棉子糖系列寡糖(RFOs)作为一种天然食品添加剂和益菌因子,已被广泛应用于食品及功能性保健食品领域[1]。RFOs是由数个半乳糖以α-(1,6)糖苷键连接在蔗糖上,所构成的一系列水溶性非还原糖。RFOs主要包括棉子糖(三糖)、水苏糖(四糖)和毛蕊花糖(五糖)等,具有改善葡萄糖耐受[2],抑制肝胆固醇[3],增强免疫[4]和改善肠道菌群[5]等多种生物活性。
RFOs广泛存在于植物界,是含量仅次于蔗糖的一类可溶性糖类成分[6]。葫芦科、豆科、唇形科、木犀科、玄参科等多种植物均利用RFOs来转运和储存能量[7]。在不同科属植物中,豆科植物种子如大豆[8]、绿豆[9]、三角豆[10]、芸豆[11]、豌豆[12]、红豆[13]等含有丰富RFOs。此外,RFOs也存在于一些药食两用植物如地黄、水苏等[14]。为了开发富含RFOs新资源,建立定性、定量分析RFOs方法,明确其在不同科属植物中的分布具有重要意义。
目前,RFOs常用分析方法包括比色法[15]、高效阴离子色谱联用脉冲安培检测器法(HPAEC-PAD)[8]、亲水相互作用色谱联用蒸发光散射检测器法(HILIC-ELSD)[16],以及亲水相互作用色谱联用示差折光检测器法(HILIC-RID)[17]等。其中,比色法简单、方便,但只能测定总糖含量,并且测定结果易受单糖类型影响。虽然HPAEC-PAD法分离度好,但糖类物质在碱性条件下易异构化和降解[18]。HILIC虽有较好的分辨率,但RID和ELSD灵敏度均较低[16, 19],并且RID不适宜梯度洗脱,影响分离能力[17]。近年来,电喷雾式检测器(CAD)作为一种新型通用检测器,已被成功应用于寡糖类化合物的分析检测[20-21]。与ELSD检测器相似,CAD响应值不依赖于分析物的结构[22],并且CAD检测器灵敏度远高于ELSD和RID检测器[23]。在前期工作中,本课题组成功应用HPLC-CAD法对菊型果寡糖[20]、木寡糖[24]等进行过分析,并研究了菊型果寡糖在不同植物中的含量。本研究应用HPLC-CAD结合高通量微波辅助提取技术,实现对不同生物来源样品中RFOs定性、定量分析。
1 仪器与试药Multiwave 3000微波消解仪(Anton Paar GmbH公司),Ultimate 3000超高效液相系统,Chromeleon 6.8色谱工作站(Dionex公司),Waters Amide色谱柱(4.6 mm×250 mm,3.5 μm;填料:亚乙基桥杂化颗粒三键键合酰胺基;Waters公司)AG135与ARB120电子分析天平(Mettler Toledo公司),Millipore Milli-Q超纯水制备系统(Millipore公司)。
样品共16批,详细信息见表 1。豆科植物种子收集自澳门与珠海当地市场,生地黄、车前草和水苏主要购买于珠海当地药店。各批药材由澳门大学乔春峰博士鉴定确认。
|
|
表 1 样品信息 Table 1 Characteristics of the investigated samples |
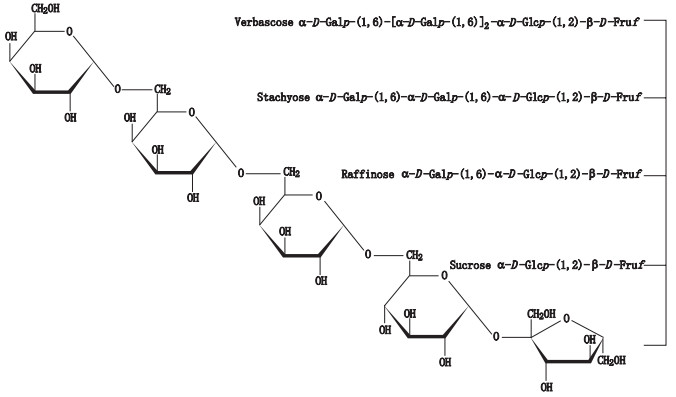
棉子糖系列寡糖,主要包括棉子糖、水苏糖、毛蕊花糖对照品由本实验室纯化制备[25],其纯度大于97%(w/w,HPLC-DAD-CAD法测定),结构信息见图 1。色谱级乙腈购于RCI Labscan公司,超纯水由Milli-Q超纯水制备系统制备,分析级乙醇购于天津市富宇精细化工有限公司。
|
图 1 棉子糖系列寡糖(棉子糖、水苏糖和毛蕊花糖)与蔗糖结构 Figure 1 Chemical structures of raffinose family oligosaccharides (raffinose, stachyose and verbascose) and sucrose |
精密称取对照品(蔗糖、棉子糖、水苏糖、毛蕊花糖)适量,置5 mL量瓶中。加60%乙醇溶解并定容至刻度,摇匀,配制成质量浓度为2.0 mg·mL-1混合对照品溶液,4 ℃储存备用。
2.2 供试品溶液制备所有样品研磨成均匀细粉,过40目筛。参照本课题组已建立的方法[20],应用Multiwave 3000微波消解仪提取寡糖。精密称取待测样品干燥细粉0.1 g,置于5 mL提取瓶中,加入60%乙醇溶液2.0 mL。微波辅助提取,功率400 W,时间4 min,温度80 ℃。提取结束后,提取液离心(5 000×g,5 min)取上清液1 mL,置于10 mL量瓶中,用60%乙醇定容至刻度,溶液过0.45 μm微孔滤膜后作HPLC-CAD分析。
2.3 HPLC-CAD分析采用Waters Amide色谱柱(4.6 mm×250 mm,3.5 μm),流动相为水(A)-乙腈(B),梯度洗脱(0~20 min,75%B→45%B,20~22 min,45%B→75%B,流速为1.0 mL·min-1,柱温30 ℃,进样量10 μL,CAD氮气压力为0.24 MPa。
2.4 重复性及稳定性试验精密称定豌豆样品粉末0.08、0.10、0.12 g各3份,按供试品溶液制备方法制备低、中、高浓度的供试品溶液,并测定含量,验证方法重复性。取中间浓度供试品溶液,在上述色谱条件下,分别于0、2、4、6、8、12、24、48 h进样分析,验证方法稳定性。
低、中、高浓度供试品溶液重复性RSD分别小于3.6%、3.8%和3.1%;稳定性试验结果(表 2)表明,供试品溶液在48 h内稳定性较好,RSD小于2.8%。说明该方法具有良好的重复性和稳定性。
|
|
表 2 4种糖重复性和稳定性考察结果 Table 2 Repeatability and stability of sucrose, raffinose, stachyose and verbascose |
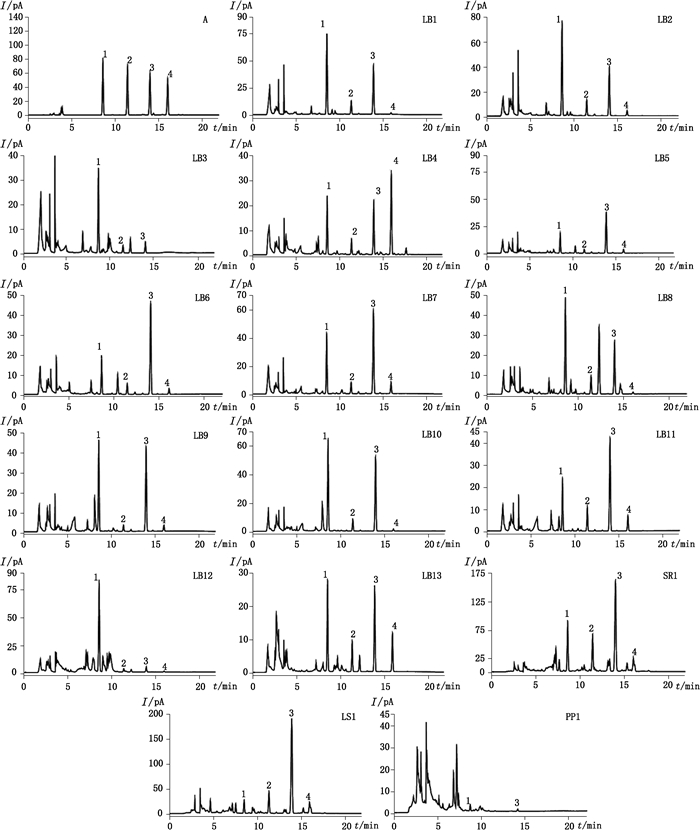
RFOs是一类可溶性非还原糖,采用HPLC-CAD方法测定不同来源(豆科、玄参科、唇形科和车前草科)样品中RFOs和蔗糖含量(图 2、表 3)。结果表明:豆科植物样品几乎都含有蔗糖和RFOs,但各种寡糖在不同来源豆科植物种子中含量差异较大,如眉豆与大豆中水苏糖和蔗糖含量最高。但在绿豆中毛蕊花糖含量最高,达总糖的45.3%。特别是RFOs在水苏和生地黄中含量非常高,总量分别达到426.19 mg·g-1和373.40 mg·g-1,但车前草中几乎检测不到。实际上,RFOs作为植物能量成分,主要储存在植物块茎(如生地黄)中,植物叶子(如车前草)中含量较少[26]。
|
1.蔗糖(sucrose)2.棉子糖(raffinose)3.水苏糖(stachyose)4.毛蕊花糖(verbascose) 图 2 对照品(A)及样品(样品编号同表 1)HPLC-CAD色谱图 Figure 2 HPLC-CAD chromatograms of mixed standards (A) and tested samples(the sample codes were the same as in Tab. 1) |
|
|
表 3 被测植物中4种糖的含量 Table 3 The contents of sucrose, raffinose, stachyose and verbascose in the investigated samples |
HILIC法是糖类化合物常用分析技术之一。通过比较不同HILIC色谱柱,本文选择Waters氨基柱(4.6 mm×250 mm,3.5 μm)用于RFOs和蔗糖分离,该色谱柱具有良好的分辨率和信噪比。另外,考察了不同梯度洗脱条件,最终建立的HILIC-CAD方法能在20 min内对待测成分实现良好分离和分析,蔗糖、棉子糖、水苏糖、毛蕊花糖均达到基线分离。
尽管已有大量研究证实RFOs存在于豆科、玄参科、唇形科和车前科植物中,但是少有研究系统比较这些科属代表植物中RFOs含量。当前市场上(特别是日本)RFOs主要来自大豆,但大豆中RFOs含量相对较低,进而限制了RFOs在健康产品中的应用[27]。本研究结果表明:水苏和地黄富含大量RFOs,有望作为开发富含RFOs的新资源,本研究建立的HPLC-CAD方法也有利于对RFOs及其产品进行质量控制。
| [1] |
TOMOMATSU H. Health effects of oligosaccharides[J]. Food Technol, 1994, 48(10): 61. |
| [2] |
THORBURN A, MUIR J, PROIETTO J. Carbohydrate fermentation decreases hepatic glucose output in healthy subjects[J]. Metabolism, 1993, 42(6): 780. DOI:10.1016/0026-0495(93)90249-N |
| [3] |
CHEN WJL, ANDERSON JW, JENNINGS D. Propionate may mediate the hypocholesterolemic effects of certain soluble plant fibers in cholesterol-fed rats[J]. Exp Biol Med, 1984, 175(2): 215. DOI:10.3181/00379727-175-41791 |
| [4] |
SCHLEY P, FIELD C. The immune-enhancing effects of dietary fibres and prebiotics[J]. Br J Nutr, 2002, 87(2): 221. |
| [5] |
ARCHER S, MENG S, WU J, et al. Butyrate inhibits colon carcinoma cell growth through two distinct pathways[J]. Surgery, 1998, 124(2): 248. DOI:10.1016/S0039-6060(98)70127-8 |
| [6] |
FRIAS J, BAKHSH A, JONES D, et al. Genetic analysis of the raffinose oligosaccharide pathway in lentil seeds[J]. J Exp Bot, 1999, 50(333): 469. DOI:10.1093/jxb/50.333.469 |
| [7] |
FRENCH D. The raffinose family of oligosaccharides[J]. Adv Carbohydr Chem, 1954, 9(12): 149. |
| [8] |
GIANNOCCARO E, WANG Y, CHEN P. Comparison of two HPLC systems and an enzymatic method for quantification of soybean sugars[J]. Food Chem, 2008, 106(1): 324. DOI:10.1016/j.foodchem.2007.04.065 |
| [9] |
DAI Z, SU D, ZHANG Y, et al. Immunomodulatory activity in vitro and in vivo of verbascose from mung beans (Phaseolus aureus)[J]. J Agric Food Chem, 2014, 62(44): 10727. DOI:10.1021/jf503510h |
| [10] |
XIAOLI X, LIYI Y, SHUANG H, et al. Determination of oligosaccharide contents in 19 cultivars of chickpea (Cicer arietinum L.) seeds by high performance liquid chromatography[J]. .Food Chem, 2008, 111(1): 215. DOI:10.1016/j.foodchem.2008.03.039 |
| [11] |
KUO TM, VANMIDDLESWORTH JF, WOLF WJ. Content of raffinose oligosaccharides and sucrose in various plant seeds[J]. J Agric Food Chem, 1988, 36(1): 32. DOI:10.1021/jf00079a008 |
| [12] |
EKVALL J, STEGMARK R, NYMAN M. Content of low molecular weight carbohydrates in vining peas (Pisum sativum) related to harvest time, size and brine grade[J]. Food Chem, 2006, 94(4): 513. DOI:10.1016/j.foodchem.2004.11.044 |
| [13] |
PETERBAUER T, RICHTER A. Galactosylononitol and stachyose synthesis in seeds of adzuki bean purification and characterization of stachyose synthase[J]. Plant Physiol, 1998, 117(1): 165. DOI:10.1104/pp.117.1.165 |
| [14] |
LIU Z, LOU Z, DING X, et al. Global characterization of neutral saccharides in crude and processed Radix Rehmanniae by hydrophilic interaction liquid chromatography tandem electrospray ionization time-of-flight mass spectrometry[J]. Food Chem, 2013, 141(3): 2833. DOI:10.1016/j.foodchem.2013.04.114 |
| [15] |
DUBOIS M, GILLES KA, HAMILTON JK, et al. Colorimetric method for determination of sugars and related substances[J]. Anal Chem, 1956, 28(3): 350. DOI:10.1021/ac60111a017 |
| [16] |
KIM TB, KIM SH, SUNG SH. Quantitation of α-galactosides in Rehmannia glutinosa by hydrophilic interaction chromatography-evaporative light scattering detector[J]. Phytochem Anal, 2012, 23(6): 607. DOI:10.1002/pca.v23.6 |
| [17] |
YANG X, ZHAO Y, HE N, et al. Isolation, characterization, and immunological effects of α-galacto-oligosaccharides from a new source, the herb Lycopus lucidus Turcz[J]. .J Agric Food Chem, 2010, 58(14): 8253. DOI:10.1021/jf101217f |
| [18] |
LEE YC. High-performance anion-exchange chromatography for carbohydrate analysis[J]. Anal Biochem, 1990, 189(2): 151. DOI:10.1016/0003-2697(90)90099-U |
| [19] |
GANGOLA MP, JAISWAL S, KHEDIKAR YP, et al. A reliable and rapid method for soluble sugars and RFO analysis in chickpea using HPAEC-PAD and its comparison with HPLC-RI[J]. Food Chem, 2014, 154: 127. DOI:10.1016/j.foodchem.2013.12.085 |
| [20] |
LI J, HU DJ, ZONG WR, et al. Determination of inulin-type fructooligosaccharides in edible plants by high-performance liquid chromatography with charged aerosol detector[J]. J Agric Food Chem, 2014, 62(31): 7707. DOI:10.1021/jf502329n |
| [21] |
INAGAKI S, MIN JZ, TOYO'OKA T. Direct detection method of oligosaccharides by high-performance liquid chromatography with charged aerosol detection[J]. Biomed Chromatogr, 2007, 21(4): 338. DOI:10.1002/(ISSN)1099-0801 |
| [22] |
EOM HY, PARK SY, KIM MK, et al. Comparison between evaporative light scattering detection and charged aerosol detection for the analysis of saikosaponins[J]. J Chromatogr A, 2010, 1217(26): 4347. DOI:10.1016/j.chroma.2010.04.047 |
| [23] |
LIGOR M, STUDZIŃSKA S, HORNA A, et al. Corona-charged aerosol detection:an analytical approach[J]. Crit Rev Anal Chem, 2013, 43(2): 64. DOI:10.1080/10408347.2012.746134 |
| [24] |
YANG ZY, WU DT, CHEN CW, et al. Preparation of xylooligosaccharides from xylan by controlled acid hydrolysis and fast protein liquid chromatography coupled with refractive index detection[J]. Sep Purif Technol, 2016, 171: 151. DOI:10.1016/j.seppur.2016.06.051 |
| [25] |
ZONG WR, CHEONG KL, WU DT, et al. Preparation and purification of raffinose family oligosaccharides from Rehmannia glutinosa Libosch.by fast protein liquid chromatography coupled with refractive index detection[J]. Sep Purif Technol, 20144, 138: 98. |
| [26] |
van den ENDE W. Multifunctional fructans and raffinose family oligosaccharides[J]. Front Plant Sci, 2013, 4: 247. |
| [27] |
CRITTENDEN R, PLAYNE MJ. Production, properties and applications of food-grade oligosaccharides[J]. Trends Food Sci Technol, 1996, 7(11): 353. DOI:10.1016/S0924-2244(96)10038-8 |
 2018, Vol. 38
2018, Vol. 38 
