1991年Watara等[1]以微乳毛细管电动色谱分离了一组具有荧光性质的芳香族化合物,开始了这种方法的应用研究。由于其进样体积小(nL)、分离效率高、分析时间快,可同时分离水溶性、脂溶性、带电或不带电的物质,促使MEEKC的分析对象涵盖了环境样品、食品、药品、维生素、生物样品、手性化合物等几乎所有的样品种类,特别在成分复杂的中药和天然产物的分析中也逐渐得到了关注和发展。
黄酮类化合物是一类在植物中广泛存在的有效成分,具有多种药理活性,比如抗肿瘤、抗炎、降压、降血脂等作用以及对心血管系统、免疫系统、中枢神经系统等保护作用。目前对黄酮类化合物的检测方法主要有分光光度法[2]、薄层色谱法(TLC)[3]、高效液相色谱法(HPLC)[4-7]、液相色谱-质谱联用技术[8]以及毛细管电泳法(CE)[9-10]等。随着毛细管电泳理论的不断发展和电泳仪的不断完善和普及,MEEKC作为一种最新的CE分离手段,在黄酮类化合物的分析中有着非常好的应用前景。本文介绍了MEEKC的组成、原理、影响分离的因素以及MEKC和MEEKC的异同,综述了MEEKC在黄酮类化合物中的研究进展并进行了总结和展望。
1 微乳液的组成微乳液是由表面活性剂、辅助表面活性剂、油相和缓冲液4种组分在适当的比例下通过超声处理形成的具有透明或半透明、低粘度等特征的稳定液体。它作为微乳体系中的假固定相可以分为水包油型(O/W)、油包水型(W/O)和双连续型(B.C.)三种类型。通常以水包油(O/W)型微乳液作为分离介质,经典的O/W微乳体系包括正构烷烃(油相)、硼酸盐或磷酸盐缓冲液(水相)、十二烷基硫酸钠SDS(常用表面活性剂)、正丁醇(短链醇辅助表面活性剂)。由于油水两相间存在很高的表面张力,两者本来互不相溶,加入表面活性剂后,由于表面活性剂具有两亲性,亲油端插入油相,亲水端在水相中,降低了油水间的表面张力。辅助表面活性剂以亲水头部朝外、疏水尾部向里的方式插入到表面活性剂的中间,进一步降低了油水间的表面张力,表面活性剂和辅助表面活性剂在油滴表面有序地排列,使得微乳体系非常稳定。微乳液的形状不一,但总体上可认为是球形。
2 分离机理在MEEKC中,微乳液滴替代了毛细管区带电泳(capillary zone electrophoresis,CZE)中的硼砂和磷酸缓冲液。当石英毛细管柱内pH大于3时,表面电离成—SiO-,管内壁带负电荷,和溶液接触时形成了双电层。当液体两端施加电压时,就会发生液体相对于固体表面的移动,产生电渗流(EOF),EOF驱使缓冲液以一定速度流向阴极。SDS作为MEEKC中最常用的阴离子表面活性剂分布于微乳液滴表面使其带负电荷,在电场作用下泳向阳极。由于电渗流速度往往比电泳速度快5~7倍,毛细管中的微乳颗粒仍能以一定速度向阴极移动。各种被分析的组分,由于它们在微乳相(假固定相)与水相中的分配差异,加之由于它们的荷电与尺寸差异性,表现出不同的表观淌度从而实现不同物质的分离,因此它们的分离过程是电泳和色谱综合作用的结果。图 1为O/W型微乳液滴组成结构图及MEEKC的分离原理示意图[11]。
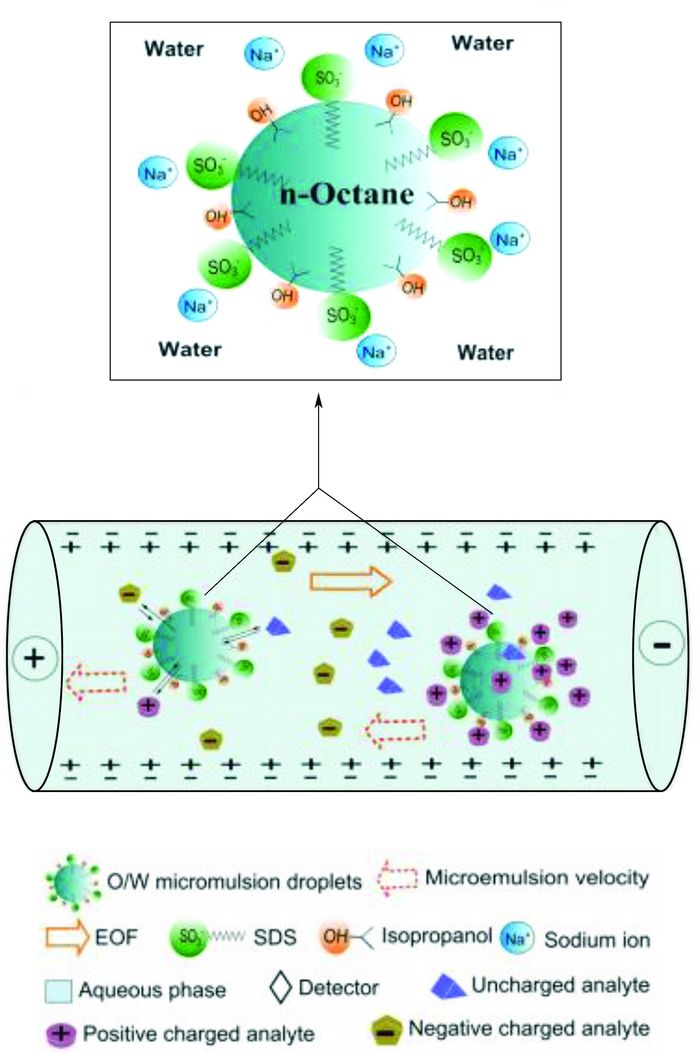
|
图 1 O/W型微乳液滴组成结构图及MEEKC的分离原理示意图 Figure 1 Schematic representations of O/W microemulsion and an MEEKC separation mechanism |
由MEEKC的分离原理可知,分析物在微乳相中的保留因子对分离和富集效率有较大的影响,因此,测定这些分析物在此微乳中的保留因子至关重要。Terabe等[12]提出的描述分析物在MEKC模式下的保留因子的方程,可以用来计算分析物在MEEKC模式下的保留因子。
以下公式用来定性和反映MEEKC分离的机制:
| $k = \frac{{{t_{\rm{r}}} - {t_0}}}{{{t_0}\left( {1 - \frac{{{t_{\rm{r}}}}}{{{t_{{\rm{me}}}}}}} \right)}}$ |
式中,k表示保留因子,tr、t0、tme分别表示分析物、电渗流标记物和微乳标记物的迁移时间。实验中一般用正丁醇和十二烷氧基苯作为电渗流和微乳的标记物。分配系数k值表明了被分析物的迁移行为及在假固定相和水相中的分配情况。被分析物的疏水性不同,在两相中的分配也不同。其脂溶性越强,在微乳液中分配越多,迁移时间就越长。
3 MEEKC与MEKC的异同MEKC是将离子型表面活性剂加入到缓冲液中,在临界胶束浓度以上形成胶束作为假固定相,被分析物在流动相与假固定相之间进行分配而实现分离。多年来研究者们经常比较这2种分离模式的异同[13-16],总体来说,MEEKC与MEKC都具有分离效率高、分析速度快、分析对象范围广以及试剂和样品耗量少等优点。它们之间最大的不同在于MEEKC中添加了不溶于水的油相,在胶束的中心形成了油核,增加了高疏水性物质的溶解性,从而使MEEKC分析的化合物范围比MEKC更广泛、样品容量更大[17-19]。然而,目前也不能一概而论MEEKC更优,对于MEEKC与MEKC在分离选择性和分离效率上的区别以及有机改性剂在2种分离模式的应用问题上一直存在争议[20]。Cao等[21]最近研究证明微乳与不加入辅助表面活性剂(正丁醇)的胶束相比,多数情况下,分离选择性和分离效率都存在着差别。但是对于采用相同辅助表面活性剂(正丁醇)调节的胶束和微乳来说,MEEKC与MEKC的分离性能很相似。这可能因为辅助表面活性剂插到表面活性剂分子中间,也能像油相一样改变胶束的结构,使之类似微乳,而甲醇、乙腈等不能插入表面活性剂分子中的有机改性剂则不能起到这样的作用。
4 影响MEEKC分离的因素在MEEKC的应用中,由于微乳液组成复杂,各种组分的变化都会影响MEEKC的分离结果,包括表面活性剂、辅助表面活性剂、缓冲液和油相的种类及浓度,缓冲液的pH,有机添加剂和样品基质的选择等。
4.1 表面活性剂种类和浓度表面活性剂对降低微乳油水表面张力从而稳定微乳系统具有举足轻重的作用。SDS是应用最广泛的阴离子表面活性剂,十六烷基三甲基溴化铵(CTAB)[22]是最常用的阳离子表面活性剂,Brij-35[23-24]、吐温类[24]等中性表面活性剂也可以用来形成微乳液,但分离选择性稍差且不能分离中性物质,目前并没有单独利用中性表面活性剂的MEEKC法分离黄酮类化合物的相关报道。
近几年来,大量的研究证明,尽管SDS是MEEKC中最常用的表面活性剂,却未必适用于所有情况。Cao等[26]建立了一种同时快速分离分析山楂中7种黄酮类化合物的ZI-MEEKC方法,实验比较了两性表面活性剂DAPS、阴离子表面活性剂SDS和中性表面活性剂Brij-35对分离的影响。结果表明,7种黄酮类化合物在DPAS-MEEKC体系下迁移时间最短、分离效果最好。Cao等[27]采用阴阳离子混合表面活性剂(SDS-DTAC)制备微乳体系用于分析黄芪中5种黄酮类化合物。结果表明微乳体系中使用混合表面活性剂相较于单一表面活性剂有更高的分离度和选择性。Cao等[28]利用MEEKC法分离茶叶中3种儿茶素,3种酚酸和2种黄酮类化合物,为了获得更低的工作电流和更短的分析时间,在微乳体系中加入由长链烷基酸和氨水反应得到的原位合成表面活性剂,结果发现电渗流明显增大,茶叶中的8种复杂化学成分在6 min内实现完全分离。
4.2 辅助表面活性剂种类和浓度辅助表面活性剂影响被分离物质在油水两相的分配、迁移时间和分离效率。浓度的不同直接影响待分析物与假固定相之间的作用力以及液滴表面的电荷密度。在MEEKC中,辅助表面活性剂一般是短链醇,例如正丁醇、异丙醇、正丙醇等,正丁醇是最常用的辅助表面活性剂,其含量范围为6.6%~16.5%,否则不能形成稳定的微乳。Siren等[29]将正丁醇作为辅助表面活性剂加入微乳中用于分离大豆苷元和染料木黄酮两种异黄酮类化合物实验发现随着微乳体系中正丁醇含量的增加,黄酮的迁移时间随之延长,分离度有所提高,但正丁醇含量在6.0%~8.6%(w/v)时微乳体系才能保持稳定。
4.3 油相种类和浓度油相的种类和浓度影响分析物与微乳假固定相之间的相互作用力,常用的一些油相有正辛烷、正庚烷、正己烷、乙酸乙酯及异丙酯等。乙酸乙酯[30]和酒石酸二丁酯[31]相比于其他油相具有更低的表面张力,形成微乳所需SDS含量少,减少了分析时间。
4.4 缓冲液种类、浓度和pH目前常用MEEKC分离黄酮类化合物的缓冲液有:硼砂、磷酸和硼砂-磷酸盐混合缓冲液。其常用的浓度范围为5~10 mmol·L-1。常用的pH范围为7.0~9.0,大部分的离子型化合物在此范围内能被电离。一些极端的pH,如pH 2.5和1.2也多有报道,酸性微乳液主要目的为抑制电渗流,用于反向MEEKC的分离应用。针对异黄酮类、苯并二氮类等极性化合物,Siren等[29]研究了碱性和酸性微乳体系对分离的影响。实验结果表明,碱性微乳体系对这些极性化合物的分离效果最好。Yu等[32]为了分离14种黄酮类化合物,考察了不同pH(6.0~9.0)10 mmol·L-1 Na2B4O7-20 mmol·L-1 H3BO3间和分离度,最终选择最优pH为7.5。Vanhoenacker等[33]在Szucs研究[34]的基础上分析了啤酒花酸性提取物和异戊烯基黄酮类化合物。实验发现,增加SDS的浓度、改变正丁醇浓度或用正戊醇代替正丁醇均不能实现基线分离。最终通过调整缓冲液的pH至9.75时,10种化合物在15 min内实现基线分离。
5 在线预浓缩目前限制毛细管电泳发展的一大障碍就是其检测器灵敏度差,仅为10-6~10-5mmol·L-1,浓度的检出限远不能满足实际样品中痕量组分的分离检测要求,目前利用在线富集技术来提高毛细管电泳的检测灵敏度的技术已经逐渐发展起来。赵有轩等[35]总结了毛细管电泳在线富集技术在黄酮类化合物分析中的应用,其中样品堆积技术[36-38]和扫集技术[38]是常用的在线富集方法。此外,富集技术还包括瞬间等速电泳(ITP)[40]、动态pH连接[41]、胶束坍塌富集[42]及选择性耗尽进样-扫集法[43-46]、无需转换极性的大体积进样一扫集富集方法[47]等联用技术。
5.1 大体积样品堆积法(large volume sample stacking,LVSS)基本原理是样品从高电场(低电导率)的样品区带迁移到低电场(高电导率)的背景缓冲液中速度骤降,导致样品区带的长度缩短,使样品组分得到富集。LVSS常需要加临时的反向电压利用电渗流将一部分样品基质排出毛细管,既提高分析灵敏度又排除样品基质的干扰。Yu等[48]在MEEKC体系条件下采用大体积样品进样对栝楼桂枝颗粒中甘草黄苷、甘草素、肉桂酸、没食子酸、原儿茶酸5种有效成分进行了定量分析。实验发现通过大体积样品堆积富集后,5种化合物在10 min内实现基线分离。相比较常规进样灵敏度大大提高,富集倍数达到14~19,检测限达到0.05~0.1 μg·mL-1。方法的回收率在94.5%~97.5%之间,且RSD小于5.0%。
5.2 反向迁移微乳扫集法(sweeping-RMME)扫集法是基于分配的原理,富集效果的高低由分析物与微乳之间的相互作用力大小决定,可同时富集中性和带电荷的化合物。扫集的富集效率是不依赖EOF的,但是在扫集还没有完成的情况下,扫集区带会在EOF的驱动下带动分析物向检测端迁移,因此,必须利用低pH缓冲溶液来抑制EOF使扫集完成。Cao等[27]采用sweeping-RMME法分析黄芪中5种黄酮类化合物。结果发现,相比常规进样灵敏度大大提高,检测限达到0.004~0.022 μg·mL-1,富集因子达到185~508。采用常规进样分析黄芪样品时无法检测到被分析物,当进行扫集之后,实现了4种有效成分的定量分析。Zhu等[49]在酸性条件下抑制电渗流,利用4种在线富集方法成功富集了枳实中6种黄酮类化合物,结果表明,sweeping-RMME得到最佳的富集效果,富集倍数达到35~79倍。
5.3 水塞、样品基质中高盐溶液的影响为了增加进样体积提高堆积效率,从而提高富集因子,通常会在进样之前,向毛细管中加入一段低电导率溶液比如水。而近几年,基于毛细管电泳的高盐堆积和扫集也成为一种在线富集手段。之前有研究表明[50-51]样品基质的选择会对样品的富集因子产生显著的影响。Cao等[27]考察了一系列的样品基质对5种黄酮类化合物富集的影响,包括磷酸、磷酸二氢钠、磷酸氢二钠、醋酸钠、硼砂,确定最优样品基质为25 mmol·L-1 pH 2磷酸后,考察了磷酸中加入不同浓度氯化钠溶液(25~150 mmol·L-1)对富集的影响,实验表明在某种程度上含盐基质提高了扫集效率,但超过一定浓度后,导致电流增加,峰型变宽,对扫集产生不利影响。
6 新型添加剂为了提高脂溶性成分的溶解度,改善分离选择性,传统的方法是在微乳体系中加入一定的有机溶剂,例如,甲醇、乙腈和异丙醇等。但是由于有机添加剂不能有效改善结构相似及复杂组分的分离度,β-环糊精被发展成为又一微乳添加剂,近几年被应用于各类手性化合物的拆分及复杂组分的分析[52-55]。目前除了有机添加剂和β-环糊精,一些新型MEEKC微乳液添加剂应用于黄酮类化合物的分析也逐渐发展起来,主要包括碳纳米管[56-57]和离子液体[58]的使用。
6.1 碳纳米管(carbon nano tubes,CNTs)碳纳米管是直径为纳米级,长度为300~2 300 nm的圆柱形碳分子微小颗粒,它在溶液中聚集形成亲水性基团从而影响分析物在毛细管柱中的分配。为了改善物质的分离效果,碳纳米管曾经也作为添加剂应用于传统毛细管电泳色谱中。目前,羧基化单壁碳纳米管(C-SWNTs)和羧基化多壁碳纳米管(C-MWNTs)作为微乳液中的添加剂已经分别应用于提高咖啡因和可可碱同系物、7种嘌呤碱和嘧啶碱之间的分离效果[59-60]。表面活性剂包覆石墨化多壁碳纳米管(SC-GMWNTs)目前已有应用于氯酚,非甾体类抗炎药,三嗪类和硝基咪唑衍生物,内酰胺类抗菌素,酰胺醇类,黄酮类,酚酸类,皂苷类,麻黄碱类和双氯醇胺的手性拆分等相关报道[61-66]。Cao等[56]将SC-SWNTs作为微乳液添加剂用于3种黄酮类化合物和6种酚酸类化合物的分析研究,不同浓度SC-SWNTs的9种混标电泳谱图如图 2所示,结果在12 min内就实现了9种待分析组分的基线分离。随后,该课题组[56]又将MWNTs应用于MEEKC中用于分离8种酚酸类化合物,4种黄酮苷类化合物和1种菲醌类化合物,结果发现MWNTs的加入能有效地提高分离度,并将该方法成功地应用于复方血栓通胶囊样品中13种成分的检测和定量。
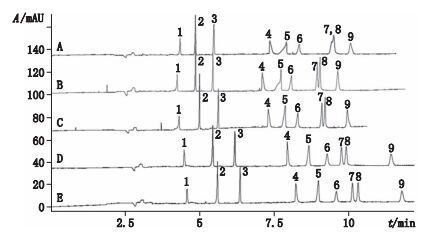
|
1.毛蕊异黄酮-7-O-β-D-葡萄糖苷(calycosin-7-O-β-D-glucoside)2.毛蕊异黄酮(calycosin)3.芒柄花黄素(formononetin)4.迷迭香酸(rosmarinic acid)5.丹参素(Danshensu)6.咖啡酸(caffeic acid)7.丹酚酸B(salvianolic acid B)8.紫草酸(lithospermic acid)9.原儿茶酸(protocatechuic acid) A. 0 mg·L-1 B. 2 mg·L-1 C. 4 mg·L-1 D. 6 mg·L-1 E. 8 mg·L-1 图 2 不同浓度(0~8 mg·L-1)SC-SWNTs条件下混合标准溶液电泳图 Figure 2 Electropherograms obtained for the separation of mixture standard in the presence of SCSWNTs(0~8 mg·L-1) |
离子液体又名室温离子液体(room temperature ionic liquids,RTILs)或室温熔融盐,是对完全由阴阳离子组成的、室温下或低温下呈液态的盐的总称,具有良好的热稳定性[67-68]、化学稳定性[69]和溶解性。1-丁基-3-甲基咪唑四氟硼酸盐(BMIM-BF4)是毛细管电泳中最常用的离子液体,BMIM-BF4的咪唑阳离子通过静电力和疏水性与微乳液滴作用。两者之间的相互作用改变了微乳的荷电属性,因此改变分析物在油水两相的分配从而改善分离。离子液体应用于MEEKC分析中药活性成分的研究近2年逐渐增多[70-72],包括对微乳液中表面活性剂的临界胶束行为的影响[72]、作为微乳液油相[74-75]或有机改性剂[76]均已有报道。另外,离子液体在区带毛细管电泳[77-80],胶束毛细管电泳[64,81]和非水毛细管电泳[82]分析黄酮类化合物的应用方面也有报道。而离子液体在毛细管微乳电动色谱分析黄酮类化合物的应用较为少见,目前只有一篇文献报道。
Zhang等[58]以离子液体BMIM-BF4作为IL-MEEKC体系的添加剂,分离测定了炒黄芩、生黄芩和清肺抑火丸3种药用制剂中的黄芩苷、汉黄芩素和黄芩素。初步实验发现当微乳液中不添加BMIM-BF4时,样品中3种被分析物不能完全分离。添加7.5 mmol·L-1 BMIM-BF4后,混标在5 min内实现基线分离。结果表明,IL-MEEKC体系相比传统微乳体系,在一定程度上可以改善分离效果。
6.3 小结由于MEEKC具有进样体积小,分离效率高,分离范围广,灵敏度高等特点,使得MEEKC在天然产物分析中的研究越来越广泛。表 1主要总结了微乳毛细管电动色谱技术在黄酮类化合物分析中的研究。主要涉及微乳液组成条件的优化、在线预浓缩技术的应用和微乳体系中新型添加剂的使用。
|
|
表 1 微乳毛细管电动色谱技术在黄酮类化合物分析中的研究 Table 1 Research on microemulsion capillary electrokinetic chromatography for the analysis of flavonoids |
MEEKC作为一种较为新型的CE分离模式,综合了电泳法和色谱法2种分离技术的特点,与HPLC和其他CE模式相比,不但具有分离效率高( > 104理论塔板数)、分析速度快(通常不超过30 min)、试样耗量少(所需样品为nL级,流动相为几毫升)等优势,同时毛细管柱的费用远低于高效液相的分析柱且没有液相色谱法在色谱柱pH耐受范围方面的限制,可调节参数更多,可分析的容量更大,分析窗口更宽,尤其适合少量样品和强疏水性化合物的分析。但是MEEKC法的研究依然存在一些难题和发展空间:(1)目前微乳色谱采用的微乳液体系还比较单一,主要是SDS/丁醇/烷烃/缓冲液体系,或者在该体系基础上进行简单调整,新的微乳系统尚待开发;(2)尽管MEEKC的应用报道较多,但很多是用标准物质混合物进行的定性分析。因此用于真实样品测定,增强方法的实用性将是MEEKC应用研究的重要内容;(3)由于微乳液中含有高浓度的表面活性剂,会产生大量的质谱吸收背景,使MS与MEEKC的联用较为困难[83-85];(4)目前MEEKC在理论认识上和技术上还不够完善,仍然存在线性范围窄、重复性差和灵敏度较低等缺陷。因此作为一种新型技术,MEEKC的研究工作还有待深入拓展,使其在药物分析、药材质量控制及相关领域作出贡献。
| [1] |
WATARAI H. Microemulsion capillary electrophoresis[J]. Chem Lett, 1991, 231(3): 391. |
| [2] |
LIU SS, MA XM, YANG HB. Determination of total flavones content in Ceratocarpusarenarius L. by spectrophotometry[J]. Agric Sci Tech, 2011, 12(11): 1612. |
| [3] |
刘德胜, 韩景田, 吕志华, 等. 落地生根黄酮类成分分析及抗氧化活性研究[J]. 安徽农业科学, 2011, 39(32): 19747. LIU DS, HAN J, LÜ ZH, et al. Analysis of flavonoids constituents in herb of air-plant and its antioxidant activity[J]. Anhui Agric Sci, 2011, 39(32): 19747. DOI:10.3969/j.issn.0517-6611.2011.32.035 |
| [4] |
YANG ZN, SUN YM, LUO SQ, et al. Quality evaluation of Houttuyniacordata Thunb. by high performance liquid chromatography with photodiode-array detection(HPLC-DAD)[J]. Pak J Pharm Sci, 2014, 27(2): 223. |
| [5] |
SEPTAMA AW, PANICHAYUPAKARANANT P. Simultan-eous HPLC analysis of three flavonoids in the extracts of Artocarpusheterophyllus heartwoods[J]. Nat Prod Sci, 2016, 22(2): 77. DOI:10.20307/nps.2016.22.2.77 |
| [6] |
章弘扬, 张弛, 王月荣, 等. 山楂中黄酮类成分的定性和定量分析[J]. 中国中药杂志, 2012, 37(5): 601. ZHANG HY, ZHANG C, WANG YR, et al. Qualitative and quantitative analysis of flavonoids in Hawthorn[J]. Chin J Mater Med, 2012, 37(5): 601. |
| [7] |
XUE X, ZHAO ZM, LI Q, et al. Determination of flavonoids by solidification of floating organic drop liquid-phase microextraction and high-performance liquid chromatography[J]. Anal Lett, 2016, 49(15): 2384. DOI:10.1080/00032719.2016.1149859 |
| [8] |
LI W, FENG YL, LI TE, et al. Rapid analysis on flavonoids in Glechomalongituba(nakai)kupr by UPLC-Q-TOF /MS couple with diagnostic ions[J]. J Chin Mass Spectrosc Soc, 2016, 37(6): 504. |
| [9] |
WANG W, LIN P, MA LH, et al. Separation and determination of flavonoids in three traditional Chinese medicines by capillary electrophoresis with amperometric detection[J]. J Sep Sci, 2016, 39(7): 1357. DOI:10.1002/jssc.v39.7 |
| [10] |
叶静, 肖美添, 汤须崇, 等. 胶束毛细管电泳测定豆粕及发酵豆粕中大豆异黄酮含量[J]. 中国药学杂志, 2010, 45(3): 223. YE J, XIAO MT, TANG XC, et al. Determination of Soybean isoflavones in Soybean Meal and Fermented Soybean Meal by micellar electrokinetic capillary chromatography[J]. Chin J Pharm, 2010, 45(3): 223. |
| [11] |
YU LS, CHU KD, YE HZ, et al. Recent advances in microemulsion electrokinetic chromatography[J]. Trends Anal Chem, 2012(4): 140. |
| [12] |
TERABE S, OTSUKA K, ICHIKAWA K, et al. Electrokinetic separations with micellar solutions and open-tubular capillaries[J]. Anal Chem, 1984, 56(1): 111. DOI:10.1021/ac00265a031 |
| [13] |
HAUNSCHMIDT M, ORTNER K, HAINZ K, et al. Investigations on the migration behavior of insulin and related synthetic analogues in CZE, MEKC and MEEKC employing different surfactants[J]. Electrophoresis, 2010, 31(9): 1560. |
| [14] |
NUSSBAUMER S, FLEURY-SOUXERAIN S, SCHAPPLER J, et al. Quality control of pharmaceutical formulations containing cisplatin, carboplatin, and oxaliplatin by micellar and microemulsionelectrokinetic chromatography(MEKC, MEEKC)[J]. J Pharm Biomed Anal, 2011, 55(2): 253. DOI:10.1016/j.jpba.2011.01.029 |
| [15] |
XIA ZN, GAN TT, CHEN H, et al. A new open tubular capillary microextraction and sweeping for the analysis of super low concentration of hydrophobic compounds[J]. J Sep Sci, 2010, 33(20): 3221. DOI:10.1002/jssc.v33:20 |
| [16] |
ORLANDINI S, GIANNINI I, NAVARRO MV, et al. Dual CD system-modified MEEKC method for the determination of clemastine and its impurities[J]. Electrophoresis, 2010, 31(19): 3296. DOI:10.1002/elps.v31:19 |
| [17] |
WEN T, ZHAO X, LUO GA, et al. Comparison of the performance of microemulsionelectrokinetic chromatography and 1-butanol modified micellar electrokinetic chromatography on the separations of amphetamine and its ephedra alkaloid impurities[J]. Chin J Anal Chem, 2006, 34(11): 1529. |
| [18] |
WEN T, ZHAO X, LUO GA, et al. Comparison of micro-emulsionelectrokinetic chromatography and solvent modified micellar electrokinetic chromatography on rapid separation of heroin, amphetamine and their basic impurities[J]. Talanta, 2007, 71(2): 854. DOI:10.1016/j.talanta.2006.05.051 |
| [19] |
YANG X, XIA Y, TAO CJ, et al. A comparative study of micellar and microemulsion EKC for the analysis of benzoylurea insecticides and their analogs[J]. Electrophoresis, 2007, 28(11): 1744. DOI:10.1002/(ISSN)1522-2683 |
| [20] |
HANSEN SH, GABEL JC, El-SHERBINY DTM, et al. Microemulsionelectrokinetic chromatography-or solvent-modified micellar electrokinetic chromatography[J]. Trends Anal Chem, 2001, 20(11): 614. DOI:10.1016/S0165-9936(01)00127-3 |
| [21] |
CAO YH, NI XJ, SHENG JW, et al. Comparison of microstructures of microemulsion and swollen micelle in electrokinetic chromatography[J]. J Chromatogr A, 2011, 1218(18): 2598. DOI:10.1016/j.chroma.2011.02.015 |
| [22] |
GONG S, LIU FJ, LI W, et al. Separation of hydrophobic solutes by organic-solvent-based micellar electrokinetic chromatography using cation surfactants[J]. J Chromatogr A, 2006, 1121(2): 274. DOI:10.1016/j.chroma.2006.04.091 |
| [23] |
DAN G, BIN T, SI L, et al. Simultaneous separation, quantitation, and determination of the dissociation constant of five components of Ixerissonchifolia by microemulsionelectrokinetic chromatography[J]. Curr Pharm Anal, 2016, 12(1): 55. |
| [24] |
LIN X, WANG YF, SUN JY, et al. Determination of uric acid in human plasma and urine by microemulsionelectrokinetic chromatography[J]. Anal Methods, 2013, 5(19): 5201. DOI:10.1039/c3ay40439k |
| [25] |
HU SQ, LUE W, M A, Y H, et al. Chiral separation of β-blockers by MEEKC using neutral microemulsion: Analysis of separation mechanism and further elucidation of resolution equation[J]. Electrophoresis, 2013, 34(2): 260. DOI:10.1002/elps.v34.2 |
| [26] |
CAO W, HU SS, LI XY, et al. Highly sensitive analysis of flavonoids by zwitterionicmicroemulsionelectrokinetic chromatography coupled with light-emitting diode-induced fluorescence detection[J]. J Chromatogr A, 2014, 1358: 277. DOI:10.1016/j.chroma.2014.06.081 |
| [27] |
CAO J, DUN WL. Separation and sweeping of flavonoids by microemulsionelectrokinetic chromatography using mixed anionic and cationic surfactants[J]. Talanta, 2011, 84(1): 155. DOI:10.1016/j.talanta.2010.12.037 |
| [28] |
CAO J, DUN WL, QU HB. Evaluation of the addition of various surfactant-suspended carbon nanotubes in MEEKC with an situ-synthesized surfactant system[J]. Electrophoresis, 2011, 32(3-4): 408. DOI:10.1002/elps.v32.3/4 |
| [29] |
HELI S, ANNE K. Microemulsionelectrokinetic chromatographic analysis of some polar compounds[J]. J Chromatogr B, 2003, 783(1): 113. DOI:10.1016/S1570-0232(02)00519-6 |
| [30] |
PUIG P, BORRULL F, AGUIlA C, et al. Sample stacking for the analysis of penicillins by microemulsionelectrokinetic capillary chromatography[J]. J Chromatogr B, 2006, 831(1-2): 196. DOI:10.1016/j.jchromb.2005.12.004 |
| [31] |
CHANG CW, CHEN YC, LIU CY. Separation and on-line preconcentration of nonsteroidal anti-inflammatory drugs by microemulsionelectrokinetic chromatography[J]. Electrophoresis, 2015, 36(21-22): 2745. DOI:10.1002/elps.201500160 |
| [32] |
YU LS, XU XQ, HUANG L, et al. Separation and determination of flavonoids using microemulsion EKC with electrochemical detection[J]. Electrophoresis, 2008, 29(3): 726. DOI:10.1002/(ISSN)1522-2683 |
| [33] |
VANHOENACKER G, RONG H, DE KD, et al. Simultaneous analysis of hop acids and prenylated flavanones by microe-mulsionelectrokinetic chromatography with diode array detection[J]. J Biomed Chromatogr, 2000, 14(1): 34. DOI:10.1002/(ISSN)1099-0801 |
| [34] |
SZUCS R, VAN HE, SANDRA P. Micellar and microemuls-ionelectrokinetic chromatography of hop bitter acids[J]. J High Resolut Chromatogr, 1996, 19(4): 189. DOI:10.1002/(ISSN)1521-4168 |
| [35] |
赵有轩, 王敦青, 李新民, 等. 毛细管电泳在线富集技术在黄酮类化合物分析中的应用[J]. 化学试剂, 2016, 38(4): 323. ZHANG YX, WANG DQ, LI XM, et al. Applications of online enrichment techniques in capillary electrophoresis analysis of flavonoids[J]. Chem Reagents, 2016, 38(4): 323. |
| [36] |
MAHER-HADIR M, ALZOMAN-NOURAH Z, ALSHEHRI-MONA M, et al. Microemulsionelectrokinetic chromatography with polarity switching stacking mode for the determination of dexamethasone and dexamethasone sodium phosphate: application to pharmacokinetic studies in rabbit plasma[J]. Anal Methods, 2015, 7(7): 3260. DOI:10.1039/C5AY00368G |
| [37] |
MA YH, ZHANG HG, WANG WF, et al. Sensitive enantioanalysis of β-blockers via field-amplified sample injection combined with water removal in microemulsion electrokinetic chromatography[J]. Electrophoresis, 2014, 35(19): 2772. DOI:10.1002/elps.v35.19 |
| [38] |
HEFNAW M, AlOMAR M, JULKHUF S, et al. Novel, selective sample stacking microemulsionelectrokinetic capillary chromatography induced by reverse migrating pseudostationary phase for the determination of the new ultra-short acting hypnotic "HIE-124" in mice serum[J]. Anal Chim Acta, 2010, 673(2): 194. DOI:10.1016/j.aca.2010.05.030 |
| [39] |
DARJI V, BOYCE MARY C, BENNETT I, et al. Determination of food grade antioxidants using microemulsion electrokinetic chromatography[J]. Electrophoresis, 2010, 31(13): 2267. DOI:10.1002/elps.v31:13 |
| [40] |
ZHENG LH, ZHANG L, TONG P, et al. Highly sensitive transient isotachophoresis sample stacking coupling with capillary electrophoresis-amperometric detection for analysis of doping substances[J]. Talanta, 2010, 81(4-5): 1288. DOI:10.1016/j.talanta.2010.02.023 |
| [41] |
BUSNEL JM, LION N, GIRAULT HH. Electrokinetic supercharging for highly efficient peptide preconcentration in capillary zone electrophoresis[J]. Electrophoresis, 2008, 29(7): 1565. DOI:10.1002/(ISSN)1522-2683 |
| [42] |
CHAO HC, LIAO HW, KUO CH, et al. Using water plug-assisted analyte focusing by micelle collapse in combination with microemulsion electrokinetic chromatography for analyzing phthalate esters[J]. J Chromatogr A, 2016, 1445: 149. DOI:10.1016/j.chroma.2016.03.086 |
| [43] |
LI S, TANG B, GUO D, et al. Determination of evodiarutaecarpa alkaloids by microemulsion electrokinetic chromatography with cation-selective exhaustive injection and sweeping[J]. Chem Res Appl, 2015, 27(7): 1009. |
| [44] |
HUANG HY, LIN YR, HSIEH SH. Sample stacking for determination of aromatic acid impurities by microe-mulsion electrokinetic chromatography[J]. Anal Chim Acta, 2009, 632(1): 148. DOI:10.1016/j.aca.2008.10.047 |
| [45] |
LIN YT, LIU YW, CHENG YJ. Analyses of sulfonamide antibiotics by a successive anion-and cation-selective injection coupled to microemulsion electrokinetic chromatography[J]. Electrophoresis, 2010, 31(13): 2260. DOI:10.1002/elps.v31:13 |
| [46] |
CHANG CW, CHEN YC, LIU CY, et al. Separation and on-line preconcentration of nonsteroidal anti-inflammatory drugs by microemulsion electrokinetic chromatography[J]. Electrophoresis, 2015, 36(21-22): 2745. DOI:10.1002/elps.201500160 |
| [47] |
ZHANG HG, ZHOU L, CHEN XG. Improving sensitivity by large-volume sample stacking combined with sweeping without polarity switching by capillary electrophoresis coupled to photodiode array ultraviolet detection[J]. Electrophoresis, 2008, 29(7): 1556. DOI:10.1002/(ISSN)1522-2683 |
| [48] |
YU LS, LIN SY, SHA M. Simultaneous determination of effective components in GualouGuizhi granules using microemulsion electrokinetic chromatography coupled with large volume sample stacking[J]. Anal Methods, 2015, 7(22): 9489. DOI:10.1039/C5AY01686J |
| [49] |
ZHU JH, QI SD, ZHANG HG. Sample stacking and sweeping in microemulsion electrokinetic chromatography under pH-suppressed electroosmotic flow[J]. J Chromatogr A, 2008, 1192(2): 319. DOI:10.1016/j.chroma.2008.04.005 |
| [50] |
LI JH, CAI ZW. Stacking and separation of urinary porphyrins in capillary electrophoresis: Optimization of concentration efficiency and resolution[J]. Talanta, 2008, 77(1): 331. DOI:10.1016/j.talanta.2008.06.033 |
| [51] |
YU LS, XU XQ, HUANG L, et al. Separation and detection of isoquinoline alkaloids using MEEKC coupled with field-amplified sample injection induced by ACN[J]. Electrophoresis, 2009, 30(4): 661. DOI:10.1002/elps.v30:4 |
| [52] |
LI M, JIN MM, LI J, et al. Analysis of corticosteroids in cosmetics by reversed microemulsion electrokinetic chromatography with BMIMBF4 ionic liquid and β-cyclodextrin[J]. Chin J Anal Lab, 2012, 31(2): 75. |
| [53] |
ABROMEIT H, WERZ O, SCRIBA, G KE. Separation of 5-lipoxygenase metabolites using cyclodextrin-modified microemulsion electrokinetic chromatography and head column field-amplified sample stacking[J]. Chromatographia, 2013, 76(17-18): 1187. DOI:10.1007/s10337-013-2517-4 |
| [54] |
郭成方, 商少明, 刘俊康, 等. β-环糊精修饰微乳毛细管电动色谱场放大-扫集法测定化妆品中的糖皮质激素[J]. 应用化工, 2016, 45(6): 1058. GUO CF, SHANG SM, LIU JK, et al. Determination of glucocorticoids in cosmetics by β-CD modified microemulsionelectrokinetic chromatography with filed amplified-sweeping method[J]. J Appl Chem, 2016, 45(6): 1058. |
| [55] |
ORLANDINI S, PASQUINI B, CAPRINI C, et al. A comprehensive strategy in the development of a cyclodextrin-modified microemulsionelectrokinetic chromatographic method for the assay of diclofenac and its impurities: Mixture-process variable experiments and quality by design[J]. J Chromatogr A, 2016, 1466: 189. DOI:10.1016/j.chroma.2016.09.013 |
| [56] |
CAO J, QU HB, CHENG YY. Separation of flavonoids and phenolic acids in complex natural products by microemulsionelectrokinetic chromatography using surfactant-coated and carboxylic single-wall carbon nanotubes as additives[J]. Electrophoresis, 2010, 31(10): 1689. DOI:10.1002/elps.v31:10 |
| [57] |
CAO J, LI P, CHEN J. Enhanced separation of compound Xueshuantong capsules using functionalized carbon nanotubes with cationic surfactant solutions in MEEKC[J]. Electrophoresis, 2013, 34(2): 324. DOI:10.1002/elps.v34.2 |
| [58] |
ZHANG HG, TIAN K, CHEN XG, et al. Analysis of baicalein, baicalin and wogonin in Scutellariae Radix and its preparation by microemulsionelectrokinetic chromatography with 1-butyl-3-methylimizolium tetrafluoborate ionic liquid as additives[J]. J Chromatogr A, 2006, 1129(2): 304. DOI:10.1016/j.chroma.2006.08.016 |
| [59] |
WANG ZH, LUO GA, CHEN JF, et al. Carbon nanotubes as separation carrier in capillary electrophoresis[J]. Electrophoresis, 2003, 24(24): 4181. DOI:10.1002/(ISSN)1522-2683 |
| [60] |
XIONG X, OUYANG J, BAEYENS WRG, et al. Enhanced separation of purine and pyrimidine bases using carboxylic multiwalled carbon nanotubes as additive in capillary zone electrophoresis[J]. Electrophoresis, 2006, 27(16): 3243. DOI:10.1002/(ISSN)1522-2683 |
| [61] |
SUAREZ B, SIMONT BM, CARDENAS S, et al. Surfactant-coated single-walled carbon nanotubes as a novel pseudostationary phase in capillary EKC[J]. Electrophoresis, 2007, 28(11): 1714. DOI:10.1002/(ISSN)1522-2683 |
| [62] |
MOLINER-MARTINEZ Y, BARRIOS M, CARDENAS S, et al. Comparative study of carbon nano tubes and C60 fullerenes as pseudostationary phases in electrokinetic chromatography[J]. J Chromatogr A, 2008, 1194(1): 128. DOI:10.1016/j.chroma.2008.04.034 |
| [63] |
MOLINER-MARTINEZ Y, CARDENAS S, VALCARCEL M. Surfactant coated fullerenes C60 as pseudostationary phase in electrokinetic chromatography[J]. J Chromatogr A, 2007, 1167(2): 210. DOI:10.1016/j.chroma.2007.07.076 |
| [64] |
CAO J, LI P, YI L. Ionic liquids coated multi-walled carbon nanotubes as a novel pseudostationary phase in electrokinetic chromatography[J]. J Chromatogr A, 2011, 1218(52): 9428. DOI:10.1016/j.chroma.2011.11.013 |
| [65] |
MOLINER-MARTINEZ Y, CARDENAS S, VALCARCEL M, et al. Evaluation of carbon nanostructures as chiral selectors for direct enantiomeric separation of ephedrines by EKC[J]. Electrophoresis, 2007, 28(15): 2573. DOI:10.1002/(ISSN)1522-2683 |
| [66] |
NA N, HU YP, JIN OY, et al. On the use of dispersed nanoparticles modified with single layer β-cyclodextrin as chiral selector to enhance enantio separation of clenbuterol with capillary electrophoresis[J]. Talanta, 2006, 69(4): 866. DOI:10.1016/j.talanta.2005.11.022 |
| [67] |
MATON C, VOS ND, STEVENS CV. Ionic liquid thermal stabilities: decomposition mechanisms and analysis tools[J]. Chem Soc Rev, 2013, 42(13): 5963. DOI:10.1039/c3cs60071h |
| [68] |
HUNT PA, ASHWORTH CR, MATTHEWS RP. Hydrogen bonding in ionic liquids[J]. Chem Soc Rev, 2015, 44(5): 1257. DOI:10.1039/C4CS00278D |
| [69] |
EARLE MJ, ESPERANC JMSS, GILEA MA, et al. The distillation and volatility of ionic liquids[J]. Nature, 2006, 439(16): 831. |
| [70] |
LI F, YANG FQ, XIA ZN. Simultaneous determination of ten nucleosides and related compounds by MEEKC with[BMIM]PF6 as oil phase[J]. Chromatographia, 2013, 76(15-16): 1003. DOI:10.1007/s10337-013-2507-6 |
| [71] |
LI F, LIU R, YANG FQ, et al. Determination of three curcuminoids in Curcuma longa by microemulsionelectrokinetic chromatography with protective effects on the analytes[J]. Anal Methods, 2014, 6(8): 2566. DOI:10.1039/C3AY42106F |
| [72] |
CAO J, QU HB, CHENG YY. The use of novel ionic liquid-in-water microemulsion without the addition of organic solvents in a capillary electrophoretic system[J]. Electrophoresis, 2010, 31(20): 3492. DOI:10.1002/elps.201000168 |
| [73] |
LU HH, AN XQ, SHEN WG. Critical behavior of a microemulsion with an ionic liquid[J]. J Phys Chem B, 2011, 115(51): 15251. DOI:10.1021/jp2018814 |
| [74] |
QIU ZM, TEXTER J. Ionic liquids in microemulsions[J]. Curr Opin Colloid Interface Sci, 2008, 13(4): 252. DOI:10.1016/j.cocis.2007.10.005 |
| [75] |
WANG Y, LI F, YANG FQ, et al. Simultaneous determination of α-, β-and γ-asarone in Acorustatarinowii by microe-mulsionelectrokinetic chromatography with[BMIM]PF6 as oil phase[J]. Talanta, 2012, 101: 510. DOI:10.1016/j.talanta.2012.10.015 |
| [76] |
TIAN K, QI SD, CHENG YQ, et al. Separation and determination of lignans from seeds of Schisandra species by micellar electrokinetic capillary chromatography using ionic liquid as modifier[J]. J Chromatogr A, 2005, 1078(1-2): 181. DOI:10.1016/j.chroma.2005.05.018 |
| [77] |
YUE ME, SHI YP. Application of 1-alkyl-3-methylimidazolium-based ionic liquids in separation of bioactive flavonoids by capillary zone electrophoresis[J]. J Sep Sci, 2006, 29(2): 272. DOI:10.1002/(ISSN)1615-9314 |
| [78] |
YUE ME, XU J, LI QQ, et al. Analysis of bioactive flavonoid-o-glycosides in Saussurea mongolica Franch. by capillary zone electrophoresis using ionic liquids as the additive[J]. J Food Drug Anal, 2011, 19(2): 146. |
| [79] |
TIAN K, WANG YS, CHE YL, et al. Application of 1-alkyl-3-methylimidazolium-based ionic liquids as background electrolyte in capillary zone electrophoresis for the simultaneous determination of five anthraquinones in Rhubarb[J]. Talanta, 2007, 72(2): 587. DOI:10.1016/j.talanta.2006.11.027 |
| [80] |
QI SD, LI YQ, HU ZD, et al. Simultaneous determination of bioactive flavone derivatives in Chinese herb extraction by capillary electrophoresis used different electrolyte systems-borate and ionic liquids[J]. J Chromatogr A, 2006, 1109(2): 300. DOI:10.1016/j.chroma.2006.01.045 |
| [81] |
王月伶, 胡中波, 袁倬斌, 等. 离子液体修饰毛细管胶束电动色谱法分离测定槲皮素、绿原酸和异槲皮苷[J]. 分析化学, 2006, 34(12): 1741. WANG YL, HU ZB, YUAN ZB. Ionic liquid modified micellar electrokinetic capillary chromatography determination of quercetin, chlorogenic acid and isoquercitrin[J]. Chin J Anal Chem, 2006, 34(12): 1741. DOI:10.3321/j.issn:0253-3820.2006.12.017 |
| [82] |
LU YQ, JIA CX, YAO QQ, et al. Analysis of flavonoids by non-aqueous capillary electrophoresis with 1-ethyl-3-methylimidazolium ionic-liquids as background electrolytes[J]. J Chromatogr A, 2013, 1319: 160. DOI:10.1016/j.chroma.2013.10.014 |
| [83] |
BYTZEK AK, REITHOFER MR, GALANSKI M, et al. The first example of MEEKC-ICP-MS coupling and its application for the analysis of anticancer platinum complexes[J]. Electrophoresis, 2010, 31(7): 1144. DOI:10.1002/elps.v31:7 |
| [84] |
HENCHOZ Y, ROMAND S, SCHAPPLER J, et al. High-throughput logP determination by MEEKC coupled with UV and MS detections[J]. Electrophoresis, 2010, 31(5): 952. DOI:10.1002/elps.v31:5 |
| [85] |
MOHORIC U, BEUTNER A, KRICKL S, et al. Surfactant-free microemulsionelectrokinetic chromatography(SF-MEEKC)with UV and MS detection -a novel approach for the separation and ESI-MS detection of neutral compounds[J]. Anal Bio Chem, 2016, 408(30): 8681. DOI:10.1007/s00216-016-0057-0 |
 2017, Vol. 37
2017, Vol. 37 
