2. 华中科技大学同济医学院公共卫生学院, 武汉 430030
2. School of Public Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, China
花生四烯酸(arachidonic acid,AA)是全顺式5,8,11,14-二十碳四烯酸,属于不饱和脂肪酸,它是一种在生物体内含量较高,分布最广的多不饱和必需脂肪酸。在花生四烯酸级联中,AA在生物体内主要有3条代谢途径:通过环氧合酶(cyclooxygenase,COXs),脂氧合酶(lipoxygenase,LOX)或细胞色素P-450(CYP-450)产生前列腺素(prostaglandin,PG)、血栓素(thromboxane,TX)、白三烯(leukotriene,LT)或羟基脂肪酸(hydroxyfatty acid,OHFA)[1](图 1)。其中,COXs分为3种形式:COX-1(组成型),COX-2(有丝分裂原诱导形式)和COX-3(COX-1的变体)[2]。AA通过磷脂酶A2s从膜磷脂中释放出来,已被证明参与细胞损伤,炎症和凋亡相关的病理生理事件[3-7]。因此,精确的定性、定量分析在AA相关疾病的发展机制、诊断和预测中具有重要意义。
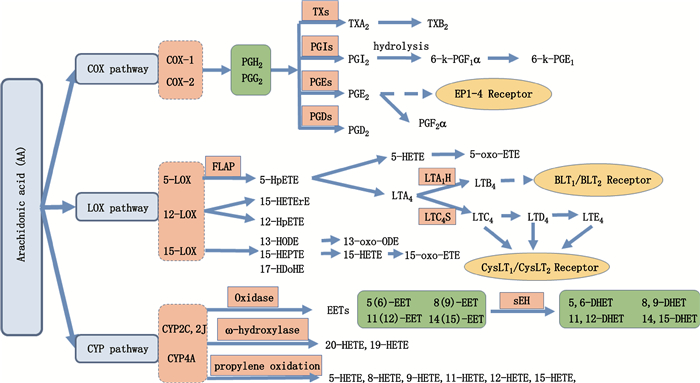
|
AA.花生四烯酸(arachidonic acid)COX.环氧酶(cyclooxygenase)LOX.脂氧酶(lipoxygenase)FLAP.花生四烯酸5-脂氧合酶激活蛋白(arachidonate 5-lipoxygenase-activating protein)oxidase.表氧化酶ω-hydroxlase.ω-羟化酶propylene oxidation:丙烯氧化TXs.血栓素A2合成酶(thromboxane A2 synthase)PGIs.前列腺素I2合成酶(prostaglandin I2 sythase)PGEs.前列腺素E2合成酶(prostaglandin E2 sythase)PGDs.前列腺素D2合成酶(prostaglandin D2 sythase)LTA1H.白三烯A4水解酶(leukotriene A4 hydrolase)LTC4s.白三烯C4合酶(leukotriene C4 sythase)sEH.环氧化物水解酶(souble epoxide hydrolase)PG.前列腺素(prostaglandin)LT.白三烯(leukotriene)TX.血栓素(thromboxane)HpETE.过氧二十碳四烯酸(hydro-peroxy-eicosatetraenoic acid)HETrE.羟基二十碳三烯酸(hydro-peroxy-eicosatetraenoic acid)HODE.羟基十八碳二烯酸(hydroxy-octadecadienoic aci)HDoHE.羟基二十二碳六烯酸(hydroxy-docosahexaenoic acid)oxo-ODE.氧代十八碳二烯酸(oxo-octadecadienoic acid)HETE.羟基二十碳四烯酸(hydroxy-eicosatetraenoic acid)oxo-ETE.氧代二十碳(oxo-eicosatetraenoic acid)EET.环氧二十碳四烯酸(epoxy-eicosatetraenoic acid)DHET.二羟基二十碳四烯酸(dihydroxy-eicosatetraenoic acid) 图 1 AA在生物体内的主要代谢途径[1] Fig.1 The main metabolic pathways of arachidonic acid in living organisms |
目前,生物样品中花生四烯酸及其代谢产物的分析方法主要有气相色谱-质谱(GC-MS)、液相色谱-质谱(LC-MS)、液相色谱-串联质谱(LC-MS /MS)、超高效液相色谱-串联质谱(UPLC-MS /MS)、液相色谱-电喷雾质谱(LC-ESI /MS)、液相色谱-核磁共振(LC-NMR)、毛细管电泳-质谱(CE-MS)和酶联免疫吸附分析(ELISA)等[8]。GC-MS分析前一般需要比较复杂的预处理步骤,包括样品纯化和衍生化,且该法不适合分析热不稳定的代谢物,如环氧二十碳四烯酸(EETs)。酶联免疫吸附分析(ELISA)是最广泛使用的技术,其侧重于终端代谢组学(PGE2,LTE4等),但该方法操作太复杂并且缺乏良好的重现性。LC-MS/MS作为代谢组学的主要技术手段已成为生物学样品中测量小分子代谢物(相对分子质量小于1 500)的有效手段[9-13],因此该分析技术在AA检测中的发展与应用一直是热门研究领域[14-15]。LC-MS分析策略对样品前处理方法并无过高要求,生物样品经蛋白沉淀、液液萃取或固相萃取纯化富集后,可直接用于LC-MS检测分析。LC-MS/MS是代谢组学中用于定量分析内源性化合物的主要技术手段,具有分析时间短,完全自动化,最少的样品制备(节点化)等优点,并且适用于相对分子质量和极性范围广泛的内源性分析物[16]。它已被广泛应用于AA代谢产物及其结构类似物[17-21]、类固醇等成分的定性和定量分析[22-24]。近年来,已有众多的该类分析方法研究成果发表,包括使用LC-MS/MS同时测定血液中23种类花生酸成分[25],同时定性和定量分析血液中AA和其32种相关代谢物[17],利用UPLC-MS/MS技术同时测定人全血中122种类花生酸衍生物[26]。最新的研究数据显示,已有专属性分析方法能够在5 min内定性和定量检测20 μL人全血中的184种AA代谢产物[27]。本文将从AA在生物体内的3个主要代谢途径的角度,对液质联用技术在AA代谢产物检测中的应用分别展开综述。
1 前列腺素代谢产物(PGs)AA的COXs代谢产物PGs是由COXs介导的、由AA转化而产生的重要的炎性介质,也是已知调节生理功能包括免疫和炎症的重要脂质介质[28-29]。PGs能增大毛细血管的通透性、增强其他炎症引起的水肿以及增强组胺、缓激肽类物质引起的疼痛和肿胀等。许多研究表明,患有炎症或已有炎症反应的机体生物样本中均可分离出PGs,而且含量远远高于正常组织,同时炎症刺激又促使AA从膜磷脂中释放出来,再通过LOX或COXs途径代谢生成炎症介质。因此,对PGs的生成和作用的抑制就可以有效控制炎症的进一步发展。PGs中的代表性物质前列腺素I2或前列环素是由血管内皮细胞产生的AA的主要代谢物[30],是具有血管舒张、血小板抑制和抗增殖特性的关键天然短效血管活性物质[31-32]。肺动脉高压患者前列环素水平降低的发现提示,它可能在肺动脉高压病理生理学中发挥重要作用,包括血管收缩,血管增生,血管壁重塑和原位血栓形成[33-34]。8-异前列腺素F2α(8-iso-PGF2α)是脂质过氧化的生物标志物,与各种氧化应激有关[35-39]。在血浆中,该化合物质量浓度在40~100 pg·mL-1范围内[40],而正常人尿中该成分质量浓度介于180~500 pg·mg-1或更高之间变化[41-43]。
HPLC方法最早应用于测定人血浆中的6-酮前列腺素F1α [44-45],然而HPLC早期仅能测定和分离前列腺素类物质(如PGE1)的初级代谢产物[46]。早期有报道使用电子碰撞电离的气相色谱-质谱测定法测定9α,11β-前列腺素F2(9α,11β-PGF2)[47]以及测定人尿中的2,3-双去甲基-6-酮前列腺素F1α [48]、PGE2和PGD2等[49]。随后Chappella等[45]建立了基于HPLC-MS的测定方法,能够检测2~3 pg·mL-1范围内的6-酮前列腺素F1α的内源性水平。虽然酶联免疫测定法[50-52]或放射免疫测定法[53-54]可用于测定人体血浆和尿液中的6-酮前列腺素F1α水平,但有相关报道发现,该类方法中的非酯化脂肪酸会严重干扰免疫测定,导致测定缺乏选择性[55-56]。同时,Enzler等[57]开发并验证了一种用于定量人血浆中前列环素稳定水解产物6-酮前列腺素F1α及其代谢物2,3-双去甲基-6酮前列腺素F1α和6,15-二酮-13,14二氢前列腺素F1α的LC-MS/MS方法。Dahl等[58]建立了10 min内快速、灵敏、准确定量分析8-iso-PGF2α的LC-MS/MS法,相对于以前的方法进行了优化,并测定了掺入尿液中的50 pg·mL-1至10 ng·mL-1的8-iso-PGF2α的线性曲线(r2 > 0.99)以及日间精密度和日内精密度。
目前,基于LC-MS/MS技术定性或定量分析的PGs成分化合物结构见图 2。
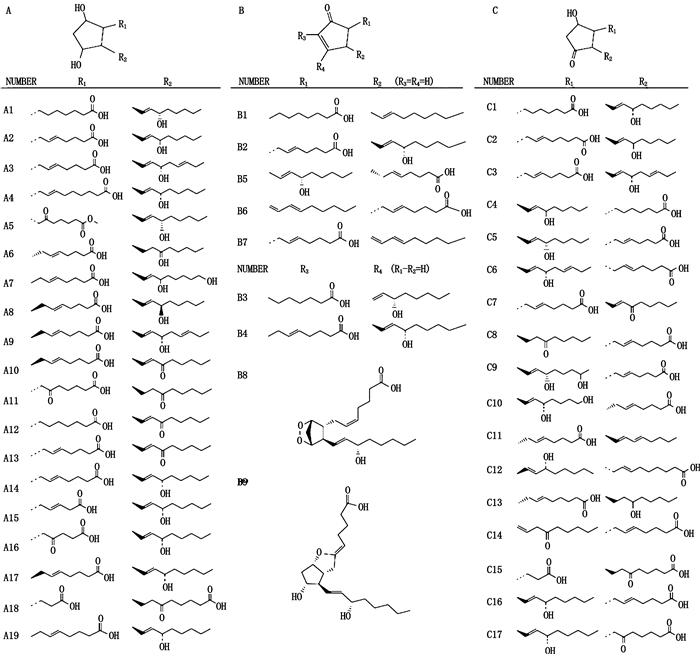
|
图 2 基于LC-MS/MS检测技术所定性或定量分析的PGs成分的化合物结构 Fig.2 The structures of prostaglandins based on qualitative or quantitative analysis by LC-MS/MS detection technique |
LTs是一类生物活性化合物,结构上含有3个共轭双键,属于脂质化剂家族,在超敏反应、炎症和增殖相关疾病过程中具有主导地位[59-63],尤其在过敏性哮喘疾病的发生和发展病程中突显重要[64]。细胞膜磷脂被磷脂酶A2水解后释放AA,随后与脂氧合酶5-LOX结合,氧化为白三烯A4(LTA4),不稳定LTA4形成后分2条路线代谢:LTA4可以通过白三烯C4合成酶进一步代谢合成白三烯C4(LTC4),在相关酶的作用下,生成白三烯E4(LTE4),并在γ-谷氨酰转肽酶作用下与谷氨酸结合生成白三烯F4(LTF4);LTA4也可以经由另一条通路被白三烯LTA4水解酶水解生成白三烯B4(LTB4),LTB4是强有力的炎症细胞趋化因子[65],可以预防广泛的炎症反应[63, 66-68]。
1995年,Yu等[69]使用HPLC测定人血浆中的LTB4、LTC4等物质,同年也有报道使用GC-MS测定人血浆中的LTB4[70]。随后Mizugaki等[71]建立了一种固相萃取结合LC-MS/MS的分析方法,测定了健康人尿液中LTE4水平,作者在样品中使用标记物LTE4-d3测定出了不同性别年龄受试者尿液中的LTE4含量。Perestrelo等[64]利用UHPLC技术建立了一种方便、快速、高灵敏度的分析方法,用于测定人尿液中LTB4含量。随后,Chappell等[72]使用UHPLC-MS/MS技术测定了健康人和哮喘患者痰液中的LTC4、LTD4、LTE4、LTB4的绝对含量,开发了能够以足够的灵敏度和选择性同时测量痰液中的半胱氨酰白三烯和LTB4的质谱分析法。结论得出LTE4是衡量痰中总半胱氨酰白三烯的良好替代标志物[72]。Lin等[73]开发验证了一种利用UFLC-MS/MS技术测定人血浆样本中白三烯B4的含量的方法,对分析方法的定量下限、检测下限、选择性、灵敏度、回收率和线性关系进行了考察,并将酶联免疫吸附法和该方法进行比较,结果发现研究建立的UFLC-MS/MS分析方法在选择性和动态范围上均优于酶联免疫吸附法。
目前,基于LC-MS/MS检测技术所定性或定量分析的LTs成分的化合物结构如图 3(D1-D18)所示。
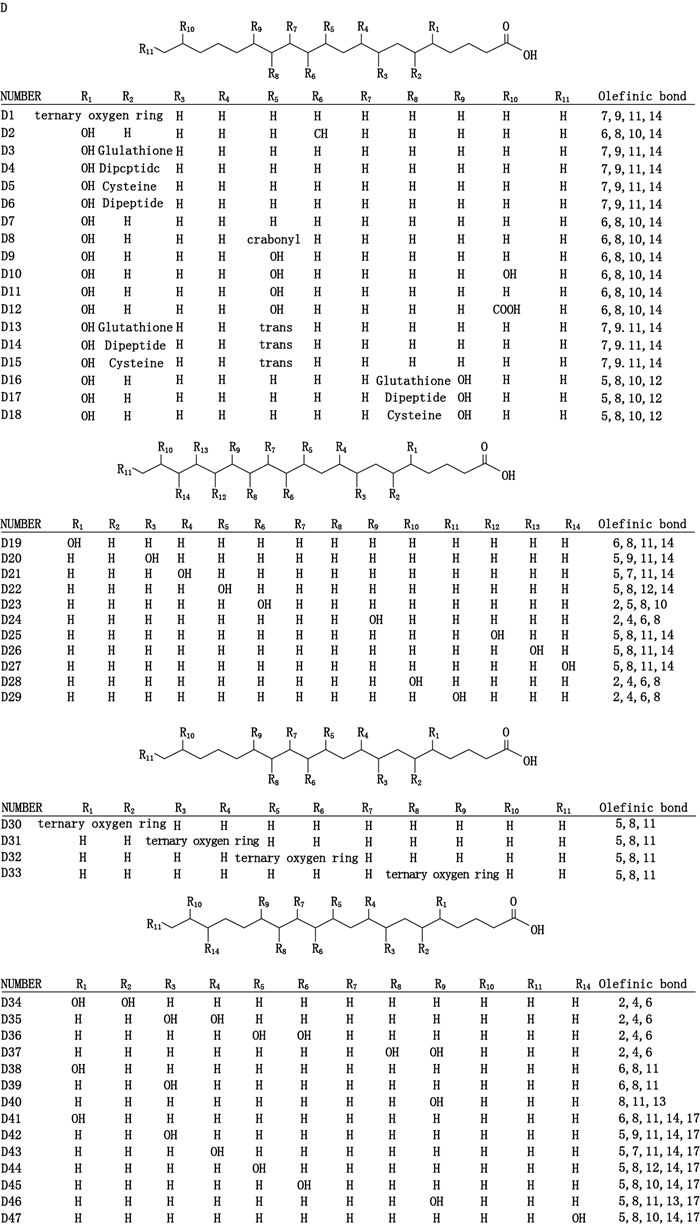
|
图 3 基于LC-MS/MS检测技术所定性或定量分析的LTs成分的化合物结构 Fig.3 The structures of leukotrienes based on qualitative or quantitative analysis by LC-MS/MS detection technique |
在人体内,AA经过过氧化物酶还原生成PGH2,而PGH2经过血栓烷合成酶生成TXs类物质。TXs由血小板产生,具有血小板凝聚及血管收缩作用。与PGs一样,TXs包括多种不同结构功能的化合物(血栓烷酸衍生物),如TXA2、TXA3、TXB1、TXB2、TXB3等。研究表明,冠心病合并糖尿病患者中,血浆11-dh-TXB2水平升高者病情重,预后差[74]。血清TXs含量还与镰状细胞病有关,Obilade等[75]用酶联免疫吸附测定法测量了受试者血清中TXA2的稳定代谢物,发现怀孕的镰状细胞病妇女血清TXB2显著异常升高。
早期Weber等[49]使用GC-MS测定了人尿液中的TXB2等物质含量Yasumoto等[76]建立了一种人血清中测定16种同位素标记物和TXB2等27种物质的LC-MS/MS方法,该方法可以在血清中测定的重现性良好,并且可以应用于临床。
目前,基于LC-MS/MS检测技术所定性或定量分析的TXs成分的化合物结构如图 4所示。
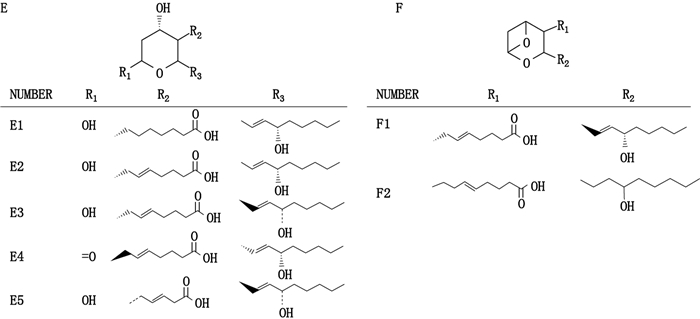
|
图 4 基于LC-MS/MS检测技术所定性或定量分析的TXs成分的化合物结构 Fig.4 The structures of thromboxanes based on qualitative or quantitative analysis by LC-MS/MS detection technique |
AA通过CYP450酶途径还可以产生OHFAs代谢产物:环氧二十碳三烯酸(EET)、羟基二十碳四烯酸(20-HETE)、二十碳四烯酸(ETE)、二羟基二十碳四烯酸(DHET)等。CYP450途径包含以下反应:双烯丙基氧化(产生5-,8-,9-,11-,12-和15-HETE),ω/ω-1羟基化(产生16-,17-,18-,19-和20-HETEs)和烯烃环氧化(产生5,6-,8,9-,11,12-和14,15-EET)[77]。据报道,HETEs,EETs和DHETs与许多疾病相关,包括炎症类疾病[78]、动脉粥样硬化[78]、癌症[79]、糖尿病[80]、肥胖[81]、脑损伤[82]和哮喘[83]。研究表明,EETs可通过影响血管生理功能来缓解肝硬化门静脉高压症的疾病发展[84],其中11,12-EET还具有拮抗内皮细胞缺氧/复氧损伤作用[85],血浆EET水平降低还与急性冠状动脉综合征发病与否相关,血浆EET水平可作为该病发生的独立预测因素[86]。有报道显示,20-HETE能够促进肾癌、前列腺癌等癌症细胞的增殖[87]。12-HETE被证实在癌症的粘附、侵袭和转移中发挥重要作用[88],同时在结核病发病机理中具有重要地位[89]。这些研究报道表明,HETEs,EETs和DHETs在各类疾病的发病机制中具有重要临床意义,可作为潜在的诊断和治疗干预生物标志物[90-91]。
Zhu等[92]开发了一种高灵敏度的基于稳定同位素探针标记的UHPLC-MS/MS分析方法,用于定性和定量分析2型糖尿病患者、白血病患者以及健康人的血清样品脂质提取物中的HETEs,EETs和DHETs。数据表明,与健康对照组相比,2型糖尿病患者样品中11个OHFAs成分在白血病患者中有显著的变化,进一步阐明OHFAs代谢物在疾病发生和形成中的生物化学机制和功能作用[92]。
目前,基于LC-MS/MS检测技术所定性或定量分析的HETEs(D19-D29)、EETs(D30-D33)、DHET(D34-D47)类等成分化学结构如图 3(D19-D47)所示。
综上所述,AA在体内参与了各种各样的代谢反应,与各种生理、病理现象密切相关,因此基于LC-MS/MS技术应用于AA代谢产物的定性和定量分析研究,对于临床疾病的诊断、治疗、病情发展监测及预后观察等具有重要的现实意义。本文围绕近年来LC-MS/MS技术在AA代谢产物检测中的应用,共整理出99个AA代谢产物。随着现代LC-MS/MS仪器设备的更新发展,势必能够发现和鉴定更多新的AA代谢产物,同时基于LC-MS/MS技术的AA代谢产物特征轮廓谱将会逐渐完善,为该类化合物的定性和定量分析提供可靠的技术支撑。
| [1] |
YAGAMI T, YAMAMOTO Y, KOMA H. Physiological and pathological Roles of 15-deoxy-delta12, 14-prostaglandin J2 in the central nervous system and neurological diseases[J]. Mol Neurobiol, 2018, 55(3): 2227. DOI:10.1007/s12035-017-0435-4 |
| [2] |
YAGAMI T, KOMA H, YAMAMOTO Y. Pathophysiological roles of cyclooxygenases and prostaglandins in the central nervous system[J]. Mol Neurobiol, 2016, 53(7): 4754. DOI:10.1007/s12035-015-9355-3 |
| [3] |
YAGAMI T, UEDA K, ASAKURA K, et al. Human group ⅡA secretory phospholipase A2 induces neuronal cell death via apoptosis[J]. J Neurochem, 2002, 61(1): 114. |
| [4] |
PHILLIS JW, O'REGAN MH. The role of phospholipases, cyclooxygenases, and lipoxygenases in cerebral ischemic/traumatic injuries[J]. Critic Rev Neurobiol, 2003, 15(1): 61. DOI:10.1615/CritRevNeurobiol.v15.i1 |
| [5] |
YAGAMI T, UEDA K, ASAKURA K, et al. Group ⅠB secretory phospholipase A2 induces cell death in the cultured cortical neurons:a possible involvement of its binding sites[J]. Brain Res, 2002, 949(1-2): 197. DOI:10.1016/S0006-8993(02)03144-X |
| [6] |
YAGAMI T, UEDA K, ASAKURA K, et al. Group ⅠB secretory phospholipase A2 induces neuronal cell death via apoptosis[J]. J Neurochem, 2002, 81(3): 449. DOI:10.1046/j.1471-4159.2002.00800.x |
| [7] |
YAGAMI T. Cerebral arachidonate cascade in dementia:Alzheimer's disease and vascular dementia[J]. Curr Neuropharmacol, 2006, 4(1): 87. DOI:10.2174/157015906775203011 |
| [8] |
金力超, 范玉明, 侯晓蓉, 等. 色谱联用技术在药物分析中的应用特点和新趋势[J]. 药物分析杂志, 2015, 35(9): 1520. JIN LC, FAN YM, HOU XR, et al. Application and tendency of chromatographic hyphenated techniques in pharmaceutical analysis[J]. Chin J Pharm Anal, 2015, 35(9): 1520. |
| [9] |
BECKER S, KORTZ L, HELMSCHRODT C, et al. LC-MS-based metabolomics in the clinical laboratory[J]. J Chromatogr B Anal Technol Biomed Life Sci, 2012, 883-884(1): 68. |
| [10] |
METZ TO, ZHANG Q, PAGE J S, et al. The future of liquid chromatography-mass spectrometry (LC-MS) in metabolic profiling and metabolomic studies for biomarker discovery[J]. Biomark Med, 2007, 1(1): 159. DOI:10.2217/17520363.1.1.159 |
| [11] |
ROUX A, LISON D, JUNOT C, et al. Applications of liquid chromatography coupled to mass spectrometry-based metabolomics in clinical chemistry and toxicology:A review[J]. Clin Biochem, 2011, 44(1): 119. DOI:10.1016/j.clinbiochem.2010.08.016 |
| [12] |
ZHANG A, SUN H, WANG P, et al. Modern analytical techniques in metabolomics analysis[J]. Analyst, 2012, 137(2): 293. DOI:10.1039/C1AN15605E |
| [13] |
ZHOU B, XIAO J F, TULI L, et al. LC-MS-based metabolomics[J]. Molecular Biosystems, 2012, 8(2): 470. DOI:10.1039/C1MB05350G |
| [14] |
HARKEWICZ R, FAHY E, ANDREYEV A, et al. Arachidonate-derived dihomoprostaglandin production observed in endotoxin-stimulated macrophage-like cells[J]. J Biol Chem, 2007, 282(5): 2899. DOI:10.1074/jbc.M610067200 |
| [15] |
YANG R, CHIANG N, OH SF, et al. Metabolomics-lipidomics of eicosanoids and docosanoids generated by phagocytes[J]. Curr Protoc Immunol, 2011, Chapter 14: Unit 14.26. |
| [16] |
GACHET MS, RHYN P, BOSCH OG, et al. A quantitiative LC-MS/MS method for the measurement of arachidonic acid, prostanoids, endocannabinoids, N-acylethanolamines and steroids in human plasma[J]. J Chromatogr B Analyt Technol Biomed Life Sci, 2015, 976-977: 6. DOI:10.1016/j.jchromb.2014.11.001 |
| [17] |
SHINDE DD, KIM KB, OH KS, et al. LC-MS/MS for the simultaneous analysis of arachidonic acid and 32 related metabolites in human plasma:basal plasma concentrations and aspirin-induced changes of eicosanoids[J]. J Chromatogr B Analyt Technol Biomed Life Sci, 2012, 911(5): 113. |
| [18] |
THOMAS A, HOPFGARTNER G, GIROUD C, et al. Quantitative and qualitative profiling of endocannabinoids in human plasma using a triple quadrupole linear ion trap mass spectrometer with liquid chromatography[J]. Rapid Commun Mass Spectrom, 2009, 23(5): 629. DOI:10.1002/rcm.v23:5 |
| [19] |
OZALP A, BARROSO B. Simultaneous quantitative analysis of N-acylethanolamides in clinical samples[J]. Anal Biochem, 2009, 395(1): 68. |
| [20] |
BALVERS MG, VERHOECKX KC, WITKAMP RF. Development and validation of a quantitative method for the determination of 12 endocannabinoids and related compounds in human plasma using liquid chromatography-tandem mass spectrometry[J]. J Chromatogr B, 2009, 877(14-15): 1583. DOI:10.1016/j.jchromb.2009.04.010 |
| [21] |
LAM PM, MARCZYLO TH, ELTALATINI M, et al. Ultra performance liquid chromatography tandem mass spectrometry method for the measurement of anandamide in human plasma[J]. Anal Biochem, 2008, 380(2): 195. |
| [22] |
TURPEINEN U, HäMäLäINEN E, STENMAN UH. Determination of aldosterone in serum by liquid chromatography-tandem mass spectrometry[J]. J Chromatogr B Analyt Technol Biomed Life Sci, 2008, 862(1-2): 113. DOI:10.1016/j.jchromb.2007.11.005 |
| [23] |
KULLE AE, WELZEL M, HOLTERHUS PM, et al. Implementation of a liquid chromatography tandem mass spectrometry assay for eight adrenal C-21 steroids and pediatric reference data[J]. Hormone Res Pediatr, 2013, 79(1): 22. DOI:10.1159/000346406 |
| [24] |
TAYLOR PJ, COOPER DP, GORDON RD, et al. Measurement of aldosterone in human plasma by semiautomated HPLC-tandem mass spectrometry[J]. Clin Chem, 2009, 55(6): 1155. |
| [25] |
BLEWETT AJ, VARMA D, GILLES T, et al. Development and validation of a high-performance liquid chromatography-electrospray mass spectrometry method for the simultaneous determination of 23 eicosanoids[J]. J Pharm Biomed Anal, 2008, 46(4): 653. DOI:10.1016/j.jpba.2007.11.047 |
| [26] |
SONG J, LIU X, WU J, et al. A highly efficient, high-throughput lipidomics platform for the quantitative detection of eicosanoids in human whole blood[J]. Anal Biochem, 2013, 433(2): 181. |
| [27] |
WANG Y, ARMANDO AM, QUEHENBERGER O, et al. Comprehensive ultra-performance liquid chromatographic separation and mass spectrometric analysis of eicosanoid metabolites in human samples[J]. J Chromatogr A, 2014, 1359: 60. DOI:10.1016/j.chroma.2014.07.006 |
| [28] |
HARRIS SG, PADILLA J, KOUMAS L, et al. Prostaglandins as modulators of immunity[J]. Trends Immunol, 2002, 23(3): 144. DOI:10.1016/S1471-4906(01)02154-8 |
| [29] |
RICCIOTTI E. Prostaglandins and inflammation[J]. Arterioscler Thromb Vasc Biol, 2011, 31: 986. DOI:10.1161/ATVBAHA.110.207449 |
| [30] |
VANE J, CORIN RE. Prostacyclin:a vascular mediator[J]. Eur J Vascul Endovasc Surg Off J Eur Soc Vascul Surg, 2003, 26(6): 571. DOI:10.1016/S1078-5884(03)00385-X |
| [31] |
WU KK, LIOU J Y. Cellular and molecular biology of prostacyclin synthase[J]. Biochem Biophys Res Commun, 2005, 338(1): 45. DOI:10.1016/j.bbrc.2005.08.021 |
| [32] |
GOMBERGMAITLAND M, OLSCHEWSKI H. Prostacyclin therapies for the treatment of pulmonary arterial hypertension[J]. Eur Respir J, 2008, 31(4): 891. DOI:10.1183/09031936.00097107 |
| [33] |
FARBER HW, LOSCALZO J. Pulmonary arterial Hypertension[J]. New England J Med, 2004, 351(16): 1655. DOI:10.1056/NEJMra035488 |
| [34] |
SCHANNWELL CM, STEINER S, STRAUER BE. Diagnostics in pulmonary hypertension[J]. J Physiol Pharmacol, 2007, 58(Suppl 5): 591. |
| [35] |
LAIGHT DW, DESAI KM, GOPAUL NK, et al. F2-isoprostane evidence of oxidant stress in the insulin resistant, obese Zucker rat:effects of vitamin E[J]. Eur J Pharmacol, 1999, 377(1): 89. DOI:10.1016/S0014-2999(99)00407-0 |
| [36] |
GRECO A, MINGHETTI L, LEVI G. Isoprostanes, novel markers of oxidative injury, help understanding the pathogenesis of neurodegenerative diseases[J]. Neurochem Res, 2000, 25(9-10): 1357. |
| [37] |
DEVARAJ S, HIRANY SV, BURK RF, et al. Divergence between LDL oxidative susceptibility and urinary F2-isoprostanes as measures of oxidative stress in type 2 diabetes[J]. Clin Chem, 2001, 47(11): 1974. |
| [38] |
PRATICò D, MY LV, TROJANOWSKI JQ, et al. Increased F2-isoprostanes in Alzheimer's disease:evidence for enhanced lipid peroxidation in vivo[J]. Faseb J, 1998, 12(15): 1777. DOI:10.1096/fasebj.12.15.1777 |
| [39] |
BASU S, HELMERSSON J. Factors regulating isoprostane formation in vivo[J]. Antioxid Redox Sig, 2005, 7(1-2): 221. DOI:10.1089/ars.2005.7.221 |
| [40] |
WANG Z, CIABATTONI G, CRMINON C, et al. Immunological characterization of urinary 8-epi-prostaglandin F2 alpha excretion in man[J]. J Pharmacol Exp Ther, 1995, 275(1): 94. |
| [41] |
MORROW JD, HARRIS TM, ND RL. Noncyclooxygenase oxidative formation of a series of novel prostaglandins:analytical ramifications for measurement of eicosanoids[J]. Anal Biochem, 1990, 184(1): 1. |
| [42] |
ROBERTS LJ, MOORE KP, ZACKERT WE, et al. Identification of the major urinary metabolite of the F2-isoprostane 8-iso-prostaglandin F2alpha in humans[J]. J Biol Chem, 1996, 271(34): 20617. DOI:10.1074/jbc.271.34.20617 |
| [43] |
TAYLOR AW, BRUNO RS, TRABER MG. Women and smokers have elevated urinary F2-isoprostane metabolites:a novel extraction and LC-MS methodology[J]. Lipids, 2008, 43(10): 925. DOI:10.1007/s11745-008-3222-1 |
| [44] |
ENGELS W, KAMPS MA, LEMMENS PJ, et al. Determination of prostaglandins and thromboxane in whole blood by high-performance liquid chromatography with fluorimetric detection[J]. J Chromatogr, 1988, 427(2): 209. |
| [45] |
CHAPPELL DL, XIAO X, RADZISZEWSKI W, et al. Development and validation of a LC/MS/MS method for 6-keto PGF1α, a metabolite of prostacyclin (PGI2)[J]. J Pharm Biomedical Anal, 2011, 56(3): 600. DOI:10.1016/j.jpba.2011.06.019 |
| [46] |
HESSE WH, SCHWEER H, SEYBERTH HW, et al. Separation and determination of prostaglandin E1 metabolites by high-performance liquid chromatography[J]. J Chromatogr, 1990, 533: 159. DOI:10.1016/S0378-4347(00)82197-0 |
| [47] |
OBATA T, NAGAKURA T, KAMMURI M, et al. Determination of 9 alpha, 11 beta-prostaglandin F2 in human urine and plasma by gas chromatography-mass spectrometry[J]. J Chromatogr B Biomed Appl, 1994, 655(2): 173. DOI:10.1016/0378-4347(94)00100-6 |
| [48] |
DANIEL VC, MINTON TA, BROWN NJ, et al. Simplified assay for the quantification of 2, 3-dinor-6-keto-prostaglandin F1 alpha by gas chromatography-mass spectrometry[J]. J Chromatogr B Biomed Appl, 1994, 653(2): 117. DOI:10.1016/0378-4347(93)E0432-P |
| [49] |
WEBER C, HOLLER M, BEETENS J, et al. Determination of 6-keto-PGF1 alpha, 2, 3-dinor-6-keto-PGF1 alpha, thromboxane B2, 2, 3-dinor-thromboxane B2, PGE2, PGD2 and PGF2 alpha in human urine by gas chromatography-negative ion chemical ionization mass spectrometry[J]. J Chromatogr, 1991, 562(1-2): 599. DOI:10.1016/0378-4347(91)80611-F |
| [50] |
DEMERS LM, DERCK DD. A radioimmunoassay for 6-keto-prostaglandin F1 alpha[J]. Adv Prostagl Thromb Res, 1980, 6(6): 193. |
| [51] |
MITCHELL MD. A sensitive radioimmunoassay for 6-keto-prostaglandin F1 alpha:preliminary observations on circulating concentrations[J]. Prostaglandins Med, 1978, 1(1): 13. DOI:10.1016/0161-4630(78)90072-1 |
| [52] |
BAXENDALE PM, MARTIN RC, HUGHES KT, et al. Development of scintillation proximity assays for prostaglandins and related compounds[J]. Adv Prostagl Thromb Leukotriene Res, 1991, 21A(1): 303. |
| [53] |
TONAI T, YOKOTA K, YANO T, et al. Enzyme immunoassay of 6-ketoprostaglandin F1alpha in a solid phase[J]. Biochem Biophy Acta (BBA)-Lipids Lipid Metabol, 1985, 836(3): 335. DOI:10.1016/0005-2760(85)90137-7 |
| [54] |
PRADELLES P, GRASSI J, MACLOUF J. Enzyme immunoassays of eicosanoids using acetylcholine esterase as label:an alternative to radioimmunoassay[J]. Anal Chem, 1985, 57(7): 1170. DOI:10.1021/ac00284a003 |
| [55] |
GOLD EW, EDGAR PR. The effect of physiological levels of non-esterified fatty acids on the radioimmunoassay of prostaglandins[J]. Prostaglandins, 1978, 16(6): 945. DOI:10.1016/0090-6980(78)90110-7 |
| [56] |
HAMBERG M, SVENSSON J, SAMUELSSON B. Thromboxanes:a new group of biologically active compounds derived from prostaglandin endoperoxides[J]. Nutri Rev, 2010, 44(6): 208. |
| [57] |
ENZLER M, SCHIPP S, NICOLAS LB, et al. Determination of 6-keto prostaglandin F1alpha and its metabolites in human plasma by LC-MS/MS[J]. J Chromatogr B Analyt Technol Biomed Life Sci, 2012, 901: 67. DOI:10.1016/j.jchromb.2012.05.044 |
| [58] |
DAHL JH, van BREEMEN RB. Rapid quantitative analysis of 8-iso-prostaglandin-F2alpha using liquid chromatography-tandem mass spectrometry and comparison with an enzyme immunoassay method[J]. Anal Biochem, 2010, 404(2): 211. |
| [59] |
PANCHAUD A, AVOIS L, ROULET M, et al. A validated liquid chromatography-mass spectrometry method for the determination of leukotrienes B4 and B5 produced by stimulated human polymorphonuclear leukocytes[J]. Anal Biochem, 2005, 341(1): 58. |
| [60] |
FORD-HUTCHINSON AW, GRESSER M, YOUNG RN. 5-lipoxygenase[J]. Annual Rev Biochem, 1994, 63(1): 383. |
| [61] |
PAUWELS RA, JOOS GF, KIPS JC. Leukotrienes as therapeutic target in asthma[J]. Allergy, 1995, 50(8): 615. DOI:10.1111/all.1995.50.issue-8 |
| [62] |
JIAN W, EDOM RW, XUE X, et al. Quantitation of leukotriene B(4) in human sputum as a biomarker using UPLC-MS/MS[J]. J Chromatogr B Analyt Technol Biomed Life Sci, 2013, 932(15): 59. |
| [63] |
DUROUDIER NP, TULAH AS, SAYERS I. Leukotriene pathway genetics and pharmacogenetics in allergy[J]. Allergy, 2009, 64(6): 823. DOI:10.1111/all.2009.64.issue-6 |
| [64] |
PERESTRELO R, SILVA CL, CAMARA JS. Determination of urinary levels of leukotriene B4 using ad highly specific and sensitive methodology based on automatic MEPS combined with UHPLC-PDA analysis[J]. Talanta, 2015, 144: 382. |
| [65] |
林力.基于AA模型和T细胞模型的抗炎中药筛选研究[D].北京: 北京中医药大学, 2005 LIN L.The Modeling and Screening study of the Anti-inflammation Troditional Chinese Medicines with AA System and T Cell[D]. Beijing: Beijing University of Chinese Medicine, 2005 |
| [66] |
MONTUSCHI P, MARTELLO S, FELLI M, et al. Ion trap liquid chromatography/tandem mass spectrometry analysis of leukotriene B4 in exhaled breath condensate[J]. Rapid Commun Mass Spectrom, 2004, 18(22): 2723. DOI:10.1002/(ISSN)1097-0231 |
| [67] |
WAN M, GODSON C, GUIRY PJ, et al. Leukotriene B4/antimicrobial peptide LL-37 proinflammatory circuits are mediated by BLT1 and FPR2/ALX and are counterregulated by lipoxin A4 and resolvin E1[J]. Faseb J, 2011, 25(5): 1697. DOI:10.1096/fj.10-175687 |
| [68] |
SAMPSON AP. The leukotrienes:mediators of chronic inflammation in asthma[J]. Clin Exp Allerg J British Soc Allerg Clin Immunol, 1996, 26(9): 995. DOI:10.1111/cea.1996.26.issue-9 |
| [69] |
YU W, POWELL WS. Analysis of leukotrienes, lipoxins, and monooxygenated metabolites of arachidonic acid by reversed-phase high-pressure liquid chromatography[J]. Anal Biochem, 1995, 226(2): 241. |
| [70] |
TAKAMOTO M, YANO T, SHINTANI T, et al. A highly sensitive and selective method for the determination of leukotriene B4 in human plasma by negative ion chemical ionization/gas chromatography/tandem mass spectrometry[J]. J Pharm Biomed Anal, 1995, 13(12): 1465. DOI:10.1016/0731-7085(95)01583-3 |
| [71] |
MIZUGAKI M, HISHINUMA T, SUZUKI N. Determination of leukotriene E4 in human urine using liquid chromatography-tandem mass spectrometry[J]. J Chromatogr B Biomed Sci Appl, 1999, 729(1-2): 279. DOI:10.1016/S0378-4347(99)00174-7 |
| [72] |
CHAPPELL GP, XIAO X, PICA-MENDEZ A, et al. Quantitative measurement of cysteinyl leukotrienes and leukotriene B4 in human sputum using ultra high pressure liquid chromatography-tandem mass spectrometry[J]. J Chromatogr B Analyt Technol Biomed Life Sci, 2011, 879(3-4): 277. DOI:10.1016/j.jchromb.2010.12.014 |
| [73] |
LIN W, HUANG MQ, XUE X, et al. A highly sensitive and selective method for the determination of leukotriene B4(LTB4) in ex vivo stimulated human plasma by ultra fast liquid chromatography-tandem mass spectrometry[J]. J Chromatogr B Analyt Technol Biomed Life Sci, 2013, 925: 54. DOI:10.1016/j.jchromb.2013.02.038 |
| [74] |
王志军, 贾岛, 周建芝, 等. 冠心病合并糖尿病患者血浆11-dh-TXB2水平变化及意义[J]. 山东医药, 2016, 56(36): 43. WANG ZJ, JIA D, ZHOU JZ, et al. Changes in levels of 11-dh-TXB2 in patients with coronary heart disease combined with diabetes mellitus[J]. Shandong Med J, 2016, 56(36): 43. DOI:10.3969/j.issn.1002-266X.2016.36.013 |
| [75] |
OBILADE OA, AKANMU AS, BROUGHTON PIPKIN F, et al. Prostacyclin, thromboxane and glomerular filtration rate are abnormal in sickle cell pregnancy[J]. PLoS One, 2017, 12(9): e0184345. DOI:10.1371/journal.pone.0184345 |
| [76] |
YASUMOTO A, TOKUOKA SM, KITA Y, et al. Multiplex quantitative analysis of eicosanoid mediators in human plasma and serum:possible introduction into clinical testing[J]. J Chromatogr B Analyt Technol Biomed Life Sci, 2017, 1068-1069: 98. DOI:10.1016/j.jchromb.2017.10.014 |
| [77] |
ZHU QF, HAO YH, LIU MZ, et al. Analysis of cytochrome P450 metabolites of arachidonic acid by stable isotope probe labeling coupled with ultra high-performance liquid chromatography/mass spectrometry[J]. J Chromatogr A, 2015, 1410: 154. DOI:10.1016/j.chroma.2015.07.100 |
| [78] |
NODE K, HUO Y, RUAN X, et al. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids[J]. Science, 1999, 285(5431): 1276. DOI:10.1126/science.285.5431.1276 |
| [79] |
CHANG NW, WU CT, CHEN DR, et al. High levels of arachidonic acid and peroxisome proliferator-activated receptor-alpha in breast cancer tissues are associated with promoting cancer cell proliferation[J]. J Nutr Biochem, 2013, 24(1): 274. |
| [80] |
ANTONIPILLAI I, NADLER J, VU EJ, et al. A 12-lipoxygenase product, 12-hydroxyeicosatetraenoic acid, is increased in diabetics with incipient and early renal disease[J]. J Clin Endocrinol Metabol, 1996, 81(5): 1940. |
| [81] |
FERREIRO-VERA C, MATA-GRANADOS JM, PRIEGO-CAPOTE F, et al. Automated targeting analysis of eicosanoid inflammation biomarkers in human serum and in the exometabolome of stem cells by SPE-LC-MS/MS[J]. Anal Bioanal Chem, 2011, 399(3): 1093. DOI:10.1007/s00216-010-4400-6 |
| [82] |
ZHANG W, OTSUKA T, SUGO N, et al. Soluble epoxide hydrolase gene deletion is protective against experimental cerebral ischemia[J]. Stroke, 2008, 39(7): 2073. DOI:10.1161/STROKEAHA.107.508325 |
| [83] |
LUNDSTRM S L, LEVNEN B, NORDING M, et al. Asthmatics exhibit altered cxylipin profiles compared to healthy individuals after subway air exposure[J]. PLoS One, 2011, 6(8): e23864. DOI:10.1371/journal.pone.0023864 |
| [84] |
黄毅军, 秦骏, 李泓杰, 等. 环氧二十碳三烯酸及其在肝硬化门静脉高压症机制中的研究进展[J]. 中国普外基础与临床杂志, 2016(8): 1005. HUANG YJ, QIN J, LI HJ, et al. Research progress of epoxyeicosatrienoic acid and its role in regulating development of cirrhotic portal hypertension[J]. Chin J Bases Clin General Surg, 2016(8): 1005. |
| [85] |
邱笑违, 王雯, 蒋东桥, 等. 11, 12-环氧二十碳三烯酸对人脐静脉内皮细胞缺氧/复氧损伤的影响[J]. 中国医学科学院学报, 2006, 28(6): 803. QIU XW, WANG W, JIANG DQ, et al. Effect of 11, 12-epoxyeicosatrienoic acids on hypoxia/reoxygenation injury in the human umbilical vein endothelial cells[J]. Acta Acad Med Sin, 2006, 28(6): 803. DOI:10.3321/j.issn:1000-503X.2006.06.015 |
| [86] |
王康君, 程兆云. 急性冠状动脉综合征患者血浆环氧二十碳三烯酸水平与血脂关系及对预后的影响[J]. 中华实用诊断与治疗杂志, 2017(8): 766. WANG KJ, CHENG ZY. Relationship between levels of plasma epoxyeicosatrienoic acids and serum lipids in patients with acute coronary syndromes and its influence on prognosis[J]. J Chin Pract Diagn Therap, 2017(08): 766. |
| [87] |
ANNA A, ANDREY S. Targeting 20-HETE producing enzymes in cancer-rationale, pharmacology, and clinical potential[J]. Oncot Therap, 2013, 6(1): 243. |
| [88] |
宋一萌, 李明真, 马潞林. AA代谢产物与肿瘤发生和发展的研究进展[J]. 临床泌尿外科杂志, 2017(3): 236. SONG YM, LI MZ, MA LL. Research advances in the association between arachchidonic acid metaolites and tumorigenesis[J]. J Clin Urol, 2017(3): 236. |
| [89] |
苏林龙, 汪文裴, 张洁云. 结核病与12-羟基二十碳四烯酸的相关性研究[J]. 中国医学工程, 2017(5): 52. SU LL, WANG WP, ZHANG JY. Relationship between tuberculosis and 12-hydroxyeicosatetraenoic acid[J]. Chin Med Eng, 2017(5): 52. |
| [90] |
SUZUKI N, HISHINUMA T, SAGA T, et al. Determination of urinary 12(S)-hydroxyeicosatetraenoic acid by liquid chromatography-tandem mass spectrometry with column-switching technique:sex difference in healthy volunteers and patients with diabetes mellitus[J]. J Chromatogr B, 2003, 783(2): 383. DOI:10.1016/S1570-0232(02)00666-9 |
| [91] |
SUZUKI N. Mass spectrometry-based quantitative analysis and biomarker discovery[J]. Yakugaku Zasshi J Pharm Soc Japan, 2011, 131(9): 1305. DOI:10.1248/yakushi.131.1305 |
| [92] |
ZHU QF, HAO YH, LIU MZ, et al. Analysis of cytochrome P450 metabolites of arachidonic acid by stable isotope probe labeling coupled with ultra high-performance liquid chromatography/mass spectrometry[J]. J Chromatogr A, 2015, 1410: 154. DOI:10.1016/j.chroma.2015.07.100 |
 2019, Vol. 39
2019, Vol. 39 
