2. 中国地质大学(北京)地球科学与资源学院, 北京 100083;
3. 东华理工大学核资源与环境国家重点实验室, 南昌 330013;
4. 西南石油大学地球科学与技术学院, 成都 610550;
5. 北京科技大学土木与资源工程学院, 北京 100083;
6. 内蒙古自治区第十地质矿产勘查开发院(有限责任公司), 赤峰 024005
2. School of Earth Sciences, China University of Geosciences(Beijing), Beijing 100083, China;
3. State Key Laboratory of Nuclear Resource and Environment, East China University of Technology, Nanchang 330013, China;
4. School of Geoscience and Technology, Southwest Petroleum University, Chengdu 610550, China;
5. Civil and Resource Engineering School, University of Science and Technology Beijing, Beijing 100083, China;
6. No. 10 Institute of Geological Exploration, Inner Mongolia Bureau of Geology and Mineral Resources, Chifeng 024005, China
As、Sb、Bi、Hg、Pb、Se、Te、Tl、Sn等元素具有亲铜性、低熔点的特点,可在低至300℃的温度下以熔体形式存在(Frost et al., 2002;Ciobanu et al., 2006;Tooth et al., 2008, 2011),并优先从流体中分离出来。因而,人们将这些元素统称为低熔点亲铜元素(LMCE)。这些低熔点亲铜元素普遍为半金属元素或具有半金属特性(Iida et al., 1975),甚至与其相关的一些化合物(如HgTe、HgSe等)也具有半金属特性。它们可与贵金属(Au、Ag、Pd、Pd等)络合形成独立矿物(Cabri, 2002),如黑铋金矿(Au2Bi)、碲金矿(AuTe2)、碲金银矿(Ag3AuTe2)、硫砷银矿(Ag2AsS2)、汞银矿(Ag11Hg2)、硒银矿(Ag2Se)、硫银铋矿(AgBiS2)、锑银矿(Ag3Sb)、砷铂矿(PtAs2)、铋钯矿(PdBi2)、碲铂矿(PtTe2)、碲铋钯矿(PdBiTe)、硒铋钯矿(PdBiSe)、碲钯矿(PdTe2)等。
Tomkins and Mavrogenes (2002)在研究南澳大利亚太古代Challenger金矿床时发现了硫化物-金-铋-黑铋金矿“液滴”,指示存在LMCE熔体,并由此认为该金矿床中95%以上的金是通过这些“液滴”在迁移过程中聚集而来的。Frost et al. (2002)在研究澳大利亚Broken Hill矿床时,也证实了在某些形成温度较低(可低至400℃以下)的矿床中存在金属熔体相。因此,LMCE熔体理论上可以在相当低的温度下形成,如铋-金熔体的熔点可低至241℃ (Okamoto and Massalski, 1983a)。
LMCE熔体的粘度很低,如Bi、Te、Pb熔体的粘度均为~10-3Pa·s (Flinn et al., 1974;Li et al., 2005),与纯水粘度相当(300℃时为10-4Pa·s),在构造应力或重力作用下极易发生迁移(Tomkins et al., 2007)。已有大量研究成果显示,LMCE熔体具有强烈吸收贵金属的能力(Douglas et al., 2000;Frost et al., 2002, 2011;Tomkins et al., 2007;Wagner, 2007;Tooth et al., 2008, 2011;Holwell and McDonald, 2010;Biagioni et al., 2013;Mavrogenes et al., 2013;Holwell et al., 2019),是形成许多矿床的重要成矿介质。
目前已知Bi熔体对Au、PGE的富集作用在许多矿床中均有发现和研究。重要的矿床类型有:(1)基性-超基性岩型Ni-Ci-PGE硫化物矿床。如加拿大Creighton矿床(Dare et al., 2010),俄罗斯Noril'sk-Talnakh矿床(Mansur et al., 2020),西班牙Aguablanca矿床(Piña et al., 2012)。(2)碱性-偏碱性侵入岩型Au矿床。如美国阿拉斯加和加拿大育空地区Tintina成矿带中Pogo、Fort Knox、Dublin Gulch矿床(McCoy, 2000;Cave et al., 2019),我国河北东坪、大白阳矿床(Gao et al., 2015; Wang et al., 2019, 2021)。(3)斑岩型Au矿床。如希腊Skouries Cu-Au-(Te-Pd)矿床(McFall et al., 2018;Holwell et al., 2019)。(4)矽卡岩型Au矿床。如我国云南北衙和姚安矿床(Zhou et al., 2017, 2018),澳大利亚Stormont矿床(Cockerton and Tomkins, 2012),津巴布韦Viceroy矿床(Oberthür and Weiser, 2008)和西班牙Río Narcea金矿带(Cepedal et al., 2006)。(5)浅成低温热液型Au-Ag矿床。如我国黑龙江三道湾子矿床(Zhai et al., 2018; Gao et al., 2021; ),罗马尼亚Larga矿床(Cook and Ciobanu, 2004)。(6)造山型Au矿床。如乌克兰Maiskoe矿床(Nechaev and Bondarenko, 1997),津巴布韦Viceroy矿床(Oberthür and Weiser, 2008),罗马尼亚Soimus Ilii矿床(Ciobanu et al., 2006)和澳大利亚Challenger矿床(Tomkins and Mavrogenes, 2002)。(7) IOCG型Au矿床。如加拿大NICO Au-Co-Bi矿床(Acosta-Góngora et al., 2015)。(8) VMS型富Au矿床。如美国Gorda洋脊Escanaba海槽块状硫化物矿床(Törmänen and Koski, 2005;Tooth et al., 2008)。
作者在研究我国河北东坪、河南大湖和金渠等Au矿床时,都观察到了自然金与Au-Ag-Bi的碲化物、铅铋硫盐等矿物共生的现象,显示成矿时可能存在自然铋、Bi的碲化物、Bi-Te-S等熔体不断抽提Au导致Au的富集(刘家军等, 2020)。因此,LMCE熔体在斑岩型矿床、矽卡岩型矿床、VMS型矿床、IOCG型矿床、造山型矿床、浅成低温热液型矿床以及岩浆Cu-Ni硫化物型矿床等均常出现(Ciobanu et al., 2010),这可能是导致这些不同类型矿床中富集Au(-Ag-PGE)的重要原因。
Se、As、Sb、Hg、Pb、Tl、Sn与Te、Bi等均为低熔点亲铜元素,也都具有半金属的特性。这些元素是否与Te、Bi一样在流体中可以形成单一或多元“液相”熔体而对Au、Ag、PGE等金属起到富集作用?即Se、As、Sb、Hg、Pb等对金属成矿是否起到一种重要的“桥梁”作用?虽然LMCE熔体与贵金属成矿关系的研究已有很多,但主要集中在地质现象描述、矿物组成特征及矿床成因等方面,少量关注实验模拟和分配系数计算。对于指示LMCE熔体存在的矿物组成与结构标志、LMCE熔体中各元素的存在形式、LMCE熔体吸收Au、Ag、PGE贵金属的机理等问题的研究程度还相当薄弱。本文通过系统梳理LMCE与贵金属成矿关系的研究现状,结合具体矿床实例,对上述问题进行初步讨论,以飨读者。
1 LMCE熔体的基本化学性质 1.1 二元体系熔体二元体系是指熔体中仅存在两种元素的理想体系,具有一个最低共熔点(图 1、图 2),表 1列出各LMCE与Au、Ag、Pd和Pt间二元体系的主要共熔温度及贵金属的含量。
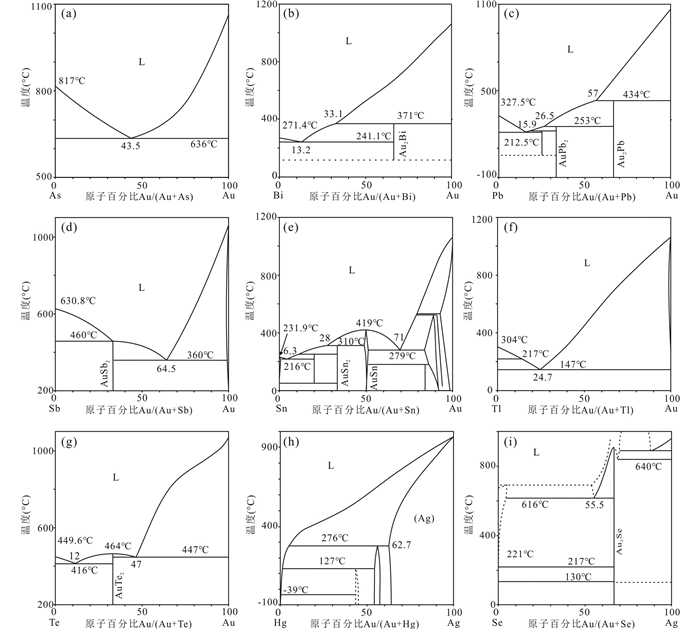
|
图 1 LMCE与Au、Ag的二元相图 (a) As-Au二元相图(Okamoto and Massalski, 1984a);(b) Bi-Au二元相图(Okamoto and Massalski, 1983a);(c) Pb-Au二元相图(Okamoto, 1993);(d) Sb-Au二元相图(Okamoto and Massalski, 1984d);(e) Sn-Au二元相图(Okamoto and Massalski, 1984b);(f) Tl-Au二元相图(Okamoto and Massalski, 1983b);(g) Te-Au二元相图(Okamoto and Massalski, 1984c);(h) Hg-Ag二元相图(Baren, 1996);(i) Se-Ag二元相图(Rajkumar and Chen, 2018) Fig. 1 Binary phase diagrams of LMCE vs. Au or Ag (a) the As-Au phase diagram (Okamoto and Massalski, 1984a); (b) the Bi-Au phase diagram (Okamoto and Massalski, 1983a); (c) the Pb-Au phase diagram (Okamoto, 1993); (d) the Sb-Au phase diagram (Okamoto and Massalski, 1984d); (e) the Sn-Au phase diagram (Okamoto and Massalski, 1984b); (f) the Tl-Au phase diagram (Okamoto and Massalski, 1983b); (g) the Te-Au phase diagram (Okamoto and Massalski, 1984c); (h) the Hg-Au phase diagram (Baren, 1996); (i) the Se-Ag phase diagram (Rajkumar and Chen, 2018) |
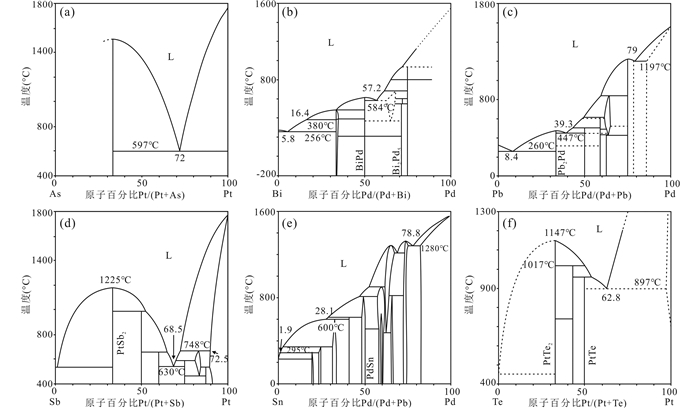
|
图 2 LMCE与Pt、Pd的二元相图 (a) As-Pt二元相图(Okamoto, 1990a);(b) Bi-Pd二元相图(Okamoto, 1994a);(c) Pb-Pd二元相图(Vassiliev et al., 1998);(d) Sb-Pt二元相图(Okamoto, 1992c);(e) Sn-Pd二元相图(Okamoto, 2012);(f) Te-Pt二元相图(Okamoto, 1994b) Fig. 2 Binary phase diagrams of LMCE vs. Pt or Pd (a) the As-Pt phase diagram (Okamoto, 1990a); (b) the Bi-Pd phase diagram (Okamoto, 1994a); (c) the Pb-Pd phase diagram (Vassiliev et al., 1998); (d) the Sb-Pt phase diagram (Okamoto, 1992c); (e) the Sn-Pd phase diagram (Okamoto, 2012); (f) the Te-Pt phase diagram (Okamoto, 1994b) |
|
|
表 1 LMCE与贵金属二元体系的共熔温度及原子分数占比 Table 1 Eutectic temperatures and atomic ratios of the LMCE and noble metals binary system |
As的熔点为817℃ (图 1a),与Au、Ag、Pd、Pt的共熔温度较高。As-Au最低共熔温度为636℃,Au含量43.5at% (图 1a),As-Pt最低共熔温度为597℃,Pt含量72at% (图 2a)。热液条件下As熔体难以形成,但岩浆条件下很容易,并且能够携带大量的贵金属,对岩浆体系中贵金属的成矿具有意义(Piña et al., 2013)。
Bi的熔点为271.4℃ (图 1b),Bi-Au最低共熔温度241.1℃,Au含量13.2at%,温度升高至371℃时Au的含量可达33.1at% (图 1b)。Bi-Ag的共熔温度为262.5℃,但Ag的含量仅为4.7at%,Bi-Pd、Bi-Pt最低共熔点处Pd、Pt的含量也较低,随温度增加Pd、Pt含量增加(图 2b),分别可达57.2at% (584℃)和57at% (730℃)。
Pb的熔点为327.5℃ (图 1c),Pb-Au最低共熔温度212.5℃,Au含量15.9at%,温度为253℃时Au含量为26.5at%,温度升高至434℃时Au含量可达43at% (图 1c)。Pb-Pd最低共熔温度260℃,Pd含量8.4at%,温度升高至447℃时Pd含量达39.3at% (图 2c)。Pb熔体的湿法冶金已存在1000多年,元素提取率>99% (Chekushin et al., 2008),但对环境污染严重。
Sb的熔点为630.8℃ (图 1d),当存在64.6at% Au时,熔点降为360℃,存在59at% Ag时,熔点为485℃。Sb熔体在723℃时可存在58at% Pd,630℃时可存在68.5at% Pt,Pd、Pt含量的微小变化会使共熔温度快速升高(图 2d)。
Sn的熔点为231.9℃ (图 1e),Sn-Au最低共熔温度216℃,Au含量15.9at%,温度为310℃时Au含量为28at%,Au含量为71at%时,共熔温度降为279℃。Sn-Ag的最低共熔温度为221℃,Ag含量3.8at%,480℃时Ag含量可达50.4at%。Sn熔体在600℃时可含28.1at% Pd (图 2e),539.6℃时含6.1at% Pt。
Tl的熔点为304℃ (图 1f),存在24.7at% Au时熔点降为147℃,存在2.6at% Ag时熔点为291℃,存在1.7at% Pd时熔点为293℃。538℃时,Tl熔体中最高可存在27at% Pd。
Te的熔点为449.6℃ (图 1g),但理想β-Te生长在300℃以下。Te-Au最低共熔温度416℃,Au含量12at%,温度447℃时Au含量达47at%。Te-Ag最低共熔温度353℃,Ag含量33.3at%。503℃时,Te熔体可含62.7at% Pd,Pd含量微小变化使共熔温度快速升高。Te-Pt共熔温度为897℃,Pt含量62.8at%,Te熔体是岩浆中Pt、Pd的重要迁移介质(Holwell et al., 2019)。
Hg的熔点为-39℃ (图 1h),418℃时Au含量可达80.2at%,276℃时Ag含量可达62.7at%。金矿选冶中的混汞法即利用Hg对Au-Ag的强烈吸收形成合金并随矿浆流走,通过蒸馏使汞挥发从而回收金(闫晓慧等, 2019)。该方法具有毒性大、污染环境等缺点,目前在我国应用较少。
Se的熔点为221℃ (图 1i),当加入Au或Ag时熔点急聚升高,到达一定温度后出现相分离,温度不再随Au或Ag含量的变化而变化。Se熔体760℃可含48at% Au,616℃时可含55.5at% Ag (图 1i)。
二元体系是理想体系,在地质环境中很少单独出现。一般情况下,当加入其它元素时二元体系的共熔温度降低(图 3)。如Pb-Ag、Sb-Au体系的熔点随Bi的加入降低(图 3a, b);Bi-Ag中加入Te时,由于Te的熔点高,体系熔点迅速上升,随后下降(图 3c);Au-Ag中加入Te,熔点逐渐降低,AgTe-AgAuTe2附近熔点最低(图 3d);体系中存在S时,As-Ag共熔温度降低为280℃,Sb-Ag共熔温度降低为450℃ (Roland, 1970)。
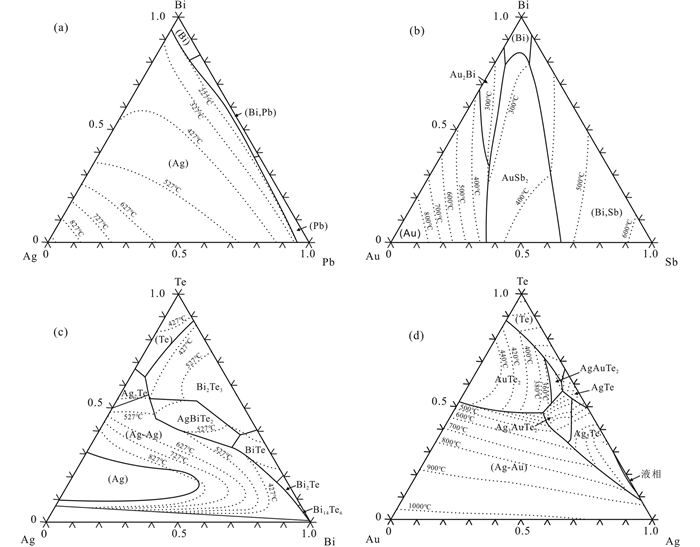
|
图 3 LMCE-Au-Ag三元液相线投影图 (a) Ag-Pb-Bi三元相线投影图(Lukas, 1980);(b) Au-Sb-Bi三元相线投影图(Wang et al., 2007);(c) Ag-Bi-Te三元相线投影图(Babanly et al., 2007);(d) Ag-Au-Te三元相线投影图(Markham, 1960) Fig. 3 Ternary phase diagram of LMCE-Au-Ag (a) liquidus projection of the Ag-Pb-Bi ternary system (Lukas, 1980); (b) liquidus projection of the Au-Sb-Bi ternary system (Wang et al., 2007); (c) liquidus projection of the Ag-Bi-Te ternary system (Babanly et al., 2007); (d) liquidus projection of the Ag-Au-Pb ternary system (Markham, 1960) |
与简单二元体系不同,多元(三元及以上)体系的熔化过程要复杂的多。由于多元体系包含多种元素,同时各元素原子间的体积差异大,在共熔温度附近相似原子会相互结合产生相分离,并形成复杂的硫化物和多金属矿物组合(Mavrogenes et al., 2013)。
PbS-Cu2S-Sb2S3体系的熔化过程中会出现两个熔体相:(1)元素简单的富Cu相;(2)元素复杂的富Sb相。在熔体固结后形成车轮矿(CuPbSbS3)和斜方辉锑铅矿(Pb13CuSb7S24) (Hoda and Chang, 1975a);Ag2S-Cu2S-Sb2S3体系在345℃初熔,到453℃时形成以9(Ag1.67Cu0.33)S·Sb2S3和9(Ag1.51Cu0.49)S·Sb2S3为端元的连续固溶体(Chen and Chang, 1974);Ag2S-Cu2S-Bi2S3熔体形成以AgBi3S5和CuBi3S5为端元的两相固溶体,固结后形成硫铜银矿(AgCuS)、辉铜银矿(Ag3CuS2)和直硫铜银矿(Ag5-xCu3+xS4) (Chen and Chang, 1974)。PbS-Ag2S-Sb2S3熔体中存在Ag2S-AgSbS2和AgSbS2-Sb2S3两个熔体相,固结后形成锑铅银矿(PbAgSb3S6)、辉锑银铅矿(Pb3Ag2Sb6S13)和杂辉锑银铅矿(Pb5Ag2Sb8S18) (Hoda and Chang, 1975b)。Ag-As-S (Roland, 1970)、Pb-Sb-S (Craig and Barton, 1973)和Fe-Sb-S (Barton, 1971)熔体分离出不混溶的硫盐熔体和贫硫的多金属熔体。Ag2S-PbS-Cu2S和PbS-Cu2S-Bi2S3熔体中均形成富Cu相到富Pb相的连续熔体,更复杂的Ag2S-PbS-Cu2S-Bi2S3四元熔体中可形成9类固溶体(Chang et al., 1988)。Govindarao et al. (2020)研究发现,500℃时Cu2S-PbS-Ag2S、Cu2S-PbS-Sb2S3和PbS-Sb2S3-Ag2S不能熔化,但Cu2S-PbS-Sb2S3-Ag2S可以熔化,表明多元体系中,元素种类越多,初始部分熔融温度就越低。Karup-Möller (1977)根据矿床矿物组合及包裹体研究,认为大多数Ag-(Cu)-Pb-Bi-S体系中硫盐矿物形成温度在200~400℃之间。Ag2S-Cu2S-PbS-Bi2S3四元体系在500℃时存在着丰富的类质同象取代与组分变化很大的固溶体,可能意味着该体系在低温时存在丰富的有序固溶体相(吴大清, 1987)。
在实际的成矿过程中,熔体的组成及固结产物要比实验复杂得多。Sinyakova et al. (2019)研究富LMCE的Cu-Fe-Ni-贵金属的超多元熔体结晶过程,发现温度降低时,熔体分离成两相,分别为(Pd, Au, Ag)-(Bi, Sb, Te)相和Cu-(S, Bi, Sb, Te)相。在前者相中贵金属含量高,后者相中以Cu为主。这些相固结形成四类包体:(1)自然金+Au-Cu-Pd矿物包体;(2)含Bi-Au的硫盐包体;(3)砷铂矿Pt(As, S)2包体;(4)由(1)、(2)、(3)类混合而成的包体。Acosta-Góngora et al. (2015)在NICO矿床中发现辉铋矿+自然金与自然金+自然铋的矿物组合共生,但辉铋矿的熔点高(775℃,Lin et al., 1996),不会在Bi-Au-S熔体中形成,因而辉铋矿+自然金可能由自然金+自然铋转化而来,如温度降低或硫逸度增高时,自然铋形成辉铋矿,导致Au或Au2Bi(黑铋金矿)从Bi熔体中结晶出来(Cockerton and Tomkins, 2012),黑铋金矿由于硫化反应分解(Ciobanu et al., 2010),形成辉铋矿+自然金的矿物组合。吴大清(1987)通过对Ag2S-Cu2S-PbS-Bi2S3体系内固溶体及铜、银、铅的铋硫盐矿物结晶化学研究表明,这些矿物相中存在的四种类型类质同象取代类型是:配对取代Ag(Cu)+Bi→2Pb,简单取代Cu→Ag和Cu→Bi(Pb),和铜原子填隙(以平衡Cu原子取代Bi或Pb时的电价差),这样形成的铋硫盐矿物存在4个系列:块硫铋银矿(9种)、硫铋铅矿(15种)、辉铋矿-针硫铋铅矿(9种)和贺硫铋铜矿(5种)。
除元素种类外,体系的熔融温度还受压力和流体的影响。如黄铁矿+方铅矿+闪锌矿的熔融温度随压力升高6℃/kbar (Mavrogenes et al., 2001),毒砂+黄铁矿的熔融温度随压力升高14℃/kbar (Sharp et al., 1985),Te、Se和As的熔化温度随压力的升高而升高,而Sb和Bi的熔化温度随压力的升高而降低(Liu and Bassett, 1986)。水直接或间接地影响体系的熔化温度,含水条件下FeS-PbS-ZnS的熔点降低35℃ (900℃降低到865℃,Wykes and Mavrogenes, 2005),Cl会降低Fe-Cu-Ni-S中单硫化物固溶体的熔点,结晶分异时Cl逐渐富集在熔体相中(Mungall and Brenan, 2003)。
2 LMCE熔体的形成环境根据形成环境与地质过程的差异,可把形成LMCE熔体的地质作用分为岩浆作用、热液作用和变质作用。
2.1 岩浆作用岩浆演化过程中会按结晶分异顺序形成不同的熔体相和硫化物相,其中单硫化物固溶体(MSS)最先分离出来,接着形成晚结晶的中间硫化物固溶体(ISS)(Naldrett, 2004),随着温度继续降低,硫化物熔体(SL)中分离出LMCE熔体(Mansur et al., 2020)。该过程已被许多研究和实验证实,如Fleet et al. (1993)发现岩浆中LMCE总量超过1wt%时,便可形成LMCE熔体并与MSS共存;Helmy et al. (2007)研究Fe-Cu-Ni-Pd-Pt-Te-S的分配过程和结晶温度,发现富Te熔体可以在1015~825℃时与高Te/S比的硫化物熔体分离,Te在MSS和ISS中的溶解度低(0.2wt%)且不受温度变化的影响,表明其与S发生了类质同像替换;Cafagna and Jugo (2016)研究Fe-Ni-Cu-Co-S的熔融实验,发现熔体结晶过程中先后形成自形黄铁矿、MSS、ISS和LMCE熔体,认为黄铁矿中的元素分带可由岩浆中硫化物熔体冷却形成,而不代表热液过程中的阶段性生长。
传统理论认为矿物结晶发生于熔体、流体达到过饱和或过冷却条件下。自然界硫化物熔体中的铂族元素(PGE)和LMCE(如As、Se、Sb、Te、Bi)含量一般在10-9~10-6范围内,仅在岩浆结晶分离晚期才能达到形成铂族元素矿物(PGM)的饱和浓度(Helmy et al., 2013)。在硫化物熔体固化过程中,因IPGE (Os、Ir、Ru)对早结晶的单硫化物固溶体(MSS)相容而富集在MSS及其出溶产物磁黄铁矿和镍黄铁矿中;但PPGE (Pt、Pd、Rh)及LMCE对MSS和ISS均不相容(Liu and Brenan, 2015),故其富集于残余硫化物熔体中,且在硫化物颗粒边缘结晶形成PGM(Godel et al., 2010)。然而,在加拿大Creighton、我国四川杨柳坪和甘肃金川Cu-Ni硫化物矿床中,均存在磁黄铁矿、镍黄铁矿和黄铜矿包含IPGE、PPGE的矿物(Song et al., 2008;Dare et al., 2010),说明PGM的形成早于或与MSS同时结晶,这明显悖于PGE地球化学行为及传统结晶理论。故矿床中PGM的形成并非简单地受过冷却和过饱和机制的控制。由于PGM主要为PGE和LMCE、S的化合物(Cabri, 2002),表明LMCE对PGM的结晶有着重要影响。
岩浆演化过程中形成的LMCE熔体有助于贵金属的富集,是岩浆硫化物矿床中富集PGE和岩浆Au矿床形成的重要成矿机制。
2.2 热液作用本节所指的热液包括岩浆热液、变质热液及加热大气降水等中高温的成矿流体。Douglas et al. (2000)进行的初步静态模拟实验发现,LMCE熔体可直接从流体中析出,且热液中的金被分配到了熔融的铋液滴中,据此首次提出了铋熔体可以从热液中提取金的模型。Tooth et al. (2008)使用水溶液-矿物-熔体系统的平衡热力学模型研究了铋熔体从热液中提取金的作用。热力学计算结果表明:在300~450℃的温度范围内,铋-金熔体中Au的浓度比热液中Au的浓度高几个数量级,任意温度下,液相铋熔体与金结合的能力远高于其他流体相(300℃时Au的溶解度可达20%; Okamoto and Massalski, 1983a)。Wagner (2007)通过热力学模拟计算发现,热液中可以形成金含量3%~5%的Bi-Te熔体和富Au的Te熔体,冷却后形成碲铋矿物+自然金和碲金矿物+自然金的矿物组合,说明Au-Bi-Te熔体可以有效从流体(即使是未饱和状态)中汲取金。Ciobanu et al. (2006)认为铋的碲化物熔体也有利于金的富集沉淀。Tooth et al. (2011)的界面耦合溶解-沉淀反应实验结果表明:在有溶解Bi的热水溶液中可直接析出Bi熔体,进而提取热水溶液中溶解的Au形成Bi-Au熔体。因此,即使热水溶液中贵金属浓度极低(例如金,即便在热液中处于欠饱和状态),在LMCE的帮助下,也可以导致贵金属的富集形成一定规模的矿床。
Meinert (2000)根据矽卡岩型Au-Cu矿床中大量发育Au-Bi-(Te)-(S)矿物组合特点认为,这些矿物本身的结晶温度远低于包裹它们的矽卡岩硅酸盐的结晶温度,这些矿物很可能呈熔体的形式存在于(岩浆)热液中。只要这种熔体能够保持液态,就会从热液中抽提Au而成为Au的“清道夫”。Te-Bi熔体能够强烈吸收Au,即使是Au在矿物晶格中也能被有效带出(Ciobanu et al., 2006)。McFall et al. (2018)推测Bi-Te熔体是希腊Skouries斑岩Cu-Au-(Te-Pd)矿床中PGE、Te共同富集的关键因素。Tooth et al. (2008)对300~450℃条件下Au-Bi-Na-Cl-S-H-O体系的计算模拟表明,熔体中Au的浓度比与其共存热液中的Au高几个数量级,即含Au熔体可能比非饱和热液的成矿贡献更大。因此,熔体从热液中提取Au的机制比饱和沉淀更为有效。“液态铋收集器模型”机制表明,以金属熔体形式存在的自然铋有助于某些金属矿床的形成(Guimarães et al., 2019)。
熔体如何从热液中产生?Tooth et al. (2011)认为热液中的Bi主要以+3价存在。当含有Bi3+的流体与还原剂(如石墨、磁黄铁矿)发生反应时,可将Bi3+还原为Bi熔体,反应方程式为:
Bi(OH)3(aq) = Bi(melt)+1.5H2O(aq)+3/4O2(aq)
(Bi2S2)2+(aq)+Reductant(s) → Bi(melt) (>271℃)
因此,在热液中产生的熔体铋通常呈乳滴状分布于还原剂(石墨、磁黄铁矿)的边缘,有利于进一步解释部分热液金矿床中自然铋与磁黄铁矿之间的密切联系(Törmänen and Koski, 2005;Wang et al., 2019)。
2.3 变质作用本节的变质作用主要针对的是那些矿床形成后由于区域变质或热变质对矿床进行改造的作用。由于变质过程中总是伴随着温度和压力的变化,当温度、压力达到一定条件时,LMCE会发生熔化。硫盐、碲化物及铋化物的熔化温度明显低于硫化物(表 2),在低变质温度下可形成LMCE熔体,黄铁矿、磁黄铁矿、闪锌矿、辉钼矿和黄铜矿以固体残留物形式存在,形成贫Fe、Zn、Mo等的LMCE熔体(Tomkins et al., 2007)。独立硫化物的熔化温度较高,但当多硫化物共存时,熔点会明显降低,如方铅矿的熔点为1114℃,PbS-Ag2S的熔点为605℃ (Urazov et al., 1983),FeS2-PbS的熔点为719℃ (Brett and Kullerud, 1967),CuFeS2-PbS-FeS-ZnS-S体系可在700~730℃时发生熔化(Stevens et al., 2005)。Sb、Bi、Ag、Tl等元素常通过2(Bi, Sb)3++□↔3Pb2+、(Ag, Cu, Tl)++(Bi, Sb)3+↔2Pb2+进入方铅矿晶格(George et al., 2015),富Sb-Bi-Ag-Tl的方铅矿会优先于纯净的方铅矿熔化(Mavrogenes et al., 2001)。
|
|
表 2 硫化物矿床中矿物及矿物组合熔点统计表 Table 2 The melting temperatures of minerals and mineral associations in sulfide ore deposits |
Mavrogenes et al. (2013)通过实验发现,在变质过程中可形成三类“硫化物”熔体:(1)硫化物熔体,结晶形成黄铁矿、磁黄铁矿、黄铜矿、方铅矿和闪锌矿;(2)硫盐熔体,Sb和As的含量高,结晶形成硫盐矿物或硫盐-硫化物的矿物集合体;(3) LMCE熔体,几乎不含S,主要由Sb、As、Bi和Te等组成,结晶形成LMCE的矿物。这三类熔体形成所需的变质条件不同:当变质为绿片岩相(400~500℃)到角闪石相(500~800℃)时硫盐和LMCE的矿物熔化形成熔体;当变质高于角闪岩相时含方铅矿的矿石熔化形成熔体;当达到麻粒岩相(700~900℃)时会形成两种熔体:(1)低温到中温的硫盐和LMCE熔体;(2)高温的硫化物熔体(Tomkins et al., 2007)。存在大量硫盐矿物矿床的变质程度达到绿片岩相至角闪石相时可形成硫盐熔体,而Pb-Zn-Cu矿床则在变质程度达到角闪岩相之上才形成硫化物熔体,但如果矿床中存在黄铁矿、毒砂,则形成熔体的温度可低于角闪石相(Tomkins et al., 2007)。
除LMCE矿物熔化形成熔体外,许多硫化物也可为LMCE熔体提供物质来源。如变质过程中黄铁矿的重结晶会释放As、Sb、Tl、Pb、Hg、Zn、Ag和Cu,促进LMCE熔体的形成(Biagioni et al., 2013, 2020;George et al., 2018);毒砂的重结晶会释放Au、Ag、Pb和Bi,可形成Pb-Bi-Au-Ag熔体(Cave et al., 2019);辉锑矿和毒砂在高硫逸度条件下会分解形成Sb-As熔体,促进元素的溶解迁移(Tomkins et al., 2004)。
3 LMCE熔体富集贵金属的机理LMCE包括As、Sb、Bi、Hg、Pb、Se、Te、Tl、Sn等元素,其熔体对贵金属的富集主要受温度、流体及成矿体系的氧逸度-组成成分等的影响(图 1、图 2),压力的影响小。温度是控制LMCE能否形成熔体的关键,如温度低于300℃时,Bi、Pb、Sn、Tl和Hg可形成熔体并富集贵金属,其余元素保持固体。高温下,Pb、Sn和Tl会与水发生反应,Sb、Bi、Hg、Te、As和Se不溶于水,因此热液流体中可以形成Sb、Bi、Hg、Te、As、Se熔体,而Pb、Sn、Tl以离子形式存在(曹锡章等, 1994)。成矿体系的氧逸度-组成成分会影响LMCE的分配系数(Li and Audétat, 2013, 2015;Zajacz et al., 2013)及熔体的形成,如高氧逸度时Bi、Te以离子形式存在,低氧逸度时形成Bi、Te熔体(Tooth et al., 2008, 2011; Grundler et al., 2013)。
3.1 岩浆作用中LMCE熔体吸收贵金属的机理岩浆中的Au、Ag、PGE和LMCE在不同熔体相和硫化物相之间进行分配(Kiseeva et al., 2017),导致元素的分异和富集。LMCE会系统性的富集在硫化物中,但分配系数差异明显,如DAuSL/SM=40~30000、DAgSL/SM=1252±1201、DBiSL/SM=663±576、DPbSL/SM=34±18、DAsSL/SM=2.4±7.6,整体变化顺序为PGE>Au≥Te>Cu≈Ag>Se≥S≈Re (Li and Audétat, 2013, 2015;Zajacz et al., 2013;Mungall and Brenan, 2014;Jégo et al., 2016)。Os-Ir-Ru富集在早结晶的MSS中,而LMCE、Pd、Pt、Au等与MSS及ISS不相容,会聚集到晚期的残余硫化物熔体中(Liu and Brenan, 2015;Mansur et al., 2020)。
相比于硫化物熔体,Pt-Pd-Au更相容于LMCE熔体。如富As熔体-硫化物熔体中,DPtAs/SL=330、DPdAs/SL=250、DAuAs/SL=310、DAgAs/SL=4、DSbAs/SL=890、DTeAs/SL=190、DBiAs/SL=50、DAgAs/SL=4、DSeAs/SL=0.6 (Piña et al., 2013),表明PGE、Au、Bi、Te和Sb与富As熔体具有强相容性,富As熔体是PGE和其他亲硫元素的重要载体(Makovicky et al., 1990);Helmy et al. (2007)实验表明高温下LMCE熔体与硫化物熔体发生相分离,形成的As-Sb-Bi-Te熔体中富集大量Pd和Pt,降温过程中砷化物和硫砷化物首先结晶,形成富Pd-Pt的Sb-Bi-Te熔体,结晶后形成Pt-Pd-Bi-Te矿物(Holwell and McDonald, 2010)。
金、碲易富集在碱性岩浆中已获得广泛共识。金主要以Au-S形式溶解在硅酸盐熔体中,在中等氧化条件下的含水岩浆系统环境下,Au与硫络合进入液相,碱金属和氯的存在可以促进该过程的发生(Zajacz et al., 2012)。Te相对于其他稀散元素较相容,在部分熔融过程中易富集在地幔中,并且洋壳中的铁锰结壳、页岩及浮游沉积物等是碲的重要储库(Cohen, 1984; Hein et al., 2003)。与碲、金成矿有关的碱性岩多形成于洋陆俯冲后的伸展环境(Jensen and Barton, 2000),大陆岩石圈地幔和洋壳的部分熔融形成低硫、高氧逸度、高挥发份的碱性岩浆(Li and Audétat, 2013; Müller and Groves, 2016)。低硫和高氧逸度可以有效抑制硫化物的结晶沉淀(Richards, 1995),促进金和碲有效地从岩浆向流体转移(Li et al., 2019),高挥发份使碲、金更易以气相进行迁移,从而在近地表富集沉淀(赵振华等, 2002),因此碲、金的成矿潜力大。
Holwell et al. (2019)针对碱性岩浆生成到侵位过程中Cu、Te、Au、Pd和Pt的富集行为进行了系统总结:(1)俯冲环境下,大陆岩石圈地幔和洋壳发生部分熔融,低熔点的富铜硫化物和Au-Pt-Pd-Te矿物优先熔化,形成富含不相容元素和贵金属的碱性岩浆;(2)岩浆到达下地壳,结晶分异形成富Co、Ni的硅酸盐矿物(橄榄石),Cu、Te、Au、Pd和Pt存在硫化物熔体中(Mavrogenes and O'Neill, 1999),未发生沉淀,并随岩浆继续向上运移;(3)岩浆到达中地壳,压力降低使硫化物在硅酸盐熔体中的溶解度增高,部分Cu、Au、Te重新进入硅酸盐熔体,而其他组份保留在硫化物熔体,这是Cu、Au能够继续向地表迁移的重要机制;(4)当温度低于900℃时,硫化物熔体与Te熔体发生相分离(图 2f,Helmy et al., 2007),Pd和Pt高度集中在Te熔体中;(5)岩浆到达上地壳,侵位结晶形成斑岩Cu-Au-Te矿床,Pd-Pt-Te熔体结晶形成贵金属矿物,岩浆排气/流体出溶使Te、Au进入气相/流体相,沿断裂继续向上迁移,形成浅成低温热液Au-Te矿床。
因此,碱性岩的幔源性、高挥发性及所形成流体的高氧逸度、中等偏碱性等特点,为Te、Au的活化、运移提供了良好的条件,从而解释了Te、Au与碱性岩浆间的成因联系(Müller and Groves, 2016; Sillitoe, 2002)。
3.2 热液作用中LMCE熔体富集贵金属的机理热液条件下,Sb、Bi、Hg、Te、As、Se不易与水发生反应,可以形成熔体。实验和计算表明LMCE熔体可强烈吸收流体中的Au (Douglas et al., 2000;Tooth et al., 2008, 2011),如Au在Bi熔体和流体中的分配系数为DAuBi/fluid=2.26×108 (450℃,Tooth et al., 2008),因此LMCE熔体对热液中Au的吸收富集是热液型金矿床重要的成矿方式之一。
LMCE熔体在流体中吸收贵金属的过程类似于液相萃取,是利用贵金属在LMCE熔体和流体中的溶解度不同而实现的(Harwood et al., 1989)。当贵金属以原子态存在时,萃取属于物理溶解过程,以离子存在时,萃取过程存在化学反应。LMCE熔体中的原子主要以共价键和金属键连接,如Sb熔体中均为金属键,Bi熔体中存在金属键和共价键,当温度升高时共价键被破坏转变为金属键,在310℃时全部为金属键连接(Geng et al., 2007),因此LMCE熔体属于非极性溶剂。由于水属于极性溶剂,故LMCE熔体与水发生不混溶(Chekushin et al., 2008)。Palomba and Carotenuto (2016)发现AuCl可高度溶解在非极性的熔体中,并在一定温度时发生分解,形成金原子(零价Au)和Cl-。因此流体中的Au倾向进入LMCE熔体,Au的含量取决于压力、温度、Eh、pH和阴离子的浓度和类型(Tooth et al., 2008)。
在Au-Bi-Te三元体系中,富Te和富Bi端元的温度分别为475~383℃和235~266℃。以Bi为主的熔体主要在还原环境中存在,Bi3+是唯一氧化态,而富Te熔体可以以还原形式存在,也可以由氧化作用形成(Tooth et al., 2011)。当温度高于271℃(自然铋的熔点温度),中等氧逸度、低含量硫的流体中可以分离出液态Bi熔体,并不断汲取流体中的Au (Tooth et al., 2011)。同时,由于铋的碲化物熔体有利于金的富集,因此当流体物理化学条件突然变化(如硫化反应、氧化还原反应等),铋的碲化物熔体就会从流体中分离而发生沉淀,从而导致Au的富集(Ciobanu et al., 2006)。
Zhou et al. (2017)认为北衙金矿床中氧逸度的变化促使流体中的Bi在+3到0价之间不断波动,高氧逸度时Bi以Bi(OH)3存在热液中并形成赤铁矿,低氧逸度时Bi以熔体存在并形成磁铁矿,Bi熔体吸收了Au在磁铁矿表面及孔隙中沉淀形成纳米级至微米级的Au-Bi矿物微粒。在熔体-流体共存条件下,由于热液本身就具有极强的流动性,熔体不需要运移便可以从流体中高效“捕获”金(Tooth et al., 2011)。同时,鉴于金在LMCE熔体与热液间的分配系数差异巨大,只需极少量熔体便可以将热液中金的富集起来,形成高品位的金矿床。
3.3 变质作用中LMCE熔体富集贵金属的机理变质作用中形成的流体相对较少,大多为高温下矿物的直接熔融。金矿床中存在大量硫盐矿物、碲化物、铋化物及汞化物,大量矿物可在低温变质条件下熔化形成富Au贫Fe、Zn、Mo等的LMCE熔体。变质过程中毒砂可与黄铁矿反应形成As-S熔体和磁黄铁矿(Sharp et al., 1985),反应式为:FeS2+FeAsS = As-Smelt+FeS (491℃),因此在许多金矿床中也会形成富Au的As-S熔体。当矿床中只存在黄铁矿、磁黄铁矿和毒砂时(如许多造山型金矿床),很难在低温变质过程中形成LMCE熔体,虽然毒砂+黄铁矿可以形成As-S熔体,但该反应需要高硫逸度和中等氧逸度(Tomkins et al., 2006);如果矿床中的黄铁矿含量低或存在大量还原性矿物(如石墨围岩或条带状铁建造),毒砂不会形成As-S熔体,需要到~770℃时发生3FeAsS (+Au) = (Au-)As-Smelt+FeAs2+2FeS反应(Sharp et al., 1985)形成Au-As-S熔体。同理,岩浆Cu-Ni-PGE矿床中存在大量砷化物及少量碲铋化物和硫盐矿物,包括毒砂(FeAsS)、辉砷镍矿(NiAsS)、辉钴矿(CoAsS)、红砷镍矿(NiAs)、斜方砷铁矿(FeAs2)等(Prichard et al., 2004),由于矿床中黄铁矿含量少(黄铁矿︰磁黄铁矿 < 1︰99),很难形成As-S熔体(Tomkins et al., 2007),仅其中的碲铋化物和硫盐矿物可形成少量LMCE熔体。
由于硫化物在矿床中的分布是不均匀的,因此变质过程中在矿床的不同部位形成LMCE熔体的规模和成分会存在很大差异。当LMCE熔体规模较小时,其迁移路径受围岩变形控制,迁移距离为几厘米到几米,并会在石英脉或围岩中形成LMCE-Au-Ag-PGE透镜体;当LMCE熔体规模较大时,其会在重力作用下向深部迁移,而不受变形控制。Tomkins et al. (2007)针对变质作用提出了“熔体辅助迁移”模型,熔体提取金等成矿元素需要熔体发生运移,迁移动力主要为重力。若是变质过程中通过部分熔融形成了硫化物熔体,则因该熔体流动性较差,其提取金的效率相对较低。要形成一定规模的富金矿床所需要的熔体量是十分巨大的(Tooth et al., 2011);若是变质过程中矿石的部分熔融形成了LMCE熔体,因该熔体的粘度小易于迁移,其与未熔矿物接触并不断吸收其中的LMCE元素形成更大的熔体。由于熔体的密度比矿石大,当熔体体积超过0.5%时,便会在重力作用下向深部迁移,不断吸收途经的LMCE-Au-Ag,最终富集成矿(Tomkins et al., 2007)。
3.4 LMCE熔体中Au的存在形式Au具有高电离势、高电负性及高氧化-还原电位特性,几乎所有还原剂都能将Au的阳离子(Au1+、Au3+)还原为Au0,因此Au常以原子状态存在(Pyykkö, 2004)。Au的熔点很高,但会随粒径减小而降低,如9.7nm Au微粒的熔点不到530℃ (Liu et al., 2019)。对于Au在熔体中的存在形式目前研究不多,且不同熔体中Au可能以不同形式存在。Fernández et al. (2007)发现Au+和Bi3+之间存在很强的吸附力,熔体中Au可能以+1价存在;Au-Cs熔体中Cs为+1价,Au为-1价(Martin et al., 1980);Au-Te熔体存在Au2Te相,Au为+1价;固体硅中Au为-1、0、+1价(Fazzio et al., 1985),Au0取代了Si的晶格位(Watkins and Williams, 1995)。
除上述形式,金还以一种价态介于0和+1间的物质存在,即金原子团簇。原子团簇由大量原子聚集而成,这些团簇的原子排列规则且有固定的化学计量比(Elliott, 1984),团簇之间没有明显的边界,原子可加入和脱离团簇,一个原子能被多个小团簇共享。以共价键和金属键为主的A-B熔体中存在大量原子团簇Ax、By及AmBn,整个熔体可以看成是由这些团簇和A、B原子组成的集合体。如Ag-Ga、Ag-Sn和Ag-Bi熔体中存在大量单元素团簇(Agx、Biy,x、y取决于元素含量)和多元素团簇(如Ag65Ga35、Ag75Sn25、Ag80Bi20) (Kaban and Hoyer, 2002);Au-Te熔体中存在Au、TeII、TeIII原子和AumTen团簇,具有四元合金的性质(Yassin and Castanet, 1998)。
以原子团簇存在的熔体在升降温过程中,具有稳定性和可逆性,小团簇很容易聚集起来融合成更大的团簇,从而成为晶体生长的基本单元,聚集堆砌后形成矿物(Greer et al., 2012)。上述过程有别于传统单原子堆积的“层生长”和“螺旋生长”理论,属于纳米晶粒定向堆砌的矿物生长,存在多个生长阶段,并且堆砌方向的纳米颗粒排列具有随机性,该机制已在熔体结晶的云母中得到证实(He et al., 2021)。在降温过程中,团簇间的聚集生长会使熔体无法达到相平衡,从而形成许多非平衡矿物组合。Au-LMCE熔体发生扰动或降温过程中,金团簇(Aux)最先发生聚集沉淀,温度继续下降,LMCE团簇(LMCEy)和Au-LMCE团簇(AumLMCEn)也聚集沉淀,形成自然金、LMCE单质/化合物及Au-LMCE矿物,如自然金+自然碲、碲金矿+碲银矿、自然金+自然铋等矿物组合均可由原子团簇聚集生长而致。Hannington et al. (2016)发现海底黑烟囱中2μm~50nm的金微粒,Prokofiev et al. (2020)发现含6000×10-6Au的流体包裹体及McLeish et al. (2021)认为金以胶体形式在流体中迁移等均表明金可以离子团簇存在成矿过程中,此时金原子不能通过传统堆积形成晶体,而是团簇聚集形成球状或片状,并由此形成巨富的金矿脉。
4 LMCE熔体参与成矿的矿物组合与结构特征标志前已述及,LMCE熔体在许多不同类型的矿床中均可出现,特别是在矽卡岩矿床、斑岩矿床、IOCG矿床、VMS矿床、造山型金矿床及浅成低温热液矿床中普遍存在(Ciobanu et al., 2005),其对Au、Ag、PGE等贵金属的富集具有重要作用。如我国云南北衙斑岩-矽卡岩型金矿床中Bi-Au熔体在磁铁矿生长阶段发生沉淀又进一步促进了自然金的沉淀(Zhou et al., 2017)。罗马尼亚Larga斑岩型金矿床中存在“水珠状的”Au-Bi合金与毒砂共生,显示Te-Bi熔体在成矿过程中充当了“清道夫”作用(Cook and Ciobanu, 2004)。澳大利亚Stormont矽卡岩金矿床中Bi熔体对Au的反复吸收作用,是金发生富集成矿的重要过程(Cockerton and Tomkins, 2012)。加拿大NICO IOCG型矿床中的Bi-Au合金产于砷化物和硫砷化物中,其形成存在两个过程:(1) Bi-Te熔体与流体同时运移时,不断抽提富集Au,并沉淀于早期矿物中;(2)矿物中的Bi-Te-Au在退变质时期重新活化,形成熔体,Au发生进一步富集,最终在毒砂中重新沉淀成矿(Acosta-Góngora et al., 2015)。美国Gorda洋脊Escanaba海槽VMS矿床中金的沉淀与主成矿期流体中Bi-Te的浓度升高有关,Bi-Te熔体可以有效地从海底喷口喷出的流体中吸收富集Au,在温度低于241℃时沉淀形成自然铋和黑铋金矿(Toörmaänen and Koski, 2005)。津巴布韦Viceroy造山型金矿床中的Bi-Te-S熔体从先前已沉淀的含金毒砂中吸收Au,形成Au-Bi-Te-S矿物,从而提高矿石中的Au品位(Oberthür and Weiser, 2008);罗马尼亚Highis地区Soimus Ilii造山型Cu-Au矿床中存在自然金-铅铋硫盐-碲化物/硒化物-黄铜矿的矿物共生组合,表明在成矿期存在自然铋熔体,该熔体在断裂中运移时不断吸收围岩中的Au、Pb、Te和S,并在扩张部位沉淀成矿(Ciobanu et al., 2006)。在其他一些金矿床中都出现Au-Bi-(Te-S)矿物被更高温的矿物所包裹,Meinert (2000)认为二者是同时沉淀形成的,LMCE熔体起到了“清道夫”的作用。
本研究团队在研究我国甘肃拉尔玛卡林型金矿床和安房坝造山型金矿床、陕西双王类卡林型金矿床、黑龙江三道湾子浅成低温热液型金矿床、河北东坪碱性-偏碱性侵入岩型金矿床、河南大湖和金渠造山型金矿床以及新疆包古图造山型金矿床的物质组成与矿床成因时,发现这些矿床中存在许多LMCE熔体参与成矿的标志性矿物组合与结构。结合国内外学者的研究成果(Frost et al., 2002, 2011;Biagioni et al., 2013;Mavrogenes et al., 2013),本文总结和提出了LMCE熔体参与成矿的矿物组合与结构特征标志,并对各结构特征形成的机理进行讨论。
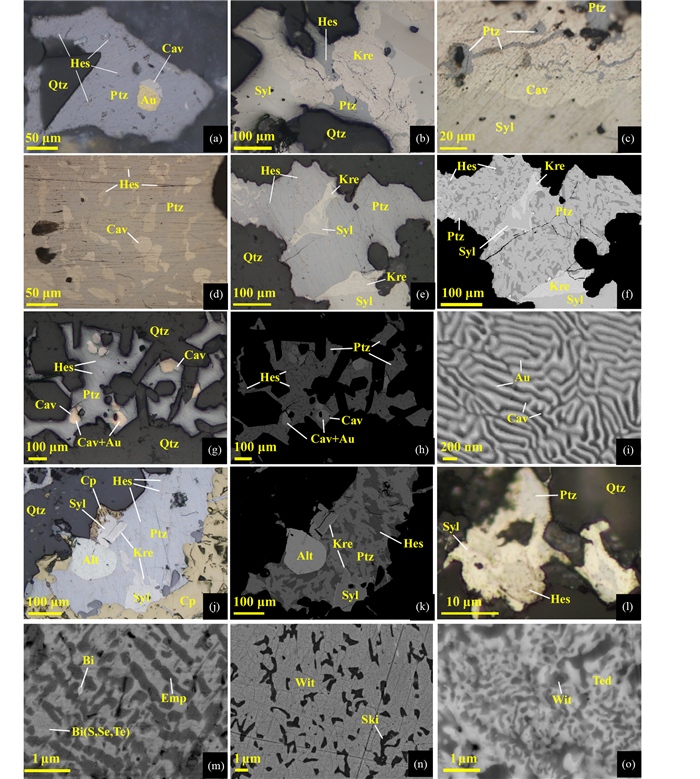
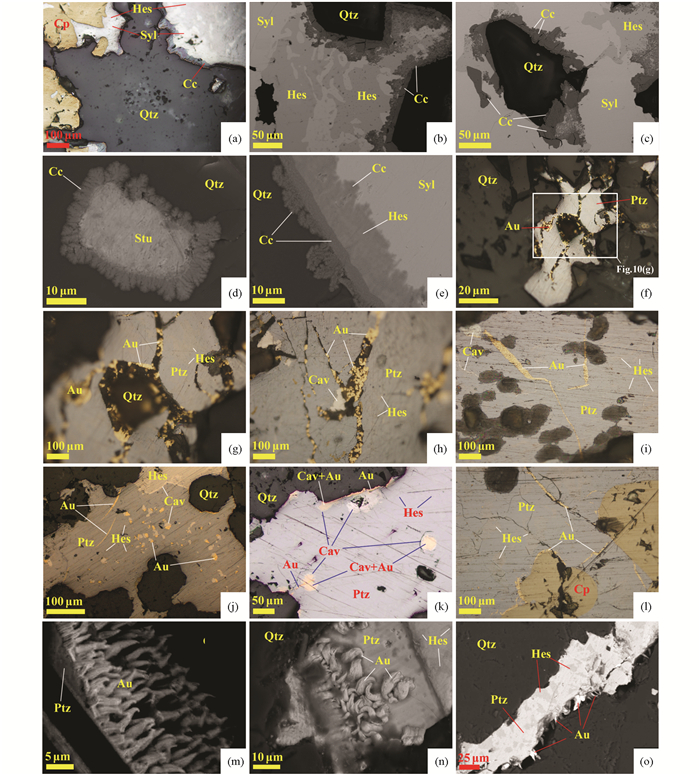
4.1 矿物形态与分布由LMCE熔体形成的矿物常以乳滴、珠滴、气泡的微粒包体产在硫化物、硒化物、碲化物、氧化物和硅酸盐矿物内或沿矿物断裂线形排列,形态多为浑圆状、近浑圆状。如河北东坪金矿床中Au-Ag-Te矿物以浑圆状独立或线形排列分布于石英、重晶石、黄铁矿及其他硫化物等矿物中(图 4),如甘肃安房坝金矿床中铋熔体固结形成的自然铋就呈液滴状分布于硫铜铋矿中(图 4a)。自然铋液滴状形态学特征也强烈指示了这些金属以熔融态形式沉淀的(Ciobanu et al., 2005)。无论是流体-熔体的分离作用还是由岩浆分异或变质熔融过程中出溶形成的LMCE熔体,都较容易发生迁移和聚集而形成不规则的片状或粒径较大的矿物集合体。如新疆包古图金矿床中由锑-金熔体、砷-金-银熔体结晶分别形成的方锑金矿+自然锑、自然砷+银金矿,在金矿石中分布较为常见。
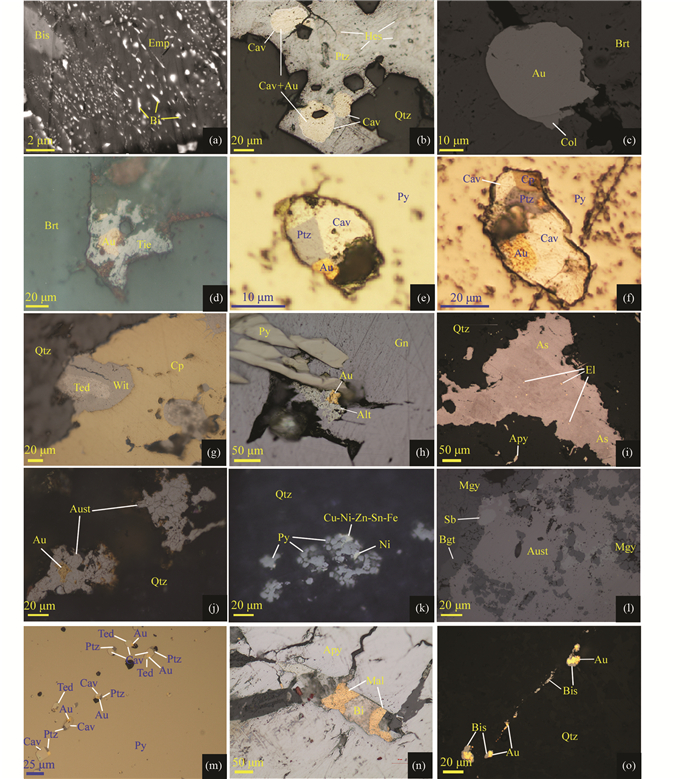
|
图 4 LMCE熔体形成矿物的形态与分布 (a)铋熔体固结形成自然铋,呈液滴状在硫铜铋矿中分布,具有明显定向性,BSE图像(安房坝);(b)碲-金熔体结晶形成碲金矿+自然金,保留了熔体的圆形、近圆形轮廓,反射光(三道湾子);(c)汞-碲-金熔体结晶形成碲汞矿+自然金,呈圆形产在重晶石中,BSE图像(寨上);(d)硒-汞-金熔体结晶形成形态不规则的硒汞矿+自然金,反射光(拉尔玛);(e)碲-金-银熔体结晶形成碲金矿+碲金银矿+自然金,呈近圆形分布在黄铁矿中,表明黄铁矿捕获了碲-金-银熔体,反射光(东坪);(f)碲-金-银-铜熔体结晶形成碲金矿+碲金银矿+自然金+黄铜矿,呈椭圆形分布在黄铁矿中,反射光(东坪);(g)碲-铋-铜熔体结晶形成近圆形的碲铋矿+硫铋铜矿,反射光(安房坝);(h)碲-铅-金熔体结晶形成碲铅矿+自然金,呈不规则状分布在方铅矿裂隙中,反射光(大湖);(i)砷-金-银熔体固结分离形成自然砷+银金矿,自然砷呈不规则粒状产出,银金矿呈微粒产在自然砷内,反射光(包古图);(j)锑-金熔体结晶形成形态不规则的方锑金矿+自然金,表明熔体中Au含量高于33.3at%,反射光(包古图);(k)铜-镍-锌-锡-铁熔体结晶形成Cu-Ni-Zn-Sn-Fe矿物和自然镍,不规则状分布在黄铁矿边缘,反射光(寨上);(l)锑-金熔体结晶形成方锑金矿+自然锑,表明熔体中Sb含量高,Au含量低于33.3at%,反射光(包古图);(m)碲-铋-金-银熔体结晶形成圆形、近圆形的碲金银矿+碲金矿+碲铋矿+自然金的矿物组合,产于黄铁矿中呈线性排列,几微米至几十微米的微粒中均具有相同的矿物组合,表明矿物微粒为黄铁矿捕获同一熔体后结晶形成,反射光(东坪);(n)铋-金熔体结晶形成黑铋金矿+自然铋,产在毒砂的裂隙中,反射光(双王);(o)辉铋矿+自然金分布在石英裂隙中,由于辉铋矿形成温度高,因此该矿物组合不能由Bi-Au-S熔体结晶形成,更可能是Bi-Au熔体中加入S导致辉铋矿沉淀,并分离出自然金,反射光(包古图). 缩写代号:Alt-碲铅矿;Apy-毒砂;As-自然砷;Au-自然金;Aust-方锑金矿;Bgt-“包古图矿”;Bi-自然铋;Bis-辉铋矿;Brt-重晶石;Cav-碲金矿;Col-碲汞矿;Cp-黄铜矿;Cu+Ni-Zn-Sn-Fe表示Cu+Ni-Zn-Sn-Fe金属互化物;黄铜矿;El-银金矿;Emp-硫铜铋矿;Gn-方铅矿;Hes-碲银矿;Kre-斜方碲金矿;Mal-黑铋金矿;Mgy-辉锑银矿;Ni-自然镍;Ptz-碲金银矿;Py-黄铁矿;Qtz-石英;Sb-自然锑;Syl-针碲金银矿;Tie-硒汞矿;Wit-硫铋铜矿;Ski-硫锑铜矿;Ted-碲铋矿 Fig. 4 Morphology and distribution of minerals fromed by LMCE melt (a) solidification of the Bi melt formed native bismuth, distributed in emplectite as droplets with significant orientation, BSE image (the Anfangba deposit); (b) crystallization of the Te-Au melt formed calaverite+native gold, which retained the rounded to subrounded shapes, reflect light image (the Sandaowanzi deposit); (c) crystallization of the Hg-Te-Au melt formed coloradoite+naive gold, distributed in the barite with rounded shape, BSE image (the Zhaishang deposit); (d) crystallization of the Se-Hg-Au melt formed tiemannite+native gold with irregular shapes, reflect light image (the La'maer deposit); (e) crystallization of the Te-Au-Ag melt formed calaverite+petzite+native gold, which distributed in pyrite with rounded shapes, indicating the pyrite captured the Te-Au-Ag melt, reflect light image (the Dongping deposit); (f) crystallization of the Te-Au-Ag-Cu melt formed calaverite+petzite+native gold+chalcopyrite, distributed in pyrite with elliptical shape, reflect light image (the Dongping deposit); (g) crystallization of the Te-Bi-Cu melt formed rounded tellurbismuth+wittichenite, reflect light image (the Anfangba deposit); (h) crystallization of the Te-Pb-Au melt formed altaite+native gold, distributed in fractures of galena with irregular shape, reflect light image (the Dahu deposit); (i) solidification and fraction of the As-Au-Ag melt formed native arsenic+electrum, the native arsenic occurred as irregular shaped grains, and the electrum distributed in the native arsenic as particulates, reflect light image (the Baogutu deposit); (j) crystallization of the Sb-Au melt formed aurostibite+native gold, indicating the Au content in the melt was higher than 33.3at%, reflect light image (the Baogutu deposit); (k) crystallization of the Cu-Ni-Zn-Sn-Te melt formed Cu-Ni-Zn-Sn-Fe mineral+native nickel, distributed in the boundaries of pyrite as irregular shapes, reflect light image (the Zhaishang deposit); (l) crystallization of the Sb-Au melt formed aurostibite+native antimony, indicating the melt contained higher Sb, Au content was lower than 33.3at%, reflect light image (the Baogutu deposit); (m) crystallization of the Te-Bi-Au-Ag melt formed rounded to subrounded petzite+calaverite+tellurbismuth+native gold mineral assemblage, which distributed linearly in pyrite, and the particles with a few to dozens of microns in sizes contain the similar mineral compositions, indicating the mineral particles were formed by crystallization of the same melt that captured by pyrite, reflect light image (the Dongping deposit); (n) crystallization of the Bi-Au melt formed maldonite+native bismuth, distributed in the fractures of arsenopyrite reflect light image (the Shuangwang deposit); (o) bismuthinite+native gold distributed in the fractures of quartz, because of the high formation temperature of Bismuthinite, this mineral composition could not be formed through crystallization of Bi-Au-S melt, which was more likely formed by additation of sulfur in the Bi-Au melt that led to the precipitation of Bismuthinite and native gold, reflect light (the Baogutu deposit). Abbreviation: Alt-altaite; Apy-arsenopyrite; As-native arsenic; Au-native gold; Aust-aurostibite; Bgt-baogutuite; Bi-native bismuth; Bis-bismuthinite; Brt-barite; Cav calaverite; Col-coloradoite; Cp-chalcopyrite; Cu-Ni-Zn-Sn-Fe means Cu+Ni-Zn-Sn-Fe intermetallic compound, EI-electrum; Emp-emplectite; Gn-galena; Hes-hessite; Kre-krennerite; Mal-maldonite; Mgy-miargyrite; Ni-native nickel; Ptz-petzite; Py-pyrite; Qtz-quartz; Sb-native antimony; Syl-sylvanite; Wit-wittichenite; Ski-skinnerite; Ted-tellurbismuth; Tie-tiemannite |
LMCE熔体形成微粒包体矿物呈分散分布的原因是:熔体在强烈扰动下形成大量金属液滴,这些液滴的沉淀固结形成大量微米到纳米级LMCE矿物。在流体演化过程中,流体沸腾作用是导致熔体扰动的主要因素。这是因为流体沸腾作用可以使流体出现空化(液体内部形成大量空泡),这时金属熔体以球体微粒(直径 < 1μm至100μm不等)分散到空泡中,随后快速冷却固化,固化的金属微粒悬浮在液相中或缓慢沉淀(Friedman et al., 2013;Yang et al., 2020)。该现象可以发生在流体-熔体、熔体-熔体中。如Bi可呈微粒球体(直径 < 0.5μm)均匀分散在Zn熔体中,发生乳化,形成乳状液,降温后形成Bi-Zn合金(Keppens et al., 1996)。
乳化是一种以极微小液滴或液晶均匀地分散在互不相溶的另一种液体中形成乳状液的过程,自然界中典型的乳状液为牛奶和原油,乳状液中的液滴直径约为0.1~10μm,具有稳定性较差和分散度低的特征(李明远和吴肇亮, 2009)。一般认为,两种密度差小的液相(如水和油)发生乳化的能量要小于空化所需能量,而两种密度差大的液相(如水和汞)则要空化后才能乳化(Li and Fogler, 1978)。在富Si流体中同时含有LMCE熔体、硫化物熔体时,因LMCE熔体与硫化物熔体密度相当,且都大于富Si流体的密度,故LMCE熔体与硫化物熔体之间易发生乳化,富Si流体中的LMCE液滴非常少,致使形成的LMCE矿物呈大片集合体出现,仅硫化物中存在少量LMCE微粒包体。但在流体沸腾发生空化时,LMCE熔体、硫化物熔体与富Si流体均可发生乳化,形成大量LMCE液滴,致使LMCE微粒包体广泛分散在硫化物和硅酸盐矿物中(Li and Fogler, 1978;Friedman et al., 2013;Yang et al., 2020)。
在Tooth et al. (2011)熔融实验获得的球形微粒自然铋(Bi,直径1~2μm,最大30μm)内,包裹有随机分布的球形黑铋金矿(Au2Bi,直径0.1~1μm)。自然铋球形微粒主要分布在磁黄铁矿表面或磁铁矿孔隙内,磁铁矿的破裂处发现大粒径的自然铋,这是磁黄铁矿、石墨等还原矿物将Bi3+、Au+还原为Bi0、Au0并形成了Bi熔体。同时Bi熔体在循环流体的扰动下与流体发生乳化,形成的Bi液滴因表面张力呈现出球形,当遇到磁铁矿等矿物时被捕获沉淀。因此磁黄铁矿、石墨等还原矿物对Bi3+和Au+的还原作用是导致Bi沉淀的关键,而乳化作用则是导致实验中形成球形微粒自然铋的关键。
LMCE熔体的固结温度低,不管岩浆、热液还是变质环境中均为最晚的熔体相,因此,若早期矿物存在裂隙时,LMCE熔体则会进入裂隙,形成的LMCE矿物集合体呈线形排列(图 4m),或呈脉状切穿或交代早期矿物(图 4o)。
4.2 矿物组合LMCE熔体具有亚稳态异质结构,不能快速淬火结晶,而是在低温下缓慢冷却达到相平衡(Sparks and Mavrogenes, 2005),分离出包含LMCE的自然元素矿物(如自然金、自然铋、自然锑、自然砷、自然硒、自然碲等)、金属互化物(如银金矿、黑铋金矿、Cu-Zn合金、Cu-Zn-Fe-Ni-Sn合金)及多相矿物(如硫化物、硒化物、碲化物、砷化物、锑化物等)(Sklyarchuk et al., 2007),导致矿床中存在复杂的LMCE的复杂的矿物组合(尤其是一些微米到纳米级的微粒包体)(图 4)。熔体缓慢冷却形成的矿物接触边较圆滑(图 4),而快速结晶的矿物边界呈多边形(Frost et al., 2002)。该现象在存在LMCE熔体的矿床中很常见,如我国云南北衙矿床中存在大量纳米级至微米级的Au-Bi矿物微粒(Zhou et al., 2017),意大利Monte Arsiccio矿床中的Tl-Hg-As-Sb-(Ag, Cu)-Pb矿物集合体(George et al., 2018),Alpi Apuane矿床中的Tl-Hg-As-Sb-(Ag, Cu)-Pb硫盐矿物组合(Biagioni et al., 2013),印度Sindesar Khurd矿床中复杂的Ag-Pb-S、Ag-Sb-S和Sb-Ag矿物组合,并形成锑硫镍矿+红锑镍矿+斜方锑镍矿+硫化物+硫盐矿物的矿物集合体(Govindarao et al., 2020)。
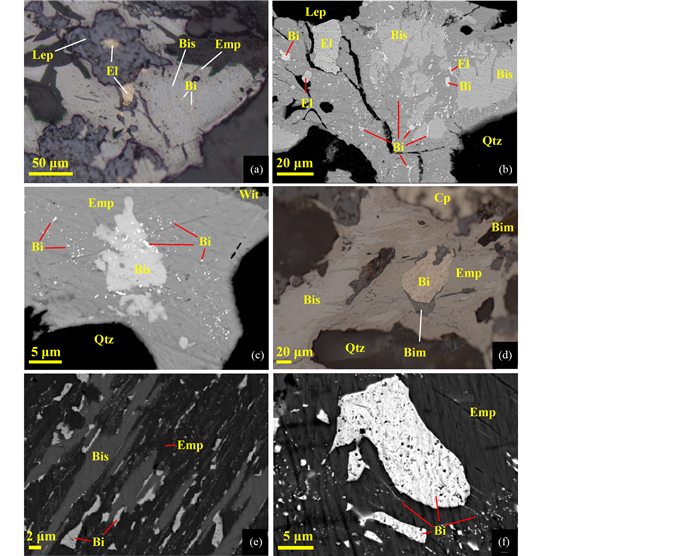
作者在研究黑龙江三道湾子碲化物型金矿床的物质组成时,确认矿床中产出含金银的碲化物包括碲金矿、斜方碲金矿、碲金银矿、针碲金银矿、碲银矿、碲铅矿、碲汞矿等。这些碲化物的生成顺序是依次以含Pb、Ag、Au和Hg碲化物矿物为主导(图 5),从早到晚出现4个主要的矿物组合,包括:碲铅矿(PbTe)、碲金银矿(Ag3AuTe2)-碲银矿(Ag2Te)-六方碲银矿(Ag5-xTe3)-粒碲银矿(AgTe)(为含银碲化物主导的矿物组合)(< 380℃)、针碲金银矿[(Au, Ag)2Te4)]-碲金矿(AuTe2)-斜方碲金矿(Au1-xAgxTe2)(以含金碲化物为主导的矿物组合)(< 380℃)和自然金-银金矿-碲汞矿(HgTe)等(表 3,Zhai and Liu, 2014)。

|
图 5 三道湾子金矿床碲化物Au-Ag-Te-Pb-Hg图解 箭头方向显示矿物组合的先后顺序;电子探针数据来自许虹等(2012)和Zhai and Liu (2014) Fig. 5 Au-Ag-Te-Pb-Hg diagram of the Sandaowanzi gold deposit The arrow direction shows the sequence of mineral assemblages; EPMA data are from Xu et al. (2012) and Zhai and Liu (2014) |
|
|
表 3 典型金矿床LMCE矿物组合统计表 Table 3 LMCE mineral compositions of typical gold deposits |
对我国其它一些碲化物型、硒化物型金矿床研究也显示其具有复杂的矿物组合。如东坪金矿床的主要碲化物物组合为自然金+碲金矿+碲金银矿±黄铜矿(< 380℃)和自然金+碲金矿+方铅矿等(表 3,Wang et al., 2019)。河南大湖金矿床的主要碲化物组合为碲铅矿-六方碲银矿、碲铅矿-碲金矿、碲铅矿-碲金银矿等(表 3,Yin et al., 2019)。四川-甘肃交界一带的拉尔玛-邛莫金矿床的主要硒化物组合为硒汞矿-硒铅矿-硒锑矿-硒镍矿-辉锑矿-自然金-重晶石等(表 3,Liu et al., 2000)。
我国新疆包古图金矿床中出现As-Bi-Sb-Au-Ag的矿物组合:自然砷-自然金(636℃)、自然铋-自然金 < 241℃)、辉锑矿-自然锑-方锑金矿(360℃)等(表 3,郑波等, 2009, 2013),本文认为自然铋-自然金及辉锑矿-自然锑-方锑金矿矿物组合的形成可用LMCE熔体进行解释。包古图金矿床的成矿温度达510℃(郑波等, 2009),该温度下,Bi和Sb均可以熔体形式存在和迁移,不断吸收Au-Ag,温度降低后形成上述矿物组合。由于As-Au的熔点高,难以形成熔体,因此自然砷-自然金的矿物组合可能是降温还原形成,但也不能排除多元素(如Bi-As-Sb)存在导致As-Au熔体降低,形成As-Au-多元素熔体的可能。
因此,自然元素矿物、金属互化物及多相矿物组合常出现在LMCE熔体参与成矿而形成的矿床中,尤其是非平衡的多相矿物组合可作为LMCE参与金、银等贵金属成矿的一个重要标志。
4.3 标志性结构LMCE熔体的冷却不仅可以形成一些具有独特的矿物组合,而且也能形成了一些标志性的结构(Frost et al., 2002, 2011;Biagioni et al., 2013;Mavrogenes et al., 2013)。有关标志性结构特征描述如下。
4.3.1 固溶体分解结构LMCE熔体在冷却过程中会形成一些亚稳定或不稳定的过渡态矿物,并随着温度下降会进一步分解,使不同类型的LMCE矿物相互交生在一起而形成固溶体分解结构或交生结构。在一些浅成低温热液和造山型金矿床中碲、铋、砷等矿物常具有这种显微结构特征。
如三道湾子金矿床中存在大量碲金银矿与碲银矿呈显微交生的固溶体分解结构(图 6a-k),这与流体中形成的χ相熔体(分子式为Ag3+xAu1-xTe2,其中0.1 < x < 0.55)在体系温度降到120℃以下的发生的分解和出溶有关;矿床中存在针碲金银矿-碲金银矿-碲银矿-六方碲银矿呈显微交生的固溶体分解结构(图 6e, f),与早先形成的γ相与碲金银矿或六方碲银矿等组成的熔体在温度降至120℃以下的分解密切有关;矿床中存在斜方碲金矿-针碲金银矿-碲金银矿-碲银矿呈显微交生的固溶体分解结构(图 6c),与γ相-χ相-碲金银矿-斜方碲金矿构成的熔体分解有关;矿床中也存在自然金与碲金矿相互交生在一起的纳米文象结构(图 6i),其形成的原因可能为:高温时,Te以熔体形式存在,吸收了高含量的金,当温度降低时,Te-Au熔体沿共熔线演化,分解形成AuTe2+Au(图 6g-i);由于温度降低过快,AuTe2与Au来不及完全分离,形成交织结构,且整体保存了熔体状态时的浑圆球状(图 6g、图 7;Liu et al., 2017)。因此,在该矿床中所发现的大量碲化物矿物的交生结构以及固溶体分解结构都与含碲的熔体相(如γ相和χ相)在降温过程中的分解有关。这些结构可作为LMCE参与金和银成矿的一个关键标志。
|
图 6 碲铋化物的固溶体出溶结构 (a)碲金银矿-碲银矿、碲金矿-自然金等固溶体出溶,反射光(三道湾子);(b)碲金银矿-碲银矿、斜方碲金矿-针碲金银矿等固溶体出溶,反射光(三道湾子);(c)碲金矿-针碲金银矿-碲金银矿等固溶体出溶,反射光(三道湾子);(d)碲金银矿-碲银矿-碲金矿等固溶体出溶,反射光(三道湾子);(e)针碲金银矿-碲金银矿-碲银矿等固溶体出溶,反射光(三道湾子);(f)同(d),BSE图像;(g)碲金矿与自然金的固溶体圆球粒包裹于碲金银矿和碲银矿中,圆球粒边缘分布有碲金矿,反射光(三道湾子);(h、i)同(g),BSE图像;(j)碲金银矿和碲银矿及其它类型碲化物等固溶体出溶,反射光(三道湾子);(k)同(j),BSE图像;(l)碲金银矿-碲银矿-针碲金银矿固溶体出溶,反射光(东坪);(m)硫铜铋矿与Bi(S, Se, Te)矿物等固溶体出溶,BSE图像(安房坝);(n)硫铋铜矿与硫锑铜矿固溶体出溶,BSE图像(安房坝);(o)硫铋铜矿与碲铋矿的固溶体出溶,BSE图像(安房坝) Fig. 6 The exsolution texture of telluride-bismuthide solid solution (a) solid solution exsolution of petzite-hessite and calaverite-native gold, reflect light image (the Sandaowanzi deposit); (b) solid solution exsolution of petzite-hessite and krennerite-sylvanite, reflect light image (the Sandaowanzi deposit); (c) solid solution exsolution of calaverite-sylvanite-petzite, reflect light image (the Sandaowanzi deposit); (d) solid solution exsolution of petzite-hessite-calaverite, reflect light image (the Sandaowanzi deposit); (e) solid solution exsolution of sylvanite-petzite-hessite, reflect light image (the Sandaowanzi deposit); (f) the same with (d), BSE image; (g) rounded calaverite and native gold solid solution covered by petzite and hessite, the boundaries of solid solution were calaverite, reflect light image (the Sandaowanzi deposit); (h, i) the same with (g), BSE image; (j) solid solution exsolution of petzite, hessite and other tellurides, reflect light image (the Sandaowanzi deposit); (k) the same with (j), BSE image; (l) solid solution exsolution of petzite-hessite-sylvanite, reflect light image (the Dongping deposit); (m) solid solution exsolution of emplectite and Bi(S, Se, Te) mineral, BSE image (the Anfangba deposit); (n) solid solution exsolution of wittichenite and skinnerite mineral, BSE image (the Anfangba deposit); (o) solid solution exsolution of wittichenite and tellurbismuth, BSE image (the Anfangba deposit) |

|
图 7 “壳-幔-核”分层结构 (a-d)黄铜矿包裹辉铋矿、硫铜铋矿、硫铋铜矿的固溶体,分布于黄铜矿中,显示“壳-幔-核”的分层结构,其中(a)、(c)为反射光,(b)、(d)为BSE图像(安房坝);(e-j)碲金矿包裹碲金矿与自然金的固溶体,分布于碲金银矿中,显示“壳-幔”的分层结构,其中(e-g)为反射光,(h-j)为BSE图像(三道湾子) Fig. 7 "Crust-mantle-core" layered texture (a-d) the solid solution of bismuth, emplectite and wittichenite is wrapped by chalcopyrite which is distributed in chalcopyrite, showing the layered texture of "shell mantle core"(the Anfangba deposit); (e-j) the solid solution of calaverite and native gold is wrapped by calaverite which is distributed in petzite, showing the layered texture of "crust mantle"(the Sandaowanzi deposit). Micrographs of (a), (c), and (e-g) are reflect light images, and the other are BSE images |
溶解-再沉淀作用(CDR)在自然界广泛存在。在很多情况下,溶解-再沉淀作用与Te-Bi熔体的吸收富集同时存在。如磁黄铁矿经历CDR反应形成多孔磁铁矿,为Bi熔体提供了运移通道和与流体充分接触的空间,从而促进了Au的富集,并且在磁铁矿孔隙中形成大量群簇状的自然铋微粒包体(Tooth et al., 2011)。当流体-LMCE熔体共存时,溶解-再沉淀过程中,LMCE熔体的吸收富集会同时发生,相互促进,并将矿物中的贵金属元素不断带出富集,在此过程中会形成特征的溶解-再沉淀结构。当不饱和流体与矿物发生接触时便会发生CDR,原矿物发生溶解,生成更加稳定的、通常具有多孔特征的矿物。如甘肃安房坝金矿床中辉铋矿经历CDR形成硫铜铋矿(图 7a-d),在两矿物的反应界面及硫铜铋矿中形成大量Bi-Au-Ag矿物(图 8),并且具有沿辉铋矿解理面定向分布的特征(图 8e),从而提高了金矿石的品位,显示出含LMCE矿物的溶解-再沉淀作用为LMCE熔体的迁移和富集贵金属提供了重要的通道和与流体充分接触的空间。故溶解-再沉淀过程中可同时发生流体-LMCE熔体对Au、Ag等元素的吸收富集。因此溶解-再沉淀结构可能是LMCE熔体富集贵金属的一个重要标志性结构。
|
图 8 安房坝金矿床中的交代作用与退火结构 (a)硫铜铋矿交代辉铋矿,反射光;(b)硫铜铋矿交代辉铋矿,并在硫铜铋矿中产出大量自然铋,其分布具有一定的方向性,BSE图像;(c)硫铜铋矿、辉铋矿中产出大量的自然铋,其分布具有一定的方向性,BSE图像;(d)硫铜铋矿沿辉铋矿解理面交代,使两矿物间的交代界面(反应前沿)呈现出定向的特征,反射光;(e)硫铜铋矿交代后形成的自然铋,沿辉铋矿的解理面分布,BSE图像;(f)硫铜铋矿中呈乳滴状产出的自然铋分布有一定的方向性,具有“拖尾”现象,BSE图像. 缩写代号:Bim-铋氧化物; Lep-纤铁矿 Fig. 8 Replacement reaction and melt annealing texture of the Anfangba gold deposit (a) replacement of bismuthinite by emplectite, reflect light image; (b) replacement of bismuthinite by emplectite, and large amount of native bismuth was formed with certain orientation distribution, BSE image; (c) large amount of native bismuth distributed in emplectite and bismuthinite with certain orientation, BSE image; (d) emplectite replaced bismuthinite along bismuthinite's cleavage plane, showing orientation characters along the replacement boundaries (reaction front), reflect light image; (e) native bismuth was formed by replacement of emplectite, and distribute along bismuthinite's cleavage plane, BSE image; (f) native bismuth distributed in emplectite as droplets with certain orientation distribution, and showing tailing texture, BSE image. Abbreviation: Bim-bismuth oxide; Lep-lepidocrocite |
需要指出的是,在一些矿床中发生的溶解-再沉淀作用,有时可能并没有LMCE熔体的参与,通常为氧化流体为主的溶解-再沉淀作用。如河北东坪金矿床原生矿石中存在微孔隙金围绕碲金矿呈“环形”生长(图 9a-l),或是微孔隙碲围绕Ag-Te-S固溶体分布(图 9m-o),形成具有溶解-再沉淀的结构特征。该结构主要出现在成矿较晚的阶段或是在成矿后的变质交代阶段,是由先形成的碲化物被相对氧化的流体溶解后再沉淀而形成的(Wang et al., 2020;刘家军等, 2020)。再如黑龙江三道湾子金矿床矿石中存在呈雪花状、羽毛状的辉铜矿(图 10a-e),其紧密围绕金银碲化物颗粒,或碲化物与石英的接触部位产出,该特点也是溶解-再沉淀作用的产物。

|
图 9 东坪金矿床中微孔隙金、微孔隙碲显微照片和扫描电镜图像 (a、f)原生矿石中微孔隙金的显微镜下的特征, 反射光;(b-e、g-l)分别对应于微孔隙金颗粒的扫描电镜图像;(m)原生矿石中微孔隙碲的显微镜下的特征, 反射光;(n、o)微孔隙碲扫描电镜图像. 缩写代号:Ag-Te-S表示Ag-Te-S矿物;MA-微孔隙金 Fig. 9 Microphotograph and SEM images of microporous gold of the Dongping gold deposit (a, f) microporous gold in primary ores, reflect light image; (b-e, g-l) SEM images of microporous gold; (m) microporous tellurium in primary ores, reflect light image; (n, o) SEM images of microporous tellurium. Abbreviation: Ag-Te-S means Ag-Te-S mineral; MA-microporous gold |
|
图 10 三道湾子Au-Te矿床中溶解-再沉淀结构显微照片和扫描电镜图像 (a)辉铜矿在针碲金银矿-碲银矿边缘生长,反射光照片;(b、c)辉铜矿在针碲金银矿-碲银矿边缘生长,表明辉铜矿形成时间晚,扫描电镜照片;(d)辉铜矿沿六方碲银矿边缘呈“羽毛状”、“雪花状”生长,呈多孔状,具有溶解-再沉淀特征,扫描电镜照片;(e)六方碲银矿被辉铜矿交代,辉铜矿中含有碲银矿,交代特征明显,原矿物颗粒边缘形成“羽毛状”、“雪花状”辉铜矿,具有溶解-再沉淀特征,扫描电镜照片;(f-i)自然金沿碲金银矿裂隙分布,反射光照片;(j)碲金银矿中的细脉状自然金,反射光照片;(k)细脉状自然金沿碲金银矿与石英颗粒接触部位分布,有的穿切浑碲金银矿中浑圆状自然金-碲金矿颗粒,反射光照片;(l)自然金产在碲金银矿裂隙中或沿碲金银矿和黄铜矿接触边分布,反射光照片;(m、n)分布在碲金银矿裂隙中的自然金呈“绒毛状”、“金芽状”生长,扫描电镜照片;(o)“绒毛状”、“金芽状”自然金在碲金银矿-碲银矿与石英的接触边生长,扫描电镜照片. 缩写代号:Cc-辉铜矿 Fig. 10 Microphotographs and SEM images of dissolution-reprecipitation textures in the Sandaowanzi Au-Te deposits (a) chalcocite distributes along boundaries of petzite-hessite, reflect light image; (b, c) chalcocite distributes along boundaries of sylvanite-hessite, showing chalcocite formed later, SEM images; (d) chalcocite distributes along boundaries of stutzite and shows penniform and snowflake-shaped, having characteristics of dissolution-reprecipitation textures, SEM image; (e) stutzite had been replaced by chalcocite, and hessite exists in chalcocite, showing characteristics of replacement texture, and penniform-snowflake chalcocite distributes along the boundaries of original mineral grain, having characteristics of dissolution-reprecipitation textures, SEM image; (f-i) native gold distributes grains in the intragranular cracks of petzite, reflect light image; (j) native gold veinlet in petzite, reflect light image; (k) native gold veinlet distributed along petzite-quartz boundaries, cutting partly the rounded native gold-calaverite exists in petzite, reflect light image; (l) native gold distributes in the intragranular cracks of petzite or along boundaries of petzite and chalcopyrite, reflect light image; (m, n) native gold distributed in fractures of petzite shows villiform, gemmiform-shaped texture, SEM images; (o) villiform, gemmiform-shaped native gold distributes along boundaries of petzite-hessite and quartz, SEM images. Abbreviation: Cc-chalcocite |
在富金流体参与的过程中,自然金通常充填于碲化物裂隙或沿碲化物表面生长。如黑龙江三道湾子金矿床,自然金在金矿石中所占比例虽然不到5%(金主要以碲化物形式产出,Liu et al., 2013; Zhai and Liu, 2014; Zhai et al., 2018),但自然金的产出形式较为独特:除了少数自然金与碲化物共生产出外,绝大多数自然金呈细脉状产于碲金银矿颗粒内的裂隙中(图 10f-o),或沿碲金银矿与石英、黄铜矿颗粒接触边分布(图 10 f-h, k, l)。裂隙中的自然金,部分以“绒毛状”或“金芽状”形态近于垂直脉壁生长(图 10m-o)。虽然作者在这些碲化物颗粒内的裂隙周围或是碲化物与其它矿物接触部位并未观察到明显的溶蚀现象,但呈细脉状、垂直脉壁产出的“金芽状”的自然金,明显是成矿晚期富金流体在裂隙中或碲化物表面再沉淀、结晶而形成的。
由于碲化物、铋化物等较硫化物不稳定,在较高氧逸度流体中易被溶解或被其他矿物交代(Wang et al., 2020),因此在相同条件下,富碲化物、铋化物LMCE的矿床更容易发生溶解-再沉淀作用,形成特征的溶解-再沉淀结构。故作者将溶解-再沉淀结构作为LMCE熔体存在的依据之一。
4.3.3 熔体退火结构在熔-流体作用晚期或退变质作用过程中,在环境温度缓慢冷却的条件下,存在于流体相中的LMCE熔体与流体相分离发生退火沉淀(Tomkins and Mavrogenes, 2002; Ciobanu et al., 2005; Törmänen and Koski, 2005),并形成特殊的结构与纹理。Tomkins and Mavrogenes (2002)对澳大利亚南部Challenger金矿中存在的多相金属硫化物(硫化物-金-铋-黑铋金矿)样品进行了退火实验(Annealing-quenching experiments)研究,实验结果显示,发生退火作用的熔融样品通常由微米级多相包裹体组成,这些包裹体通常沿着退火径迹形成串珠状结构,并在局部发育拖尾。每条退火径迹上的包裹体形态各异,从乳滴状到棒状,再到退火断口内部的近乎片状。这些由退火作用形成的乳滴状串珠矿物集合体被认为是其在熔融状态下沉淀的最直接的岩相学证据(Ciobanu et al., 2005)。作者在我国安房坝金矿床原生矿石中观察到自然铋具有典型的退火结构特点:自然铋多呈乳滴状分布于硫铜铋矿中或辉铋矿与硫铜铋矿交接面上,具有定向分布特点(图 4a),局部出现拖尾现象(图 8f),说明自然铋是以熔体形式发生沉淀的。需要指出的是,不同于安房坝金矿床中发育单一相铋熔体,在多数金矿床中存在的熔体往往是多组分的。众所周知,金属和硫化物熔体不会直接淬火成玻璃,而会形成不同成分交织分布的“马赛克”(Mavrogenes et al., 2001; Tomkins and Mavrogenes, 2002)或乳滴衍生的斑片状(Ciobanu et al., 2005)。这些结构特征也被视为是熔体存在的证据(Ciobanu et al., 2005; Törmänen and Koski, 2005; Tooth et al., 2008; Cockerton and Tomkins, 2012)。
4.3.4 二面角结构二面角包括矿物二面角和矿物-熔体二面角。矿物二面角是指当3个矿物共棱接触时相邻两个矿物接触面之间所夹的二面角,矿物-熔体二面角指两个矿物与熔体共棱接触时相邻的固液界面间的二面角(Smith, 1948)。矿物二面角受每种矿物表面能控制,表面能小的矿物形成的二面角较小,反之较大。具有相同表面能的矿物间的二面角为120°(Frost et al., 2011),一般结晶矿物的二面角介于100°~140° (Vernon, 2004),如方铅矿与闪锌矿-闪锌矿间的二面角在300℃时为111°,700℃时变为88° (Lusk et al., 2002)。矿物-熔体二面角大小取决于固-固界面能与固-液界面能之比,比值越大二面角越小(邵同宾等, 2011)。例如氧化条件下的富Fe硫化物熔体与橄榄石间的二面角小,因为富Fe硫化物熔体中存在可与橄榄石表面相互作用的FeO配合物,但在还原条件下,二面角会变大(Gaetani and Grove, 1999)。如果富Fe硫化物熔体中难进入橄榄石的元素含量越来越高,则二面角也会越来越大(Rose and Brenan, 2001)。矿物-熔体二面角大小可以反映液体间的连通度,当二面角小于60°时,固液界面凸向熔体,无论熔体含量多少,矿物间的熔体能够彼此连通,当二面角大于60°时,固液界面凸向矿物,只有熔体含量大于某阈值时,粒间熔体才可能相互连通(侯渭等, 2004; 邵同宾等, 2011)。当LMCE熔体存在时,会与矿物形成远小于正常结晶矿物的二面角,如果熔体处于流动状态,则二面角小于60°,一般为40°~60° (Frost et al., 2011)。Spry et al. (2008)认为后期变质过程中矿物-熔体二面角会被破坏,不能作为判断熔体是否存在的必要条件。
4.4 富LMCE熔-流体包裹体在一些与岩浆岩有密切联系的斑岩型、矽卡岩型Cu-Au矿床中,人们时常观察到熔体-流体包裹体,较为完整地记录了岩浆活动与成矿作用过程的许多信息,且流体包裹体成分显示出高Au、Te、As、Sb含量(Pudack et al., 2009),表明了这些低熔点亲铜元素与金具有较好的相关性。与流体包裹体类似,熔体-流体包裹体可分为原生包裹体和次生包裹体,原生包裹体常沿寄主矿物结晶方向面状排列或独立分布,次生包裹体沿寄主矿物裂隙线性排列。熔体-流体包裹体具有以下特点可以与含LMCE矿物流体包裹体区别:(1)熔体-流体包裹体由同时捕获熔体与流体形成,因此熔体固结物不会超出包裹体边界;(2)熔体较流体的表面张力大,熔体与流体的接触边主要呈凸向(LMCE熔体与寄主矿物不浸润,如Bi熔体与石英)或凹向(LMCE熔体与寄主矿物浸润,如Bi熔体与黄铁矿,Giuranno et al., 2003; Ma et al., 2008)流体的弧形,不会出现尖角的接触边;(3)熔体的密度大于流体,因此熔体会集中分布在包裹体的一侧,同一时间捕获的熔体-流体包裹体群中熔体占据包裹体的位置应一致。
冯浩轩(2021)在研究辽宁五龙金矿床过程中发现,石英中含有大量Bi熔体-流体包裹体,Bi熔体中存在黑铋金矿或自然金,Bi熔体-流体包裹体与流体包裹体及富Bi微粒包体共存,沿石英生长边呈面状排列。五龙金矿床的成矿流体经历了强烈的沸腾作用(成曦晖等, 2017),导致Bi熔体与流体发生乳化,Bi以熔滴分散在流体中,并被石英和硫化物捕获(Wei et al., 2021; 冯浩轩, 2021),形成Bi熔体-流体包裹体及Bi微粒包体。Jian et al. (2021)对小秦岭造山型金矿床中的金-银-碲矿物包体(含少量的Fe、S、Cu和Pb) 的熔融实验分析表明,金-银-碲矿物包体在135℃时就开始熔化,进一步阐述了碲熔体捕获金的模式是金的沉淀的富集机制。
因此,矿床中存在的熔体-流体包裹体是LMCE熔体参与成矿最为直接的证据,沸腾导致的熔体与流体间的乳化是形成热液脉(如石英脉)中熔体-流体包裹体的关键。
5 结论与认识通过研究与分析,获得的主要认识如下:
(1) 低熔点亲铜元素(LMCE)具有亲铜性、低熔点、半金属特性,在成矿作用过程中可以形成LMCE熔体,对Au、Ag、PGE等贵金属高效沉淀富集可以起到一种重要的桥梁作用,是岩浆PGE(-Au)矿床、(岩浆)热液Au-Ag矿床及变质Au-Ag矿床重要的成矿机制之一。
(2) LMCE熔体中存在大量原子团簇,团簇间的聚集生长会使熔体难以达到相平衡,形成许多非平衡矿物组合,如包含LMCE的自然元素、金属互化物及含LMCE的多相矿物。Au-LMCE熔体中可以形成Aux、LMCEy及AumLMCEn的原子团簇,富金团簇聚集形成球状或片状,并形成富金矿体。
(3) LMCE微粒包体是熔体扰动导致熔-熔或熔-液间发生乳化所致,由LMCE熔体形成的矿物常以浑圆状、近浑圆状、不规则状的乳滴、珠滴、气泡等微粒包体存在,产在硫化物、硒化物、碲化物、氧化物和硅酸盐矿物内或沿矿物裂隙线形排列。流体沸腾是熔体扰动的主要机制。
(4) 矿床存在的熔体-流体包裹体是LMCE熔体参与成矿作用最为直接的证据,固溶体分解结构、熔体退火结构、矿物-熔体二面角结构、溶蚀-充填结构等也是LMCE熔体参与成矿的标志性结构。
致谢 感谢本期组稿专家合肥工业大学的周涛发教授和范裕教授给予本文的大力支持!承蒙孙晓明教授、秦克章研究员等对本文的认真审阅并提出重要的修改建议以及本刊编辑的细致编辑,极大地提高了文稿的质量。在相关研究与成文过程中,作者曾与中国地质大学(北京)张德会教授进行了讨论,获益良多。在此一并致以诚挚的谢意!
Acosta-Góngora P, Gleeson SA, Samson IM, Ootes L and Corriveau L. 2015. Gold refining by bismuth melts in the iron oxide-dominated NICO Au-Co-Bi (±Cu±W) deposit, NWT, Canada. Economic Geology, 110(2): 291-314 DOI:10.2113/econgeo.110.2.291
|
Ashtakala S, Pelton AD and Bale CW. 1981. The Ag-Pb (Silver-Lead) system. Bulletin of Alloy Phase Diagrams, 2(1): 81-83 DOI:10.1007/BF02873709
|
Babanly MB, Shykhyev YM, Babanly NB and Yusibov YA. 2007. Phase equilibria in the Ag-Bi-Te system. Russian Journal of Inorganic Chemistry, 52(3): 434-440 DOI:10.1134/S0036023607030242
|
Baren MR. 1989. The Ag-Tl (silver-thallium) system. Bulletin of Alloy Phase Diagrams, 10(6): 641-643 DOI:10.1007/BF02877633
|
Baren MR. 1990. The Ag-As (silver-arsenic) system. Bulletin of Alloy Phase Diagrams, 11(2): 113-116 DOI:10.1007/BF02841692
|
Baren MR. 1996. The Ag-Hg (silver-mercury) system. Journal of Phase Equilibria, 17(2): 122-128 DOI:10.1007/BF02665787
|
Barton PB. 1969. Thermochemical study of the system Fe-As-S. Geochimica et Cosmochimica Acta, 33(7): 841-857 DOI:10.1016/0016-7037(69)90031-3
|
Barton PB. 1971. The Fe-Sb-S system. Economic Geology, 66(1): 121-132 DOI:10.2113/gsecongeo.66.1.121
|
Bhan S, Gödecke T, Panday PK and Schubert K. 1968. über die mischungen palladium-thallium und platin-thallium. Journal of the Less Common Metals, 16(4): 415-425 DOI:10.1016/0022-5088(68)90140-9
|
Biagioni C, D'Orazio M, Vezzoni S, Dini A and Orlandi P. 2013. Mobilization of Tl-Hg-As-Sb-(Ag, Cu)-Pb sulfosalt melts during low-grade metamorphism in the Alpi Apuane (Tuscany, Italy). Geology, 41(7): 747-750 DOI:10.1130/G34211.1
|
Biagioni C, D'Orazio M, Fulignati P, George LL, Mauro D and Zaccarini F. 2020. Sulfide melts in ore deposits from low-grade metamorphic settings: Insights from fluid and Tl-rich sulfosalt microinclusions from the Monte Arsiccio mine (Apuan Alps, Tuscany, Italy). Ore Geology Reviews, 123: 103589 DOI:10.1016/j.oregeorev.2020.103589
|
Brett R and Kullerud G. 1967. The Fe-Pb-S system. Economic Geology, 62(3): 354-369 DOI:10.2113/gsecongeo.62.3.354
|
Bryndzia LT and Kleppa OJ. 1988. High-temperature reaction calorimetry of solid and liquid phases in the quasi-binary system Ag2S-Sb2S3. Geochimica et Cosmochimica Acta, 52(1): 167-176 DOI:10.1016/0016-7037(88)90064-6
|
Bryndzia LT and Davis AM. 1989. Liquidus phase relations on the quasi-binary join Cu2S-Sb2S3: Implications for the formation of tetrahedrite and skinnerite. American Mineralogist, 74(1-2): 236-242
|
Cabri LJ. 1965. Phase relations in the Au-Ag-Te systems and their mineralogical significance. Economic Geology, 60(8): 1569-1606 DOI:10.2113/gsecongeo.60.8.1569
|
Cabri LJ, Harris DC and Gait RI. 1973. Michenerite (PdBiTe) redefined and froodite (PdBi2) confirmed from the Sudbury area. The Canadian Mineralogist, 11(5): 903-912
|
Cabri LJ. 2002. The platinum-group minerals. In: The Geology, Geochemistry, Mineralogy and Mineral Beneficiation of the Platinum-Group Elements. Canadian Institute of Mining, Metallurgy and Petroleum, 54: 13-129
|
Cafagna F and Jugo PJ. 2016. An experimental study on the geochemical behavior of highly siderophile elements (HSE) and metalloids (As, Se, Sb, Te, Bi) in a mss-iss-pyrite system at 650℃: A possible magmatic origin for Co-HSE-bearing pyrite and the role of metalloid-rich phases in the fractionation of HSE. Geochimica et Cosmochimica Acta, 178: 233-258 DOI:10.1016/j.gca.2015.12.035
|
Cao XZ, Song TY and Wang XQ. 1994. Inorganic Chemistry. 3rd Edition. Beijing: Higher Education Press (in Chinese)
|
Cave BJ, Barnes SJ, Pitcairn IK, Sack PJ, Kuikka H, Johnson SC and Duran CJ. 2019. Multi-stage precipitation and redistribution of gold, and its collection by lead-bismuth and lead immiscible liquids in a reduced-intrusion related gold system (RIRGS); Dublin Gulch, western Canada. Ore Geology Reviews, 106: 28-55 DOI:10.1016/j.oregeorev.2019.01.010
|
Cepedal A, Fuertes-Fuente M, Martín-Izard A, González-Nistal S and Rodríguez-Pevida L. 2006. Tellurides, selenides and Bi-mineral assemblages from the Río Narcea Gold Belt, Asturias, Spain: Genetic implications in Cu-Au and Au skarns. Mineralogy and Petrology, 87(3): 277-304
|
Chang LLY, Wu DQ and Knowles CR. 1988. Phase relations in the system Ag2S-Cu2S-PbS-Bi2S3. Economic Geology, 83(2): 405-418 DOI:10.2113/gsecongeo.83.2.405
|
Chang M, Liu JJ, Carranza EJM, Yin C, Zhai DG, Wu T and Wang DZ. 2020. Gold-telluride-sulfide association in the Jinqu Au deposit, Xiaoqinling region, central China: Implications for ore-forming conditions and processes. Ore Geology Reviews, 125: 103687 DOI:10.1016/j.oregeorev.2020.103687
|
Chekushin VS, Oleinikova NV and Tychenko AI. 2008. The extraction of gold from sulfide concentrates into molten lead. Russian Journal of Non-Ferrous Metals, 49(5): 340-345 DOI:10.3103/S1067821208050052
|
Chen TT and Chang LLY. 1974. Investigations in the systems Ag2S-Cu2S-Bi2S3 and Ag2S-Cu2S-Sb2S3. The Canadian Mineralogist, 12(6): 404-410
|
Cheng XH, Xu JH, Yang FQ, Zhang H and Bian CJ. 2017. Study on fluid inclusions in the Wulong gold deposit, eastern Liaoning. Acta Mineralogica Sinica, 37(Suppl.): 460-461 (in Chinese)
|
Ciobanu CL, Cook NJ and Pring A. 2005. Bismuth tellurides as gold scavengers. In: Mao JW and Bierlein FP (eds. ). Mineral Deposit Research: Meeting the Global Challenge. Berlin, Heidelberg: Springer, 1383-1386
|
Ciobanu CL, Cook NJ and Spry PG. 2006. Preface: Special Issue: Telluride and selenide minerals in gold deposits: How and why?. Mineralogy and Petrology, 87(3): 163-169
|
Ciobanu CL, Birch WD, Cook NJ, Pring A and Grundler PV. 2010. Petrogenetic significance of Au-Bi-Te-S associations: The example of Maldon, Central Victorian gold province, Australia. Lithos, 116(1-2): 1-17 DOI:10.1016/j.lithos.2009.12.004
|
Cockerton ABD and Tomkins AG. 2012. Insights into the liquid bismuth collector model through analysis of the Bi-Au stormont skarn prospect, Northwest Tasmania. Economic Geology, 107(4): 667-682 DOI:10.2113/econgeo.107.4.667
|
Cohen BL. 1984. Anomalous behavior of tellurium abundances. Geochimica et Cosmochimica Acta, 48(1): 203-205 DOI:10.1016/0016-7037(84)90363-6
|
Cook NJ and Ciobanu CL. 2004. Bismuth tellurides and sulphosalts from the Larga hydrothermal system, Metaliferi Mts, Romania: Paragenesis and genetic significance. Mineralogical Magazine, 68(2): 301-321 DOI:10.1180/0026461046820188
|
Craig JR and Kullerud G. 1967. The Cu-Fe-Pb-S system. Carnegie Institution of Washington Yearbook, 65: 344-352
|
Craig JR and Barton PB. 1973. Thermochemical approximations for sulfosalts. Economic Geology, 68(4): 493-506 DOI:10.2113/gsecongeo.68.4.493
|
Dare SAS, Barnes SJ and Prichard HM. 2010. The distribution of platinum group elements (PGE) and other chalcophile elements among sulfides from the Creighton Ni-Cu-PGE sulfide deposit, Sudbury, Canada, and the origin of palladium in pentlandite. Mineralium Deposita, 45(8): 765-793 DOI:10.1007/s00126-010-0295-6
|
Douglas N, Mavrogenes J, Hack A and England R. 2000. The liquid bismuth collector model: An alternative gold deposition mechanism. In: Geological Society of Australia, Abstracts No. 59. Sydney, 135
|
Echmaeva EA and Osadchii EG. 2009. Determination of the thermodynamic properties of compounds in the Ag-Au-Se and Ag-Au-Te systems by the EMF method. Geology of Ore Deposits, 51(3): 247-258 DOI:10.1134/S1075701509030076
|
Elliot RP. 1965. Constitution of Binary Alloys, First Supplement. New York: McGraw-Hill
|
Elliott SR. 1984. Physics of Amorphous Materials. London, New York: Longman Group Ltd., 1-386
|
Fazzio A, Caldas MJ and Zunger A. 1985. Electronic structure of copper, silver, and gold impurities in silicon. Physical Review B, 32(2): 934-954 DOI:10.1103/PhysRevB.32.934
|
Feng HX. 2021. Metallogenic age and mineralization of the Wulong and Baiyun large gold deposits in eastern Liaoning: A new perspective of U-rich and Bi-rich accessory minerals. Ph. D. Dissertation. Beijing: University of Chinese Academy of Sciences (in Chinese with English summary)
|
Fernández EJ, Laguna A, López-de-Luzuriaga JM, Monge M, Nema M, Olmos ME, Pérez J and Silvestru C. 2007. Experimental and theoretical evidence of the first Au(i)…Bi(iii) interaction. Chemical Communications, (6): 571-573 DOI:10.1039/B613365G
|
Fleet ME, Chryssoulis SL, Stone WE and Weisener CG. 1993. Partitioning of platinum-group elements and Au in the Fe-NiCu-S system: Experiments on the fractional crystallization of sulfide melt. Contributions to Mineralogy and Petrology, 115(1): 36-44 DOI:10.1007/BF00712976
|
Flinn JM, Gupta PK and Litovitz TA. 1974. Mechanism of volume viscosity in the liquid metal system lead-bismuth. The Journal of Chemical Physics, 60(11): 4390-4395 DOI:10.1063/1.1680916
|
Freidrich K. 1907. The binary systems FeS-ZnS and FeS-PbS. Metallurgie, 4: 479-485
|
Friedman H, Reich S, Popovitz-Biro R, von Huth P, Halevy I, Koltypin Y, Gedanken A and Porat Z. 2013. Micro- and nano-spheres of low melting point metals and alloys, formed by ultrasonic cavitation. Ultrasonics Sonochemistry, 20(1): 432-444 DOI:10.1016/j.ultsonch.2012.08.009
|
Frost BR, Mavrogenes JA and Tomkins AG. 2002. Partial melting of sulfide ore deposits during medium- and high-grade metamorphism. The Canadian Mineralogist, 40(1): 1-18 DOI:10.2113/gscanmin.40.1.1
|
Frost BR, Swapp SM and Mavrogenes J. 2011. Textural evidence for extensive melting of the Broken Hill orebody. Economic Geology, 106(5): 869-882 DOI:10.2113/econgeo.106.5.869
|
Gaetani GA and Grove TL. 1999. Wetting of mantle olivine by sulfide melt: Implications for Re/Os ratios in mantle peridotite and late-stage core formation. Earth and Planetary Science Letters, 169(1-2): 147-163 DOI:10.1016/S0012-821X(99)00062-X
|
Gao S, Xu H, Zhang DS, Shao HN and Quan SL. 2015. Ore petrography and chemistry of the tellurides from the Dongping gold deposit, Hebei Province, China. Ore Geology Reviews, 64: 23-34 DOI:10.1016/j.oregeorev.2014.06.010
|
Gao S, Hofstra AH, Zou XY, Valley JW, Kitajima K, Marsh EE, Lowers HA, Adams DT, Qin KZ and Xu H. 2021. Oxygen isotope evidence for input of magmatic fluids and precipitation of Au-Ag-tellurides in an otherwise ordinary adularia-sericite epithermal system in NE China. American Mineralogist DOI:10.2138/am-2021-7825
|
Geng HR, Sun CJ, Wang R, Qi XG and Zhang N. 2007. Temperature dependence of densities of Sb and Bi melts. Chinese Science Bulletin, 52(15): 2031-2034 DOI:10.1007/s11434-007-0309-7
|
George L, Cook NJ, Ciobanu CL and Wade BP. 2015. Trace and minor elements in galena: A reconnaissance LA-ICP-MS study. American Mineralogist, 100(2-3): 548-569 DOI:10.2138/am-2015-4862
|
George LL, Biagioni C, D'Orazio M and Cook NJ. 2018. Textural and trace element evolution of pyrite during greenschist facies metamorphic recrystallization in the southern Apuan Alps (Tuscany, Italy): Influence on the formation of Tl-rich sulfosalt melt. Ore Geology Reviews, 102: 59-105 DOI:10.1016/j.oregeorev.2018.08.032
|
Giuranno D, Gnecco F, Ricci E and Novakovic R. 2003. Surface tension and wetting behaviour of molten Bi-Pb alloys. Intermetallics, 11(11-12): 1313-1317 DOI:10.1016/S0966-9795(03)00173-0
|
Godel B, Barnes SJ, Barnes SJ and Maier WD. 2010. Platinum ore in three dimensions: Insights from high-resolution X-ray computed tomography. Geology, 38(12): 1127-1130 DOI:10.1130/G31265.1
|
Govindarao B, Pruseth KL and Mishra B. 2020. Experimentally produced Cu-Pb-Ag-Sb-S melts at 500℃: Implications to partial melting of massive sulfide ores. Ore Geology Reviews, 121: 103560 DOI:10.1016/j.oregeorev.2020.103560
|
Greer HF, Yu FJ and Zhou WZ. 2011. Early stages of non-classic crystal growth. Science China (Chemistry), 54(12): 1867-1876 DOI:10.1007/s11426-011-4441-5
|
Grundler PV, Brugger J, Etschmann BE, Helm L, Liu WH, Spry PG, Tian Y, Testemale D and Pring A. 2013. Speciation of aqueous tellurium (IV) in hydrothermal solutions and vapors, and the role of oxidized tellurium species in Te transport and gold deposition. Geochimica et Cosmochimica Acta, 120: 298-325 DOI:10.1016/j.gca.2013.06.009
|
Guimarães FS, Cabral AR, Lehmann B, Rios FJ, ávila MAB, de Castro MP and Queiroga GN. 2019. Bismuth-melt trails trapped in cassiterite-quartz veins. Terra Nova, 31(4): 358-365
|
Guminski C. 1990. The Hg-Pt (mercury-platinum) system. Bulletin of Alloy Phase Diagrams, 11(1): 26-32 DOI:10.1007/BF02841581
|
Hannington M, Hareardóttir V, Garbe-Schönberg D and Brown KL. 2016. Gold enrichment in active geothermal systems by accumulating colloidal suspensions. Nature Geoscience, 9(4): 299-302 DOI:10.1038/ngeo2661
|
Hansen M, Anderko K and Salzberg HW. 1958. Constitution of binary alloys. Journal of the Electrochemical Society, 105(12): 260C DOI:10.1149/1.2428700
|
Harwood LM, Moody CJ and Percy JM. 1989. Experimental Organic Chemistry: Principles and Practice. 2nd Edition. Oxford: Blackwell Scientific Publications, 1-778
|
He HP, Yang YP, Ma LY, Su XL, Xian HY, Zhu JX, Teng HH and Guggenheim S. 2021. Evidence for a two-stage particle attachment mechanism for phyllosilicate crystallization in geological processes. American Mineralogist, 106(6): 983-993 DOI:10.2138/am-2021-7529
|
Hein JR, Koschinsky A and Halliday AN. 2003. Global occurrence of tellurium-rich ferromanganese crusts and a model for the enrichment of tellurium. Geochimica et Cosmochimica Acta, 67(6): 1117-1127 DOI:10.1016/S0016-7037(02)01279-6
|
Helmy HM, Ballhaus C, Berndt J, Bockrath C and Wohlgemuth-Ueberwasser C. 2007. Formation of Pt, Pd and Ni tellurides: Experiments in sulfide-telluride systems. Contributions to Mineralogy and Petrology, 153(5): 577-591 DOI:10.1007/s00410-006-0163-7
|
Helmy HM, Ballhaus C, Fonseca ROC and Nagel TJ. 2013. Fractionation of platinum, palladium, nickel, and copper in sulfide-arsenide systems at magmatic temperature. Contributions to Mineralogy and Petrology, 166(6): 1725-1737 DOI:10.1007/s00410-013-0951-9
|
Hoda SN and Chang LLY. 1975a. Phase relations in the pseudo-ternary system PbS-Cu2S-Sb2S3 and the synthesis of meneghinite. The Canadian Mineralogist, 13(4): 388-393
|
Hoda SN and Chang LLY. 1975b. Phase relations in the systems PbS-Ag2S-Sb2S3 and PbS-Ag2S-Bi2S3. American Mineralogist, 60(7-8): 621-633
|
Holwell DA and McDonald I. 2010. A review of the behaviour of platinum group elements within natural magmatic sulfide ore systems. Platinum Metals Review, 54(1): 26-36 DOI:10.1595/147106709X480913
|
Holwell DA, Fiorentini M, McDonald I, Lu YJ, Giuliani A, Smith DJ, Keith M and Locmelis M. 2019. A metasomatized lithospheric mantle control on the metallogenic signature of post-subduction magmatism. Nature Communications, 10: 3511 DOI:10.1038/s41467-019-11065-4
|
Hou W, Zhou WG, Xie HS and Liu YG. 2004. The morphology of melt (and fluid) in intergranular pores of rock under high-temperature and high-pressure and some development of experamental studies of this branch. Advances in Earth Science, 19(5): 767-773 (in Chinese with English abstract)
|
Iida T, Morita ZI and Takeuchi S. 1975. Viscosity measurements of pure liquid metals by the capillary method. Journal of the Japan Institute of Metals and Materials, 39(11): 1169-1175 DOI:10.2320/jinstmet1952.39.11_1169
|
Jégo S, Nakamura M, Kimura JI, Iizuka Y, Chang Q and Zellmer GF. 2016. Is gold solubility subject to pressure variations in ascending arc magmas?. Geochimica et Cosmochimica Acta, 188: 224-243 DOI:10.1016/j.gca.2016.05.034
|
Jensen EP and Barton MD. 2000. Gold deposits related to alkaline magmatism. Reviews in Economic Geology, 13: 279-314
|
Jian W, Mao JW, Lehmann B, Cook NJ, Xie GQ, Liu P, Duan C, Alles J and Niu ZJ. 2021. Au-Ag-Te-rich melt inclusions in hydrothermal gold-quartz veins, Xiaoqinling lode gold district, central China. Economic Geology, 116(5): 1239-1248 DOI:10.5382/econgeo.4811
|
Kaban I and Hoyer W. 2002. Interplay between atomic and electronic structure in liquid noble-polyvalent metal systems. Journal of Non-Crystalline Solids, 312-314: 41-46 DOI:10.1016/S0022-3093(02)01647-2
|
Karakaya I and Thompson WT. 1987. The Ag-Sn (silver-tin) system. Bulletin of Alloy Phase Diagrams, 8(4): 340-347 DOI:10.1007/BF02869270
|
Karakaya I and Thompson WT. 1990. The Ag-Se (silver-selenium) system. Bulletin of Alloy Phase Diagrams, 11(3): 266 DOI:10.1007/BF03029297
|
Karakaya I and Thompson WT. 1991. The Ag-Te (silver-tellurium) System. Journal of Phase Equilibria, 12(1): 56-63 DOI:10.1007/BF02663676
|
Karakaya I and Thompson WT. 1993. The Ag-Bi (silver-bismuth) System. Journal of Phase Equilibria, 14(4): 525-530 DOI:10.1007/BF02671975
|
Karup-Möller S. 1977. Mineralogy of some Ag-(Cu)-Pb-Bi sulphide associations. Bulletin of the Geological Society of Denmark, 26: 41-68
|
Keppens V, Mandrus D, Rankin J and Boatner LA. 1996. The formation of metal/metal-matrix nanocomposites by the ultrasonic dispersion of immiscible liquid metals. MRS Online Proceedings Library, 457(1): 243-248
|
Kiseeva ES, Fonseca ROC and Smythe DJ. 2017. Chalcophile elements and sulfides in the upper mantle. Elements, 13(2): 111-116 DOI:10.2113/gselements.13.2.111
|
Kutoglu A. 1969. Röntgenographische und termische Untersuchungen im quasibinären system PbS-As2S3. Neues Jahrbuch für Mineralogie Monatshefte, 2: 68-72
|
Lbibb R, Castanet R and Rais A. 2000. Thermodynamic investigation of Pt-Pb binary alloys. Journal of alloys and compounds, 302(1): 155-158
|
Li C, Su CH, Lehoczky SL, Scripa RN, Lin B and Ban H. 2005. Thermophysical properties of liquid Te: Density, electrical conductivity, and viscosity. Journal of Applied Physics, 97(8): 083513 DOI:10.1063/1.1868881
|
Li MK and Fogler HS. 1978. Acoustic emulsification. Part 1. The instability of the oil-water interface to form the initial droplets. Journal of Fluid Mechanics, 88(3): 499-511 DOI:10.1017/S0022112078002232
|
Li MY and Wu ZL. 2009. Petroleum Emulsion. Beijing: Science Press, 1-108 (in Chinese)
|
Li Y and Audétat A. 2013. Gold solubility and partitioning between sulfide liquid, monosulfide solid solution and hydrous mantle melts: Implications for the formation of Au-rich magmas and crust-mantle differentiation. Geochimica et Cosmochimica Acta, 118: 247-262 DOI:10.1016/j.gca.2013.05.014
|
Li Y and Audétat A. 2015. Effects of temperature, silicate melt composition, and oxygen fugacity on the partitioning of V, Mn, Co, Ni, Cu, Zn, As, Mo, Ag, Sn, Sb, W, Au, Pb, and Bi between sulfide phases and silicate melt. Geochimica et Cosmochimica Acta, 162: 25-45 DOI:10.1016/j.gca.2015.04.036
|
Li Y, Feng L, Kiseeva ES, Gao ZH, Guo HH, Du ZX, Wang FY and Shi LL. 2019. An essential role for sulfur in sulfide-silicate melt partitioning of gold and magmatic gold transport at subduction settings. Earth and Planetary Science Letters, 528: 115850 DOI:10.1016/j.epsl.2019.115850
|
Lin JC, Sharma RC and Chang YA. 1996. The Bi-S (bismuth-sulfur) system. Journal of Phase Equilibria, 17(2): 132-139 DOI:10.1007/BF02665790
|
Liu JJ, Dai HZ, Zhai DG, Wang JP, Wang YH, Yang LB, Mao GJ, Liu XH, Liao YF, Yu C and Li QZ. 2015. Geological and geochemical characteristics and formation mechanisms of the Zhaishang Carlin-like type gold deposit, western Qinling Mountains, China. Ore Geology Reviews, 64: 273-298 DOI:10.1016/j.oregeorev.2014.07.016
|
Liu JJ, Zhai DG, Dai HZ, De Fourestier J, Yu C, Gu XP, Wang YH, Yu H, Wang JP and Liu ZJ. 2017. Nanoscale characterization of Au2Te grains from the Sandaowanzi gold deposit, Northeast China. The Canadian Mineralogist, 55(2): 181-194 DOI:10.3749/canmin.1600077
|
Liu JJ, Zheng MH, Liu JM and Su WC. 2000. Geochemistry of the La'erma and Qiongmo Au-Se deposits in the western Qinling Mountains, China. Ore Geology Reviews, 17(1-2): 91-111 DOI:10.1016/S0169-1368(00)00008-1
|
Liu JJ, Zhai DG, Wang DZ, Gao S, Yin C, Liu ZJ, Wang JP, Wang YH and Zhang FF. 2020. Classification and mineralization of the Au-(Ag)-Te-Se deposits. Earth Science Frontiers, 27(2): 79-98 (in Chinese with English abstract)
|
Liu JL, Zhao SJ, Cook NJ, Bai XD, Zhang ZC, Zhao ZD, Zhao HB and Lu J. 2013. Bonanza-grade accumulations of gold tellurides in the Early Cretaceous Sandaowanzi deposit, Northeast China. Ore Geology Reviews, 54: 110-126 DOI:10.1016/j.oregeorev.2013.03.003
|
Liu JL, Fan XF, Shi YF, Singh DJ and Zheng WT. 2019. Melting of nanocrystalline gold. The Journal of Physical Chemistry C, 123(1): 907-914 DOI:10.1021/acs.jpcc.8b10149
|
Liu LG and Bassett WA. 1986. Elements, Oxides, and Silicates: High Pressure Phases with Implications for the Earth's Interior. New York: Oxford University Press, 1-250
|
Liu YJ, Wang G, Wang J, Chen Y and Long ZH. 2012. Phase equilibria and thermodynamic functions for Ag-Hg and Cu-Hg binary systems. Thermochimica Acta, 547(10): 83-88
|
Liu YN and Brenan J. 2015. Partitioning of platinum-group elements (PGE) and chalcogens (Se, Te, As, Sb, Bi) between monosulfide-solid solution (MSS), intermediate solid solution (ISS) and sulfide liquid at controlled fO2-fS2 conditions. Geochimica et Cosmochimica Acta, 159: 139-161 DOI:10.1016/j.gca.2015.03.021
|
Lukas HL. 1980. The Ag-Bi-Pb system (silver-bismuth-lead). Bulletin of Alloy Phase Diagrams, 1(1): 64-67 DOI:10.1007/BF02883294
|
Lusk J, Calder BOE and Freeman TE. 2002. Temperatures from triple-junction angles in sulfides. American Mineralogist, 87(10): 1390-1400 DOI:10.2138/am-2002-1015
|
Ma GF, Liu N, Zhang HF, Li H and Hu ZQ. 2008. Wetting of molten Bi-Sn alloy on amorphous Fe78B13Si9. Journal of Alloys and Compounds, 456(1-2): 379-383 DOI:10.1016/j.jallcom.2007.02.086
|
Makovicky E, Karup-Möller S, Makovicky M and Rose-Hansen J. 1990. Experimental studies on the phase systems Fe-Ni-Pd-S and Fe-Pt-Pd-As-S applied to PGE deposits. Mineralogy and Petrology, 42(1-4): 307-319 DOI:10.1007/BF01162697
|
Mansur ET, Barnes SJ, Duran CJ and Sluzhenikin SF. 2020. Distribution of chalcophile and platinum-group elements among pyrrhotite, pentlandite, chalcopyrite and cubanite from the Noril'sk-Talnakh ores: Implications for the formation of platinum-group minerals. Mineralium Deposita, 55(6): 1215-1232 DOI:10.1007/s00126-019-00926-z
|
Markham NL. 1960. Synthetic and natural phases in the system Au-Ag-Te. Economic Geology, 55(6): 1148-1178 DOI:10.2113/gsecongeo.55.6.1148
|
Martin W, Freyland W, Lamparter P and Steeb S. 1980. Structure and density of gold-cesium-melts. II. Neutron diffraction with molten gold-cesium-alloys. Physics and Chemistry of Liquids, 10(1): 61-76 DOI:10.1080/00319108008078457
|
Maske S and Skinner BJ. 1971. Studies of the sulfosalts of copper: I. Phases and phase relations in the system Cu-As-S. Economic Geology, 66(6): 901-918
|
Mavrogenes J, Frost R and Sparks HA. 2013. Experimental evidence of sulfide melt evolution via immiscibility and fractional crystallization. The Canadian Mineralogist, 51(6): 841-850 DOI:10.3749/canmin.51.6.841
|
Mavrogenes JA and O'Neill HSC. 1999. The relative effects of pressure, temperature and oxygen fugacity on the solubility of sulfide in mafic magmas. Geochimica et Cosmochimica Acta, 63(7-8): 1173-1180 DOI:10.1016/S0016-7037(98)00289-0
|
Mavrogenes JA, MacIntosh IW and Ellis DJ. 2001. Partial melting of the Broken Hill Galena-Sphalerite ore: Experimental studies in the system PbS-FeS-ZnS-(Ag2S). Economic Geology, 96(1): 205-210 DOI:10.2113/gsecongeo.96.1.205
|
McCoy DT. 2000. Mid-Cretaceous plutonic-related gold deposits of interior Alaska: Characteristics, metallogenesis, gold-associative mineralogy, and geochronology. Ph. D. Dissertation. Fairbanks: University of Alaska Fairbanks
|
McFall KA, Naden J, Roberts S, Baker T, Spratt J and McDonald I. 2018. Platinum-group minerals in the Skouries Cu-Au (Pd, Pt, Te) porphyry deposit. Ore Geology Reviews, 99: 344-364 DOI:10.1016/j.oregeorev.2018.06.014
|
McLeish DF, Williams-Jones AE, Vasyukova OV, Clark JR and Board WS. 2021. Colloidal transport and flocculation are the cause of the hyperenrichment of gold in nature. Proceedings of the National Academy of Sciences of the United States of America, 118(20): e2100689118 DOI:10.1073/pnas.2100689118
|
Medvedeva ZS, Klochko MA, Kuznetsov VG and Andreeva SN. 1961. Structural diagram of the Pd-Te alloy system. Zhurnal Neorganicheskoi Khimii, 6: 1737-1739
|
Meinert LD. 2000. Gold in skarns related to epizonal intrusions. Reviews in Economic Geology, 13: 347-375
|
Müller D and Groves DI. 2016. Potassic Igneous Rocks and Associated Gold-Copper Mineralization. 4th Edition. Springer
|
Mungall JE and Brenan JM. 2003. Experimental evidence for the chalcophile behavior of the halogens. The Canadian Mineralogist, 41(1): 207-220 DOI:10.2113/gscanmin.41.1.207
|
Mungall JE and Brenan JM. 2014. Partitioning of platinum-group elements and Au between sulfide liquid and basalt and the origins of mantle-crust fractionation of the chalcophile elements. Geochimica et Cosmochimica Acta, 125: 265-289 DOI:10.1016/j.gca.2013.10.002
|
Naldrett A. 2004. Magmatic Sulfide Deposits: Geology, Geochemistry and Exploration. Berlin: Springer, 1-725
|
Nechaev SV and Bondarenko SN. 1997. Ore mineral assemblages of the Maiskoe gold deposit. Geochemistry International, 35(6): 516-526
|
Oberthür T and Weiser TW. 2008. Gold-bismuth-telluride-sulphide assemblages at the Viceroy Mine, Harare-Bindura-Shamva greenstone belt, Zimbabwe. Mineralogical Magazine, 72(4): 953-970 DOI:10.1180/minmag.2008.072.4.953
|
Okamoto H and Massalski TB. 1983a. The Au-Bi (gold-bismuth) system. Bulletin of Alloy Phase Diagrams, 4(4): 401-407 DOI:10.1007/BF02868093
|
Okamoto H and Massalski TB. 1983b. The Au-Ti (gold-thallium) system. Bulletin of Alloy Phase Diagrams, 4(2): 199-204 DOI:10.1007/BF02884879
|
Okamoto H and Massalski TB. 1984a. The As-Au (arsenic-gold) system. Bulletin of Alloy Phase Diagrams, 5(1): 56-59 DOI:10.1007/BF02868726
|
Okamoto H and Massalski TB. 1984b. The Au-Sn (gold-tin) system. Bulletin of Alloy Phase Diagrams, 5(5): 492-503 DOI:10.1007/BF02872904
|
Okamoto H and Massalski TB. 1984c. The Au-Te (gold-tellurium) system. Bulletin of Alloy Phase Diagrams, 5(2): 172-177 DOI:10.1007/BF02868955
|
Okamoto H and Massalski TB. 1984d. The Au-Sb (gold-antimony) system. Bulletin of Alloy Phase Diagrams, 5(2): 166-171 DOI:10.1007/BF02868954
|
Okamoto H and Massalski TB. 1989. The Au-Hg (gold-mercury) system. Bulletin of Alloy Phase Diagrams, 10(1): 50-58 DOI:10.1007/BF02882176
|
Okamoto H. 1990a. The As-Pt (arsenic-platinum) system. Journal of Phase Equilibria, 11(5): 508-510 DOI:10.1007/BF02898270
|
Okamoto H. 1990b. The As-Pd (arsenic-palladium) system. Journal of Phase Equilibria, 11(5): 503-507 DOI:10.1007/BF02898269
|
Okamoto H. 1991. The Bi-Pt (bismuth-platinum) system. Journal of Phase Equilibria, 12(2): 207-210 DOI:10.1007/BF02645718
|
Okamoto H. 1992a. The pd-se (palladium-selenium) system. Journal of Phase Equilibria, 13(1): 69-72 DOI:10.1007/BF02645382
|
Okamoto H. 1992b. The pd-te system (palladium-tellurium). Journal of Phase Equilibria, 13(1): 73-78 DOI:10.1007/BF02645383
|
Okamoto H. 1992c. Pt-Sb (platinum-antimony). Journal of Phase Equilibria, 13(5): 580-581 DOI:10.1007/BF02665778
|
Okamoto H. 1993. Au-Pb (gold-lead). Journal of Phase Equilibria, 14(5): 648-649 DOI:10.1007/BF02669159
|
Okamoto H. 1994a. The Bi-Pd (bismuth-palladium) system. Journal of Phase Equilibria, 15(2): 191-194 DOI:10.1007/BF02646365
|
Okamoto H. 1994b. Comment on Pt-Te (platinum-tellurium). Journal of Phase Equilibria, 15(4): 456-457
|
Okamoto H. 2003. Pt-Sn (platinum-tin). Journal of Phase Equilibria, 24(2): 198-198 DOI:10.1361/105497103770330938
|
Okamoto H. 2007. Ag-Sb (silver-antimony). Journal of Phase Equilibria and Diffusion, 28(4): 403-403 DOI:10.1007/s11669-007-9113-y
|
Okamoto H. 2012. Pd-Sn (palladium-tin). Journal of Phase Equilibria and Diffusion, 33(3): 253-254 DOI:10.1007/s11669-012-0025-0
|
Okamoto H. 2015. Supplemental literature review of binary phase diagrams: Al-Mg, Bi-Sr, Ce-Cu, Co-Nd, Cu-Nd, Dy-Pb, Fe-Nb, Nd-Pb, Pb-Pr, Pb-Tb, Pd-Sb, and Si-W. Journal of Phase Equilibria and Diffusion, 36(2): 183-195 DOI:10.1007/s11669-014-0359-x
|
Osadchii EG. 1990. The kesterite-velikite (Cu2Zn1-xHgxSnS4) and shalerite-metacinnabarite (Zn1-xHgxS) solid solutions in the system Cu2SnS3-Zn-HgS at temperatures of 850, 700 and 550℃. Neues, Jahrbuch für Mineralogie, Monatshefte, H1: 13-34
|
Palomba M and Carotenuto G. 2016. Precipitation of lamellar gold nanocrystals in molten polymers. AIP Conference Proceedings, 1736(1): 020151
|
Piña R, Gervilla F, Barnes SJ, Ortega L and Lunar R. 2012. Distribution of platinum-group and chalcophile elements in the Aguablanca Ni-Cu sulfide deposit (SW Spain): Evidence from a LA-ICP-MS study. Chemical Geology, 302-303: 61-75 DOI:10.1016/j.chemgeo.2011.02.010
|
Piña R, Gervilla F, Barnes SJ, Ortega L and Lunar R. 2013. Partition coefficients of platinum group and chalcophile elements between arsenide and sulfide phases as determined in the Beni Bousera Cr-Ni mineralization (North Morocco). Economic Geology, 108(5): 935-951 DOI:10.2113/econgeo.108.5.935
|
Prichard HM, Hutchinson D and Fisher PC. 2004. Petrology and crystallization history of multiphase sulfide droplets in a Mafic Dike from Uruguay: Implications for the origin of Cu-Ni-PGE sulfide deposits. Economic Geology, 99(2): 365-376 DOI:10.2113/gsecongeo.99.2.365
|
Prokofiev VY, Banks DA, Lobanov KV, Selektor SL, Milichko VA, Akinfiev NN, Borovikov AA, Lüders V and Chicherov MV. 2020. Exceptional Concentrations of Gold Nanoparticles in 1.7Ga Fluid Inclusions From the Kola Superdeep Borehole, Northwest Russia. Scientific Reports, 10: 1108 DOI:10.1038/s41598-020-58020-8
|
Pudack C, Halter WE, Heinrich CA and Pettke T. 2009. Evolution of magmatic vapor to gold-rich epithermal liquid: The porphyry to epithermal transition at Nevados de Famatina, Northwest Argentina. Economic Geology, 104(4): 449-477 DOI:10.2113/gsecongeo.104.4.449
|
Pyykkö P. 2004. Theoretical chemistry of gold. Angewandte Chemie International Edition, 43(34): 4412-4456 DOI:10.1002/anie.200300624
|
Rajkumar VB and Chen S. 2018. Ag-Se phase diagram calculation associating ab-initio molecular dynamics simulation. Calphad, 63: 51-60 DOI:10.1016/j.calphad.2018.08.004
|
Richards JP. 1995. Alkalic-type epithermal gold deposits: A review. Mineralogical Association of Canada Short Course, 23: 367-400
|
Richter KW and Ipser H. 1994. Transition metal-chalcogen systems XI: The platinum-selenium phase diagram. Journal of Phase Equilibria, 15(2): 165-170 DOI:10.1007/BF02646360
|
Roland GW. 1970. Phase relations below 575℃ in the system Ag-As-S. Economic Geology, 65(3): 241-252 DOI:10.2113/gsecongeo.65.3.241
|
Rose LA and Brenan JM. 2001. Wetting properties of Fe-Ni-Co-Cu-O-S melts against olivine: Implications for sulfide melt mobility. Economic Geology, 96(1): 145-157 DOI:10.2113/gsecongeo.96.1.145
|
Salanci B. 1979. Contribution to the system PbS-Sb2S3 in relation to lead-antimony-sulfosalts. Neues Jahrbuch für Mineralogie, Abhandlungen, 135: 315-326
|
Shao TB, Ji SC and Wang Q. 2011. Rheology of partially molten rocks: A state-of-the-art overview. Geological Review, 57(6): 851-869 (in Chinese with English abstract)
|
Sharp ZD, Essene EJ and Kelly WC. 1985. A re-examination of the arsenopyrite geothermometer, pressure considerations and applications to natural assemblages. The Canadian Mineralogist, 23(4): 517-534
|
Sillitoe RH. 2002. Some metallogenic features of gold and copper deposits related to alkaline rocks and consequences for exploration. Mineralium Deposita, 37(1): 4-13 DOI:10.1007/s00126-001-0227-6
|
Sinyakova EF, Kosyakov VI and Goryachev NA. 2019. Formation of drop-shaped inclusions based on Pt, Pd, Au, Ag, Bi, Sb, Te, and As under crystallization of an intermediate solid solution in the Cu-Fe-Ni-S system. Doklady Earth Sciences, 489(1): 1301-1305 DOI:10.1134/S1028334X19110072
|
Sklyarchuk V, Plevachuk Y, Gerbeth G and Eckert S. 2007. Melting-solidification process in Pb-Bi melts. Journal of Physics: Conference Series, 79(1): 012019 DOI:10.1088/1742-6596/79/1/012019/pdf
|
Smith CS. 1948. Grains, phases, and interfaces: An interpretation of microstructure. Transactions of the AIME, 175: 15-51
|
Sobott RJG. 1984. Sulfosalts and Tl2S-As2S3-Sb2S3-S phase relations. Neues Jahrbuch für Mineralogie-Abhandlungen, 150: 54-59
|
Song XY, Zhou MF, Tao Y and Xiao JF. 2008. Controls on the metal compositions of magmatic sulfide deposits in the Emeishan large igneous province, SW China. Chemical Geology, 253(1-2): 38-49 DOI:10.1016/j.chemgeo.2008.04.005
|
Sparks HA and Mavrogenes JA. 2005. Sulfide melt inclusions as evidence for the existence of a sulfide partial melt at Broken Hill, Australia. Economic Geology, 100(4): 773-779 DOI:10.2113/gsecongeo.100.4.773
|
Springer G and Laflamme JHG. 1971. The system Bi2S3-Sb2S3. Canadian Mineralogist, 10(5): 847-853
|
Spry PG, Plimer IR and Teale GS. 2008. Did the giant Broken Hill (Australia) Zn-Pb-Ag deposit melt?. Ore Geology Reviews, 34(3): 223-241 DOI:10.1016/j.oregeorev.2007.11.001
|
Stevens G, Prinz S and Rozendaal A. 2005. Partial melting of the assemblage sphalerite+galena+pyrrhotite+chalcopyrite+sulfur: Implications for high-grade metamorphosed massive sulfide deposits. Economic Geology, 100(4): 781-786 DOI:10.2113/gsecongeo.100.4.781
|
Tomkins AG and Mavrogenes JA. 2002. Mobilization of gold as a polymetallic melt during pelite anatexis at the Challenger deposit, South Australia: A metamorphosed Archean gold deposit. Economic Geology, 97(6): 1249-1271
|
Tomkins AG, Pattison DRM and Zaleski E. 2004. The Hemlo gold deposit, Ontario: An example of melting and mobilization of a precious metal-sulfosalt assemblage during amphibolite facies metamorphism and deformation. Economic Geology, 99(6): 1063-1084 DOI:10.2113/gsecongeo.99.6.1063
|
Tomkins AG, Frost BR and Pattison DRM. 2006. Arsenopyrite melting during metamorphism of sulfide ore deposits. The Canadian Mineralogist, 44(5): 1045-1062 DOI:10.2113/gscanmin.44.5.1045
|
Tomkins AG, Pattison DRM and Frost BR. 2007. On the initiation of metamorphic sulfide anatexis. Journal of Petrology, 48(3): 511-535
|
Tooth B, Brugger J, Ciobanu C and Liu WH. 2008. Modeling of gold scavenging by bismuth melts coexisting with hydrothermal fluids. Geology, 36(10): 815-818 DOI:10.1130/G25093A.1
|
Tooth B, Ciobanu CL, Green L, O'Neill B and Brugger J. 2011. Bi-melt formation and gold scavenging from hydrothermal fluids: An experimental study. Geochimica et Cosmochimica Acta, 75(19): 5423-5443 DOI:10.1016/j.gca.2011.07.020
|
Toörmaänen TO and Koski RA. 2005. Gold enrichment and the Bi-Au association in pyrrhotite-rich massive sulfide deposits, Escanaba Trough, Southern Gorda Ridge. Economic Geology, 100(6): 1135-1150 DOI:10.2113/gsecongeo.100.6.1135
|
Urazov GG, Sokolova MA and Brandes EA. 1983. The System Ag2S-PbS, Metals Reference Book. London Butterworths: Heinemann, 1-1664
|
Vassiliev V, Voronin GF, Borzone G, Mathon M, Gambino M and Bros JP. 1998. Thermodynamics of the Pb-Pd system. Journal of alloys and compounds, 269(1-2): 123-132 DOI:10.1016/S0925-8388(98)00120-0
|
Vernon RH. 2004. A Practical Guide to Rock Microstructure. Cambridge: Cambridge University Press, 1-594
|
Wagner T. 2007. Thermodynamic modeling of Au-Bi-Te melt precipitation from high-temperature hydrothermal fluids: Preliminary results. In: Andrew CJ (ed. ). Mineral Exploration and Research: Digging Deeper. Proceedings of the 9th Biennal Meeting of the Society for Geology Applied to Mineral Deposits. Dublin, Ireland, 769-772
|
Walia DS and Chang LLY. 1973. Investigations in the systems PbS-Sb2S3-As2S3 and PbS-Bi2S3-As2S3. Canadian Mineralogist, 12: 113-119
|
Wang DZ, Liu JJ, Zhai DG, Carranza EJM, Wang YH, Zhen SM, Wang J, Wang JP, Liu ZJ and Zhang FF. 2019. Mineral paragenesis and ore-forming processes of the Dongping gold deposit, Hebei Province, China. Resource Geology, 69(3): 287-313 DOI:10.1111/rge.12202
|
Wang DZ, Liu JJ, Zhai DG, De Fourestier J, Wang YH, Zhen SM, Wang JP, Liu ZJ and Zhang FF. 2020. Textures and formation of microporous gold in the Dongping gold deposit, Hebei Province, China. Ore Geology Reviews, 120: 103437 DOI:10.1016/j.oregeorev.2020.103437
|
Wang DZ, Zhen SM, Liu JJ, Carranza JME, Wang J, Zha ZJ, Li YS and Bai HJ. 2021. Mineral paragenesis and hydrothermal evolution of the Dabaiyang tellurium-gold deposit, Hebei Province, China: Constraints from fluid inclusions, H-O-He-Ar isotopes, and physicochemical conditions. Ore Geology Reviews, 130: 103904 DOI:10.1016/j.oregeorev.2020.103904
|
Wang J, Meng FG, Liu HS, Liu LB and Jin ZP. 2007. Thermodynamic modeling of the Au-Bi-Sb ternary system. Journal of Electronic Materials, 36(5): 568-577 DOI:10.1007/s11664-007-0090-z
|
Wang L, Liu JJ, Zhu WB, Dai HZ, Liu CH and Liu HN. 2016. Mineral association and mechanism of mineral precipitation of Lianzigou gold-telluride deposit in Xiaoqinling gold orefield, Shaanxi Province. Mineral Deposits, 35(3): 456-474 (in Chinese with English abstract)
|
Watkins GD and Williams PM. 1995. Vacancy model for substitutional Ni-, Pd-, Pt-, and Au0 in silicon. Physical Review B, 52(23): 16575-16580 DOI:10.1103/PhysRevB.52.16575
|
Wei B, Wang CY, Wang ZC, Cheng H, Xia XP and Tan W. 2021. Mantle-derived gold scavenged by bismuth-(tellurium)-rich melts: Evidence from the mesozoic wulong gold deposit in the North China Craton. Ore Geology Reviews, 131: 104047 DOI:10.1016/j.oregeorev.2021.104047
|
Wu DQ. 1987. Phase relations in the system Ag2S-Cu2S-PbS-Bi2S3 (Ⅱ): The crystal chemistry and classification of the Ag-, Cu-, and Pb-bismuth sulfosalt minerals. Acta Mineralogica Sinica, 7(3): 210-220 (in Chinese with English abstract)
|
Wykes JL and Mavrogenes JA. 2005. Hydrous sulfide melting: Experimental evidence for the solubility of H2O in sulfide melts. Economic Geology, 100(1): 157-164 DOI:10.2113/100.1.0157
|
Xu H, Yu YX, Wu XK, Ynag LJ, Tian Z, Gao S and Wang QS. 2012. Intergrowth texture in Au-Ag-Te minerals from Sandaowanzi gold deposit, Heilongjiang Province: Implications for ore-forming environment. Chinese Science Bulletin, 57(21): 2778-2786 DOI:10.1007/s11434-012-5170-7
|
Yan XH, Li GC and Meng Q. 2019. Research progress of gold extraction technology in gold deposits. Applied Chemical Industry, 48(11): 2719-2723 (in Chinese with English abstract)
|
Yang LX, Zhao X, Xu S, Lu YY, Chang H and Liu J. 2020. Oxide transformation and break-up of liquid metal in boiling solutions. Science China (Technological Sciences), 63(2): 289-296 DOI:10.1007/s11431-018-9444-5
|
Yassin A and Castanet R. 1998. Investigation of the short-range order in Au-Te melts. Journal of Alloys and Compounds, 281(2): 237-240 DOI:10.1016/S0925-8388(98)00786-5
|
Yin C, Liu JJ, Carranza EJM, Zhai DG and Guo YC. 2019. Mineralogical constraints on the genesis of the Dahu quartz vein-style Au-Mo deposit, Xiaoqinling gold district, China: Implications for phase relationships and physicochemical conditions. Ore Geology Reviews, 113: 103107 DOI:10.1016/j.oregeorev.2019.103107
|
Zajacz Z, Candela PA, Piccoli PM, Wälle M and Sanchez-Valle C. 2012. Gold and copper in volatile saturated mafic to intermediate magmas: Solubilities, partitioning, and implications for ore deposit formation. Geochimica et Cosmochimica Acta, 91: 140-159 DOI:10.1016/j.gca.2012.05.033
|
Zajacz Z, Candela PA, Piccoli PM, Sanchez-Valle C and Wälle M. 2013. Solubility and partitioning behavior of Au, Cu, Ag and reduced S in magmas. Geochimica et Cosmochimica Acta, 112: 288-304 DOI:10.1016/j.gca.2013.02.026
|
Zhai DG and Liu JJ. 2014. Gold-telluride-sulfide association in the Sandaowanzi epithermal Au-Ag-Te deposit, NE China: Implications for phase equilibrium and physicochemical conditions. Mineralogy and Petrology, 108(6): 853-871 DOI:10.1007/s00710-014-0334-6
|
Zhai DG, Williams-Jones AE, Liu JJ, Tombros SF and Cook NJ. 2018. Mineralogical, fluid inclusion, and multiple isotope (H-O-S-Pb) constraints on the genesis of the Sandaowanzi epithermal Au-Ag-Te deposit, NE China. Economic Geology, 113(6): 1359-1382 DOI:10.5382/econgeo.2018.4595
|
Zhao ZH, Xiong ZL, Wang Q, Bao ZW, Zhang YQ, Xie YW and Ren SK. 2002. Mineralization of alkali-rich igneous rock related large-super large gold-copper deposits in China. Science in China (Series D), 32(Suppl.1): 1-10 (in Chinese)
|
Zheng B, An F and Zhu YF. 2009. Native bismuth found in Baogutu gold deposit and its geological significance. Acta Petrologica Sinica, 25(6): 1426-1436 (in Chinese with English abstract)
|
Zheng B, Zhang JG, Chen G and Zhu YF. 2013. Special mineral assemblage in L7 vein of No. 4 ore district within Baogutu gold deposit of Xinjiang and its ore-forming significance. Mineral Deposits, 32(6): 1117-1138 (in Chinese with English abstract)
|
Zhou HY, Sun XM, Cook NJ, Lin H, Fu Y, Zhong RC and Brugger J. 2017. Nano-to micron-scale particulate gold hosted by magnetite: A product of gold scavenging by bismuth melts. Economic Geology, 112(4): 993-1010 DOI:10.2113/econgeo.112.4.993
|
Zhou HY, Sun XM, Wu ZW, Yang TJ, Li DS, Ren YZ, Liu QF, Zhu KJ and Yu HJ. 2018. Mineralogy of Bi-sulfosalts and tellurides from the Yaoan gold deposit, Southwest China: Metallogenic implications. Ore Geology Reviews, 98: 126-140 DOI:10.1016/j.oregeorev.2018.05.004
|
曹锡章, 宋天佑, 王杏乔. 1994. 无机化学. 第3版. 北京: 高等教育出版社, 495-508.
|
成曦晖, 徐九华, 杨富全, 张辉, 边春静. 2017. 辽东五龙金矿流体包裹体研究. 矿物学报, 37(增): 460-461. |
冯浩轩. 2021. 辽东五龙和白云大型金矿床成矿时代与成矿作用: 富U和富Bi副矿物新视角. 博士学位论文. 北京: 中国科学院大学
|
Greer HF, 于丰娇, 周午纵. 2012. 非传统晶体生长的早期观察. 中国科学(化学), 42(1): 74-83. |
侯渭, 周文戈, 谢鸿森, 刘永刚. 2004. 高温高压岩石粒间熔体(和流体)形态学及其研究进展. 地球科学进展, 19(5): 767-773. DOI:10.3321/j.issn:1001-8166.2004.05.013 |
李明远, 吴肇亮. 2009. 石油乳状液. 北京: 科学出版社, 1-108.
|
刘家军, 翟德高, 王大钊, 高燊, 尹超, 柳振江, 王建平, 王银宏, 张方方. 2020. Au-(Ag)-Te-Se成矿系统与成矿作用. 地学前缘, 27(2): 79-98. |
邵同宾, 嵇少丞, 王茜. 2011. 部分熔融岩石流变学. 地质论评, 57(6): 851-869. |
王雷, 刘家军, 朱文兵, 代鸿章, 刘冲昊, 刘华南. 2016. 陕西小秦岭镰子沟碲金矿床物质组成特征及矿质沉淀机理研究. 矿床地质, 35(3): 456-474. |
吴大清. 1987. Ag2S-Cu2S-PbS-Bi2S3四元体系相关系研究(Ⅱ): 铜银铅铋硫盐矿物结晶化学及分类. 矿物学报, 7(3): 210-220. |
许虹, 余宇星, 吴祥珂, 杨利军, 田竹, 高燊, 王秋舒. 2012. 黑龙江三道湾子金矿Au-Ag-Te矿物交生结构对成矿条件的指示. 科学通报, 57(23): 2200-2210. |
闫晓慧, 李桂春, 孟齐. 2019. 金矿中提金技术的研究进展. 应用化工, 48(11): 2719-2723. DOI:10.3969/j.issn.1671-3206.2019.11.042 |
赵振华, 熊小林, 王强, 包志伟, 张玉泉, 谢应雯, 任双奎. 2002. 我国富碱火成岩及有关的大型-超大型金铜矿床成矿作用. 中国科学(D辑), 32(增1): 1-10. |
郑波, 安芳, 朱永峰. 2009. 新疆包古图金矿中发现的自然铋及其找矿勘探意义. 岩石学报, 25(6): 1426-1436. |
郑波, 张晋国, 陈刚, 朱永峰. 2013. 新疆包古图金矿四矿区L7号脉中的特殊矿物组合及其成矿意义. 矿床地质, 32(6): 1117-1138. DOI:10.3969/j.issn.0258-7106.2013.06.003 |
 2021, Vol. 37
2021, Vol. 37

