312 g cm-3, μ(Cu-Ka)=1.54178Å, completeness θmax=98.6%, 7924 independent reflections, Rint=0.0559, 2932 reflections with [F]2≥2σ[F]2, 0 restraint, R1obs=0.0559, wR2obs=0.1785, S=0.974, GOF=1.050. Absolute structure parameter 0.3(5). The crystal structure of 1 was solved by direct method using the program SHELXS-97(G. M. Sheldrick, SHELXS 97 and SHELXL97, University of Gottingen, Germany, 1997) and subsequent Fourier difference techniques, and refined anisotropically by full matrix least-squares on F2 using SHELXL-97(G. M. Sheldrick, SHELXTL, Version 6.10, Bruker AXS Inc., Madison, Wisconsin, USA, 2000). Crystallographic data for the structure of 1 with copper radiation has been deposited in the Cambridge Crystallographic Data Centre(deposition number: CCDC 929724). |
| [12] | J.A. Marco, J.F. Sanz-Cervera, J.M. Pareja, et al., Sesquiterpene lactones from north african Artemisia species, Phytochemistry 37(1994) 477-485. |
| [13] | Artemivestinolide B(2): white amorphous powder; [α]D10+70.36(c 0.21, MeOH); UV(MeOH) λmax(logε): 203(1.31) nm; IR(KBr, cm-1): ν 3458, 2930, 2865, 1778, 1707, 1659, 1463, 1384,1282, 1209, 1075, 974, 845; CD λ(MeOH)(nm(Δε)) 200.4(-48.59), 220.8(-10.62); HR-ESI-MS: m/zobsd 331.1526 [M+Na]+(m/zcalcd |
| [14] | N. Mengi, S.C. Taneja, V.P. Mahajan, et al., Eudesmanolides from Senecio chrysanthemoides, Phytochemistry 30(1991) 2329-2330. |
| [15] | Artemivestinolide C(3): Colorless gum; αD10+113.64(c 0.26, MeOH); UV(MeOH) λmax(log ε): 206(1.86) nm; IR(KBr, cm-1): ν 3425, 2933, 2864, 1764, 1707, 1651, 1455, 1386,1216, 1202, 1058, 920, 810; CD λ(MeOH)(nm(Δε)) 193.6(+32.80), 214.6( 0.80); HR-ESI-MS: m/zobsd 289.1429 [M+Na]+(m/zcalcd [C17H24O5]+289.1416). |
| [16] | W. Stocklin, T.G. Waddell, T.A. Geissman, Circular dichroism and optical rotatory dispersion of sesquiterpene lactones, Tetrahedron 26(1970) 2397-2410. |