早期的野外观察和随后的一系列岩石学实验,证明压力(P, pressure)、温度(T, temperature)不仅决定了变质岩、岩浆岩中各种“相”(矿物、流体、熔体)出现和消失的先后顺序,还直接制约它们化学成分的变化。例如,花岗岩中共生的斜长石和碱性长石的成分变化情况,就明显受到温度条件的控制,据此人们建立了温度与两种长石化学成分之间的函数关系,即二长石温度计(Barth, 1934; 转引自Putirka, 2008)。二长石温度计是人类历史上最早建立的地质温度计。另一方面,如果压力占据了主导地位即压力条件是控制矿物组合中各种相化学成分的主要因素,就可据此建立地质压力计。有些地质温度计和压力计,建立在涉及流体的“模式反应”(model reaction)基础上,例如绿泥石-白云母-黑云母压力计(Bucher-Nurminen, 1987)。但是,绝大多数地质温度计、压力计与流体无关,因此分别称为矿物温度计、矿物压力计。
天然变质岩、岩浆岩中的大多数矿物,都是矿物晶格中混有“杂质”离子的“固态溶液”(固溶体,solid solution)。在一定的P-T范围内,温度或压力的变化,会导致矿物组合中各种矿物的化学成分发生变化,但既不会有旧相的消失,也不会有新相的出现。矿物温度计、压力计就是利用了此类“缓冲”特性建立的。矿物成分连续变化的此种模式矿物反应,又称为连续反应(continuous reaction)、滑动反应(sliding reaction)、缓冲反应(buffer reaction)。
例如,变质泥质岩中常用的石榴子石-黑云母(GB)温度计,是建立在石榴子石-黑云母之间Fe2+-Mg2+离子交换的滑动反应(Ferry and Spear, 1978)基础上:
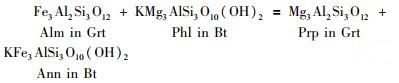
|
(1) |
滑动反应(1)左侧为相对低温矿物组合,右侧为相对高温矿物组合(本文采用沈其韩(2009)、Whitney and Evans (2010)推荐的矿物缩写代码)。对天然岩石的研究及石榴子石-黑云母之间Fe2+-Mg2+离子交换的相平衡实验都证实,给定压力条件下,随着温度的升高,石榴子石中Fe2+/Mg2+比值越来越小,黑云母中Fe2+/Mg2+比值越来越大,实际上就是石榴子石(Grt)中的铁铝榴石(Alm)、镁铝榴石(Prp)与黑云母(Bt)中的铁云母(Ann)、金云母(Phl)之间Fe2+、Mg2+浓度的此消彼长过程。石榴子石、黑云母化学成分的变化,受压力条件的影响很小。通常1kabr的压力变化,引起石榴子石-黑云母温度计的温度变化仅有3~5℃(Holdaway, 2000)。
天然变质泥质岩中,石榴子石+黑云母组合稳定的温度范围很宽,从绿片岩相(Ghent, 1975; McLellan, 1985; Stäubli, 1989; Owona et al. , 2011)、角闪岩相、麻粒岩相(Indares and Martignole, 1990; Bhowmik and Spiering, 2004; Vrána et al. , 2005; Millonig et al. , 2008; Estrada-Carmona et al. , 2009; Hallett and Spear, 2014; Yin et al. , 2014)乃至超高温麻粒岩相(Mohan and Windley, 1993; Patiño Douce et al. , 1993; Guo et al. , 2012)条件下,它们都可稳定共生。这表明,石榴子石-黑云母矿物对只要随时保持Fe2+-Mg2+离子交换的平衡状态,即不停地调整各自的化学成分,它们就能够自绿片岩相一直“携手”共存、滑动演化到麻粒岩相乃至超高温麻粒岩相条件下。因此,石榴子石-黑云母温度计适用的温度范围很宽广。实际上,天然石榴子石中还含有Ca2+、Mn2+离子,黑云母中还含有Al3+、Ti4+离子。为了准确刻画它们之间Fe2+-Mg2+离子交换的热力学效应、建立准确的石榴子石-黑云母温度计,需要准确描述铁铝榴石、镁铝榴石、铁云母、金云母的活度(activity),即它们参与平衡反应的“有效浓度”或曰“活性”。
变质泥质岩中常用的石榴子石-Al2SiO5矿物-斜长石-石英(GASP)压力计,建立在GASP矿物组合的滑动反应(Ghent, 1976)基础上:

|
(2) |
滑动反应(2)左侧为相对低压矿物,右侧为相对高压矿物组合。天然变质泥质岩中,Al2SiO5矿物(红柱石,And;蓝晶石,Ky;夕线石,Sil)和石英(Qz)为不含“杂质”的纯物质。该滑动反应表明,在矿物组合达到热力学平衡状态下,如果固定了温度条件,那么随着压力的升高,石榴子石中Ca2+离子浓度逐渐升高,斜长石中Ca2+离子浓度逐渐降低。压力逐渐降低的情况下,矿物成分演变规律与之相反。对于GASP矿物组合,温度条件对石榴子石、斜长石化学成分的控制程度较弱。通常50℃的温度变化,引起的GASP的压力变化为0.8~1.0kbar(Holdaway, 2001)。
对天然变质泥质岩的研究表明,GASP矿物组合稳定的压力范围较大。从低压(1~2kbar; Briggs and Foster, 1992; Percival and Skulski, 2000; Zeh and Holness, 2003)、中压(5~8kbar)到高压(10~17kbar; Lemennicier et al. , 1996; Liu and Zhong, 1997; Ding and Zhong, 1999; Ghebreab, 1999; Foster et al. , 2002; Vrána et al. , 2005, 2013; Owona et al. , 2011; Yin et al. , 2014; 范文寿等, 2018)条件下,该矿物组合都可稳定共生,因此GASP压力计应用的压力范围也比较宽广。石榴子石、斜长石中并非只含有Ca2+离子,还含有其它种类离子。只有准确刻画石榴子石中钙铝榴石的活度,以及斜长石中钙长石的活度,才能建立准确的GASP压力计。
基于常量元素建立的矿物温度计,所涉及的矿物都是固溶体。基于常量元素建立的每种压力计中,至少有一种矿物为固溶体。矿物固溶体中离子混合的种类和性质,决定了矿物的活度。矿物中离子混合的热力学效应,比液态溶液的情况要复杂。例如,单斜辉石固溶体中的硬玉(NaM2AlM1Si2TO6)组分,其摩尔浓度指的究竟是M2八面体位置Na+离子的浓度(XNaM2)?还是M1八面体位置Al3+离子的浓度(XAlM1)?抑或是Na+离子浓度和Al3+离子浓度的乘积(XNaM2·XAlM1)?这就是要深入研究固溶体活度的原因。如果活度描述不准确的话,将会直接影响温度计、压力计的准确度和精确度。实际上,人们很早就意识到活度在温度计与压力计中的重要作用(Applegate and Hodges, 1994; Dachs, 1994)。
变质泥质岩中,除了少数温度计(Green and Usdansky, 1986; Vidal et al. , 2005; Wu and Chen, 2015a, b)与石榴子石无关外,常用的温度计(石榴子石-黑云母温度计, Holdaway, 2000; 石榴子石-堇青石温度计, Dwivedi et al. , 1998; 石榴子石-紫苏辉石温度计, Aranovich and Berman, 1997)都与石榴子石直接有关;除了极少数压力计(Bucher-Nurminen, 1987; Wu, 2020)与石榴子石、斜长石无关外,其余压力计(Ghent and Stout, 1981; Bohlen et al. , 1983; Robinson, 1983; Hodges and Crowley, 1985; Bohlen and Liotta, 1986; Koziol and Newton, 1988; Hoisch, 1990, 1991; Koziol and Bohlen, 1992; Nichols et al. , 1992; Holdaway, 2001; Wu et al. , 2004a, b; Wu and Zhao, 2006a, b, 2007a, b; Wu, 2015, 2017, 2018, 2019)都离不开石榴子石或斜长石。因此,石榴子石、黑云母、斜长石活度的准确限定,对温度计和压力计的准确度至关重要。本文以变质泥质岩中常用的GB温度计和GASP压力计为例,探讨活度的效应。当然,限于实例数量,本文只能起到抛砖引玉的作用。
1 活度的描述 1.1 固溶体的活度混合前后,如果溶液(溶剂+溶质)总的体积不发生改变、混合过程中又没有热效应,这就是理想溶液(包括液态溶液、固态溶液)。理想固溶体当然也要求矿物晶体中不同种类阳离子(离子对)或阴离子(阴离子对)发生相互替代、混合时,没有引起矿物晶体体积的变化,混合过程中也没有热效应的存在。实际上,只有稀溶液的热力学性质才接近理想溶液。一系列研究证明,在允许误差条件下,采用理想固溶体模型研究地质问题,也能取得比较符合地质事实的结论。但是,很多情况下,由于固溶体中离子(离子对)置换、混合的热力学效应并非一定是“理想”的,采用理想固溶体模型会引起较大的误差,此时必须采用“非理想”固溶体模型来描述一般固溶体中离子(对)置换、混合的热力学效应,即引入活度。
对于非理想溶液,人们采用“活度”替代理想溶液中的浓度,活度因此类比为“有效浓度”。一般将活度表达为理想项(“理想活度”Xi)与非理想项(“活度系数”γi)两项的乘积:ai=Xiγi。这是个已经广泛接受的习惯作法,是对活度的近似数学表述。活度系数γi一般描述为矿物成分和P-T条件的多项式。也可以说,活度系数把固溶体偏离理想溶液性质的所有非理想因素(“超额项”),都“一股脑”地包括进去了。
1.2 石榴子石、黑云母、斜长石固溶体的晶体化学特征石榴子石固溶体的结构化学通式可写作(Fe2+, Mg2+, Ca2+, Mn2+)3A(Al3+, Fe3+, Cr3+)2M(Si4+, Ti4+, Zr4+, 4H+)3TO12。在3个等效的六面体结晶位置(结点)A上,发生二价阳离子Fe2+、Mg2+、Ca2+、Mn2+的混合。在2个等效的八面体结点M上,发生三价阳离子Fe3+、Al3+、Cr3+的混合。在3个等效的四面体结点T上,发生4价阳离子Si4+、Ti4+、Zr4+、4H+的混合。变质泥质岩中的石榴子石,八面体和四面体位置一般比较“干净”,即分别只有Al3+、Si4+离子充填;离子混合一般只发生在六面体位置上。因此,目前变质泥质岩中的石榴子石活度模型,只描述六面体位置离子混合的热力学效应。
黑云母固溶体的结构化学通式可写作(K+, Na+, □)C(Fe2+, Mg2+, Mn2+, □)M2(Fe3+, Mg2+, Al3+, Fe3+, Cr3+, Ti4+)2M1(Si4+, Al3+)2T2(Si4+)2T1O10(OH-, F-, Cl-1)2。可发生离子混合的结点包括层间位置C、两个非等效八面体位置M1和M2、两个非等效四面体位置T1和T2,以及羟基位置。限于资料不足,目前描述变质泥质岩中黑云母的活度时,一般只考虑八面体位置上二价阳离子混合的热力学效应,并且把非等效的八面体结点作为等效结点看待。
斜长石固溶体的结构化学通式可写作(K+, Na+, Ca2+)C(Al3+, Si4+)4TO8,其中C、T分别指可发生离子混合的1个层间结点及4个等效的四面体结点。对斜长石活度的描述,考虑了C、T结点上Ca2+、Na+、K+、Al3+、Si4+离子混合的热力学效应。
2 活度对石榴子石-黑云母温度计的作用 2.1 根据不同的活度模型组合建立石榴子石-黑云母温度计温度计中的矿物固溶体模型及其热力学参数,大多是在建立温度计的同时确定的,所以许多固溶体矿物的活度是相互关联的。此种条件下,单独讨论某种矿物固溶体活度对温度计的影响,往往难以实现。
这里以建立石榴子石-黑云母温度计的逆转反应(reversed experiment)实验数据(Ferry and Spear, 1978; Perchuk and Lavrent’eva, 1983; Gessmann et al. , 1997)为例。采用不同的石榴子石、黑云母活度模型组合,算得温度计中各种热力学参数(表 1),由此可以建立不同版本的石榴子石-黑云母温度计(图 1)。表 1中,“理想固溶体”的活度等于浓度;“Holdaway (2000)”指的是该文献所描述的Fe-Mg-AlⅥ-Ti四元非理想黑云母固溶体模型,以及Fe-Mg-Ca-Mn四元非理想石榴子石活度模型。
|
|
表 1 基于实验数据,采用不同活度模型获得的石榴子石-黑云母温度计的热力学参数 Table 1 Thermodynamic parameters of different garnet-biotite thermometers obtained by adopting different activity models of garnet and biotite, based on experimental data |
|
图 1 采用不同活度模型组合(表 1)标定的石榴子石-黑云母温度计计算温度与实验温度对比 各小图图号分别对应表 1中各行所示的活度模型组合代码. 图中代码FS78、PL83、G97分别代表Ferry and Spear (1978)、Perchuk and Lavrent’eva (1983)、Gessmann et al. (1997)实验数据. 短线代表温度计重现实验温度的误差范围(±50℃) Fig. 1 Experimental versus calculated temperatures determined by different garnet-biotite geothermometers adopting different activity models listed in Table 1 Names of the sub-figures correspond to the activity sets listed in Table 1. Symbols FS78, PL83, G97 represent the experimental temperatures of Ferry and Spear (1978), Perchuk and Lavrent'eva (1983) and Gessmann et al. (1997), respectively. Dashed lines represent ±50℃ deviations |
采用不同的活度组合所建立的4种石榴子石-黑云母温度计,在回算实验温度方面的质量有所不同(表 1、图 1)。单从计算温度与实验温度的相关系数、标准差而论,活度模型组合自优而劣的顺序是:(d) > (b) > (a) > (c)。将石榴子石、黑云母同时作为非理想固溶体(d)得到的温度计,重现实验温度的效果最佳;将石榴子石作为理想固溶体的计算效果最差,这意味着石榴子石的确是非理想固溶体。
2.2 不同版本石榴子石-黑云母温度计的应用情况矿物温度计的准确度,表现在它能准确反映客观地质事实,也是其实用性的必要条件。例如,优质的矿物温度计应该能准确反映递增变质带、倒转变质带、热接触变质晕圈中,不同变质地带变质温度的规律性变化(Wu and Cheng, 2006)。这里就以表 1中根据4种不同活度组合建立的石榴子石-黑云母温度计为例,通过对天然变质带的应用,判断不同版本温度计的实用性。以下P-T计算中,石榴子石-黑云母温度计与GASP压力计迭代求解,即同时计算P-T条件。
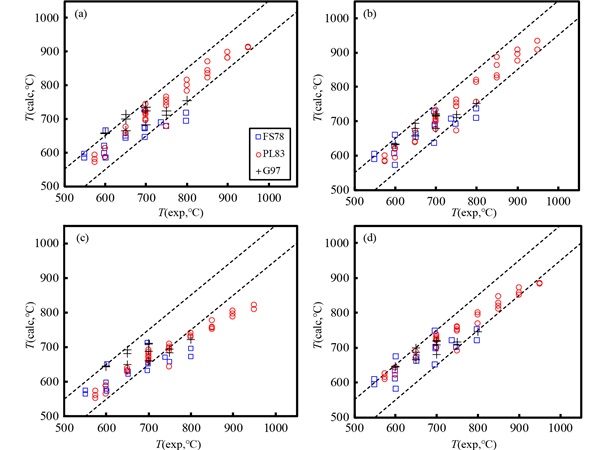
2.2.1 递增变质带巴罗型(Barrovian type)递增变质带属于中压变质相系,例如美国北爱达荷州Snow Peak地区(图 2a)、中国四川丹巴地区(图 2b)、美国纽约州Dutchess地区(图 2c)、哥伦比亚Silgará地区(图 2d)。巴肯型(Buchan type)递增变质带属于低压变质相系,例如加拿大Wopmay造山带(图 2e)、美国北卡罗来纳州蓝岭地区(图 2f)。不同版本的石榴子石-黑云母温度计都反映了各个递增变质带温度逐渐增加的地质事实(图 2)。

|
图 2 不同版本石榴子石-黑云母温度计应用于递增变质带的情况 图中横坐标自左至右表示变质程度逐渐增加的变质带划分情况,不同颜色线条分别对应表 1中各行所示的活度模型组合代码. (a)美国北爱达荷州Snow Peak地区巴罗型递增变质带(Lang and Rice, 1985);(b)中国四川丹巴巴罗型递增变质带(Huang et al., 2003);(c)美国纽约州Dutchess地区巴罗型递增变质带(Whitney et al., 1996);(d)哥伦比亚Santander地体Silgará地区巴罗型递增变质带(Ríos et al., 2003);(e)加拿大Wopmay造山带巴肯型递增变质带(St-Onge and Davis, 2017);(f)美国北卡罗来纳州蓝岭地区巴肯型递增变质带(Eckert et al., 1989) Fig. 2 Application of the different versions of the garnet-biotite geothermometers to prograde metamorphic terranes The metamorphic grade increases from left to right, as depicted in the abscissa. Different color curves in each sub-figure correspond to the activity sets listed in Table 1. (a) Barrovian-type metamorphic sequence of the Snow Peak area, Northern Idaho, USA (Lang and Rice, 1985); (b) Barrovian-type metamorphic sequence of the Danba area, Sichuan, China (Huang et al., 2003); (c) Barrovian-type metamorphic sequence of the Dutchess County, New York, USA (Whitney et al., 1996); (d) Barrovian-type metamorphic sequence of the Silgará Formation, Santander Massif, Colombian Andes (Ríos et al., 2003); (e) Buchan-type metamorphic sequence of the Wopmay Orogen, Canada (St-Onge and Davis, 2017); and (f) Buchan-type metamorphic sequence of the southern Blue Ridge, North Carolina, USA (Eckert et al., 1989) |
但是,不同温度计之间仍然存在明显的差别。除了美国蓝岭地区递增变质带(图 2f)之外,模型(d)计算的温度比其它组合得到的温度都高,而模型(a)得出的温度普遍比其它模型都低(图 2)。其中,模型(d)建立的石榴子石-黑云母温度计回算实验温度最准确。由此看来,模型(a)建立的温度计计算结果系统性偏低,即把石榴子石、黑云母同时作为理想固溶体的作法,是不可取的。
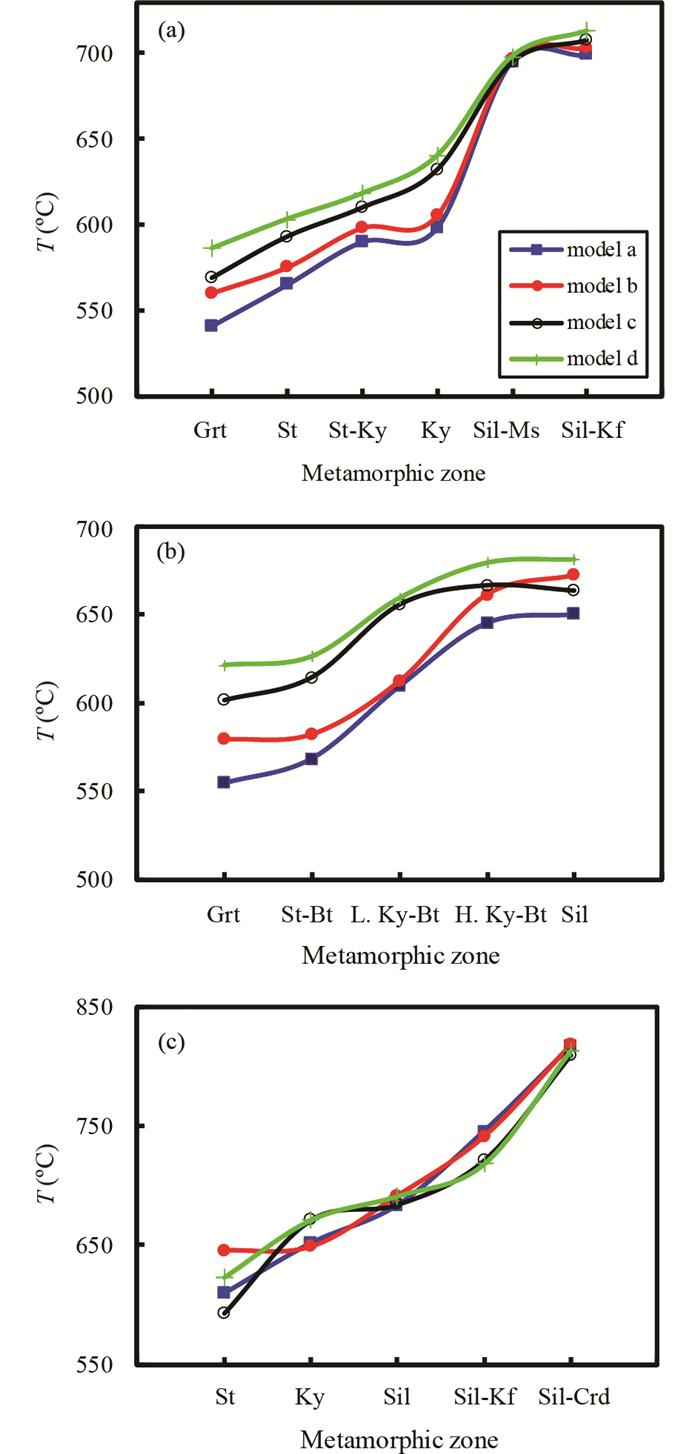
2.2.2 倒转变质带倒转变质带是指以今天的相对高程作为参照,出露位置越高的岩石变质程度越高的现象。这种现象与地下“层位越低,岩石变质程度越高”的现象相悖,因此被称为倒转变质带(inverted metamorphic sequence/terrane),其实质一般是正常的递增变质带被后期的地质构造改变所致。倒转变质带的实例有锡金高喜马拉雅倒转变质带(图 3a)、美国阿拉斯加州Juneau地区倒转变质带(图 3b)、尼泊尔高喜马拉雅倒转变质带(图 3c)。可以看出,4种版本的石榴子石-黑云母温度计都准确识别了自高而低的温度递降现象(图 3, 自右向左)。
|
图 3 不同版本石榴子石-黑云母温度计应用于倒转变质带的情况 图中横坐标自右至左表示变质程度逐渐降低的变质带划分情况,不同颜色线条分别对应表 1中各行所示的活度模型组合代码. (a)锡金高喜马拉雅倒转变质带(Dasgupta et al., 2004);(b)美国阿拉斯加州Juneau地区倒转变质带(Himmelberg et al., 1991);(c)尼泊尔高喜马拉雅倒转变质带(Imayama et al., 2010) Fig. 3 Application of the different versions of the garnet-biotite geothermometers to inverted metamorphic terranes The metamorphic grade decreases from right to left, as depicted in the abscissa. Different color curves in each sub-figure correspond to the activity sets listed in Table 1. (a) inverted metamorphic sequence in the Sikkim Himalayas (Dasgupta et al., 2004); (b) inverted metamorphic isograds in the western metamorphic belt, Juneau, Alaska, USA (Himmelberg et al., 1991); and (c) inverted metamorphic sequence of the Nepal Himalaya (Imayama et al., 2010) |
除了尼泊尔高喜马拉雅倒转变质带(图 3c)之外,模型(a)得出的温度普遍比其它模型都低(图 3)。这里再次看到把石榴子石、黑云母同时作为理想固溶体(模型a)的温度计,计算温度系统性偏低。
2.2.3 接触变质带接触变质作用是指侵入岩体烘烤围岩,在岩体外接触带岩石中形成变质程度向岩体逐渐递增的变质带。尽管接触变质带宽度不大(一般在500m以内),但岩石变质程度的分带现象一般比较明显。这里以法国东Rouergue地区接触变质带(图 4a)、爱尔兰Ardara接触变质带(图 4b)、美国缅因州Farmington区域热接触变质带(图 4c)为例。从图 4可以看出,虽然不同活度模型组合建立的石榴子石-黑云母温度计都很好地区别了变质温度逐渐增加的现象,但模型(a)计算的温度仍然系统性偏低(图 4)。

|
图 4 不同版本的石榴子石-黑云母温度计应用于接触变质带的情况 图中横坐标自左至右表示变质程度逐渐增加的变质带划分情况,不同颜色线条分别对应表 1中各行所示的活度模型组合代码. (a)法国French Massif Central东Rouergue地区接触变质带(Delor et al., 1984);(b)爱尔兰Ardara接触变质带(Homam, 2005);(c)美国缅因州Farmington区域热接触变质带(Holdaway et al., 1988) Fig. 4 Application of the different versions of the garnet-biotite geothermometers to thermal contact aureoles The metamorphic grade increases from left to right, as depicted in the abscissa. Different color curves in each sub-figure correspond to the activity sets listed in Table 1. (a) thermal aureole of the east Rouergue area, French Massif Central (Delor et al., 1984); (b) the Ardara aureole, NW Ireland (Homam, 2005); and (c) regional thermal contact aureoles in the Farmington area, west-central Maine, USA (Holdaway et al., 1988) |
数十年来,人们进行过多次涉及GASP组合的相平衡实验(Hays, 1966; Hariya and Kennedy, 1968; Hensen et al. , 1975; Cressey et al. , 1978; Schmid et al. , 1978; Wood, 1978, 1988; Goldsmith, 1980; Gasparik, 1984; Koziol and Newton, 1988, 1989; Koziol, 1990, 1996; Tropper et al. , 2005),其中有少数实验采用了含微量钙铝榴石成分的石榴子石固溶体。
为了分别考察石榴子石、斜长石活度对该压力计的效应,本文采用由纯钙铝榴石和纯钙长石进行的逆转反应(Hays, 1966; Hariya and Kennedy, 1968; Goldsmith, 1980; Gasparik, 1984; Koziol and Newton, 1988)所建立的GASP压力计。这些实验结果相互恰合得很好,该平衡反应的焓(ΔH)、熵(ΔS)变化量分别为-41.0kJ/mol、-146.0J/K·mol(McKenna and Hodges, 1988)。这两个数据与根据最新内洽性热力学数据库(Holland and Powell, 2011)推出的数据(-41.6kJ/mol、-138.070J/K·mol)在误差范围内一致。因此,本文采用其平均值(-41.3kJ/mol、-142.035J/K·mol),同时采用GASP压力计模式反应的体积变化量为-6.605J/bar。因此,只含端元矿物的GASP(Ky)压力计表达式为(其中平衡常数K=1):
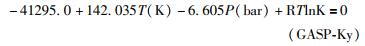
|
由于天然石榴子石、斜长石都是固溶体矿物,GASP (Ky)压力计表达为:
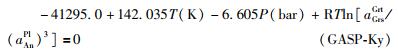
|
其中aGrsGrt、aAnPl分别为石榴子石中钙铝榴石、斜长石中钙长石的活度。
结合热力学数据库(Holland and Powell, 2011),得到含有夕线石、红柱石的GASP压力计表达式分别如下:

|

|
人们已建立了不少石榴子石活度模型,其中有三种比较准确的模型(Berman and Aranovich, 1996; Ganguly et al. , 1996; Mukhopadhyay et al. , 1997)。这三种石榴子石活度模型都采用了非对称参数来描述石榴子石中阳离子混合的热力学性质。不同的是,Berman and Aranovich (1996)、Mukhopadhyay et al. (1997)模型描述的是Fe-Mg-Ca三元非理想石榴子石固溶体,但不同活度模型中对应的各种热力学参数却有较大差别。Ganguly et al. (1996)模型描述的是Fe-Mg-Ca-Mn四元非理想石榴子石固溶体,但其Fe-Mg-Ca次级三元系活度模型对应的热力学参数同前两种也有大的差异。常用的三种斜长石固溶体活度模型(Fuhrman and Lindsley, 1988; Elkins and Grove, 1990; Benisek et al. , 2010)描述的是Ca-Na-K三元非理想斜长石固溶体,但斜长石不同活度模型中对应的各种热力学参数也各自明显不同。因此,不同的石榴子石、斜长石活度模型组合能够“组装”出不同版本的GASP压力计表达式。
与此同时,在建立不同版本的GASP压力计时,Holdaway (2001)采用了Fe-Mg-AlVI-Ti四元非理想黑云母活度模型(H00),但与不同石榴子石活度模型匹配的黑云母热力学参数略有不同,以减少温度计匹配压力计时的误差。表 2列出了13种石榴子石、斜长石、黑云母活度模型的组合,对应13种版本的GASP压力计。
|
|
表 2 GASP压力计中不同的活度模型组合 Table 2 Different activity models in different GASP geobarometers |
根据保持了热力学平衡状态下矿物成分的一些石榴子石+黑云母+斜长石+石英+Al2SiO5矿物组合,包括含红柱石(Hoisch, 1991; Zeh and Holness, 2003; 张阿利等, 2004; Pattison and Vogl, 2005)、夕线石(Grew, 1981; Hoisch, 1991; Raase and Schenk, 1994; Percival and Skulski, 2000; Zeh et al. , 2004; Estrada-Carmona et al. , 2009)和蓝晶石(McClelland et al. , 1991; Lemennicier et al. , 1996; Fraser et al. , 2000; Rütti, 2001; Hacker and Gans, 2005)的变质泥质岩样品,通过应用判断GASP压力计的准确度。从图 5a-g可以看出,除了活度组合(b)、(d)外,其余将石榴子石或斜长石当作理想固溶体的GASP压力计,都明显过高估算了压力,将含红柱石或夕线石的变质泥质岩错误地投进了蓝晶石稳定域(图 5a, c, e-g)。采用非理想活度模型组合的各种GASP压力计,都将变质泥质岩准确投入了Al2SiO5矿物各自的稳定域(图 5h-m)。

|
图 5 不同活度模型组合建立的GASP压力计应用于含Al2SiO5矿物变质泥质岩的情况 小图编号(a)-(m)分别对应于表 2中的活度模型组合. Al2SiO5矿物平衡线:实线据Holdaway and Mukhopadhyay (1993),虚线据Pattison (1992) Fig. 5 Application of the different GASP geobarometers to Al2SiO5 phase-bearing metapelite Names of the sub-figures (a)-(m) correspond to the respective activity sets (a)-(m) listed in Table 2. Al2SiO5 phase diagram: the sold lines are from Holdaway and Mukhopadhyay (1993), whereas the dashed line is from Pattison (1992) |
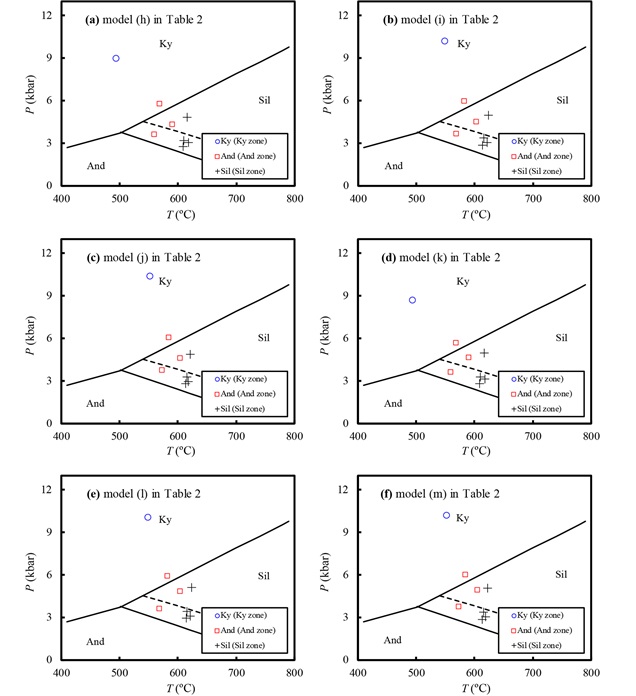
范围不宽的接触变质带,其变质压力条件应该基本一致(Wu and Cheng, 2006)。爱尔兰Ardara接触变质带宽度约500m,分为蓝晶石带、红柱石带、夕线石带(Homam, 2005)。不同版本的石榴子石-黑云母温度计都反映了温度的递增变化(图 4b、图 6)。除蓝晶石带以外,六种GASP压力计均反映这些岩石的变质压力基本一致(4.03±1.07kbar, 图 6)。蓝晶石带压力明显高于红柱石带和夕线石带(图 6),说明蓝晶石带可能来自接触变质带的更深部位(?),并且热源较浅即中-上地壳层次?值得注意的是,这6种版本的GASP压力计估算的含红柱石岩石变质压力均偏高(图 6)。
|
图 6 不同活度模型组合建立的GASP压力计应用于爱尔兰Ardara接触变质带的情况 小图编号(a)-(f)分别对应于表 2中的活度模型组合(h)-(m). Al2SiO5矿物平衡线:实线据Holdaway and Mukhopadhyay (1993),虚线据Pattison (1992) Fig. 6 Application of the different GASP geobarometers to metapelite in the Ardara aureole, NW Ireland (Homam, 2005) Names of the sub-figures correspond to activity models (h)-(m) listed in Table 2. Al2SiO5 phase diagram: the sold lines are from Holdaway and Mukhopadhyay (1993), whereas the dashed line is from Pattison (1992) |
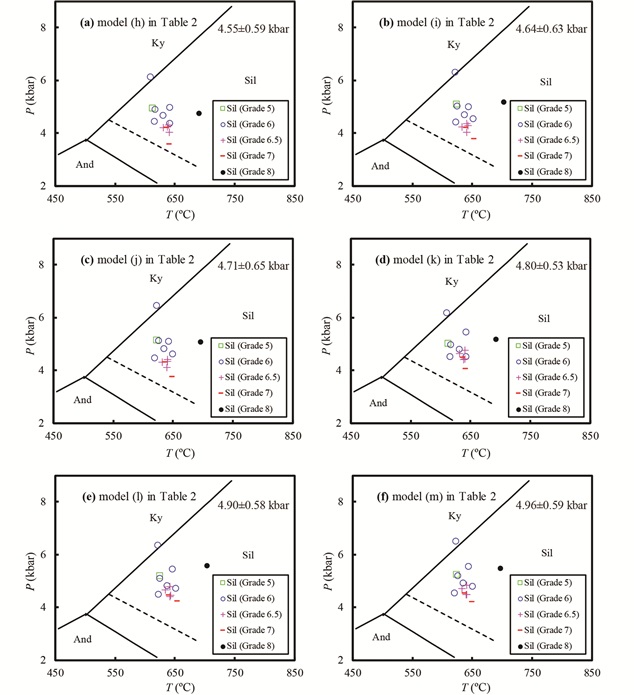
该地区有多个泥盆纪-石炭纪花岗岩体侵入,围绕这些岩体外接触带形成一系列接触变质带,称为“区域热接触变质带”(Holdaway et al. , 1988)。六种版本的GASP压力计得出的变质压力介于4.55±0.59kbar~4.96±0.59kbar之间(图 7),在误差范围内基本一致,也都正确反映了这些岩石位于夕线石稳定域的地质事实(图 7)。
|
图 7 不同活度模型组合建立的GASP压力计应用于区域热接触变质带的情况 小图编号(a)-(f)分别对应于表 2中的活度模型组合(h)-(m). Al2SiO5矿物平衡线:实线据Holdaway and Mukhopadhyay (1993),虚线据Pattison (1992) Fig. 7 Application of the different GASP geobarometers to metapelite in the regional contact aureoles, west-central Maine (Holdaway et al., 1988) Names of the sub-figures (a)-(f) correspond to activity models (h)-(m) listed in Table 2. Al2SiO5 phase diagram: the sold lines are from Holdaway and Mukhopadhyay (1993), whereas the dashed line is from Pattison (1992) |
尽管矿物温度计、压力计模式反应的焓(ΔH)、熵(ΔS)、体积(ΔV)等变化量随P-T而变化,但大量研究实践表明,纯固相平衡反应系统的这些参数随P-T演变的变化量并不大。非超高温、非超高压条件下,将它们当作常数,带来的误差可以忽略不计。因此,矿物温度计、压力计研究中一般采用矿物“标准状态下”的热力学参数,忽略矿物的热膨胀系数、压缩系数、热容。我们在讨论活度模型(表 1)对于GB温度计的影响、活度模型(表 2)对GASP压力计的影响时,也是这么处理的。
温度升高的情况下,矿物晶格增大。这样,半径差别大的离子或者电价不同的离子,相互替代更为容易。因此,麻粒岩相、超高温麻粒岩相情况下,矿物固溶体的混合性质接近理想溶液。升压的情况与之相反。对于一般P-T条件下形成的变质岩石,矿物固溶体不再是理想溶液,其活度需要尽可能准确描述。
4.1 活度误差被掩盖的情况建立GB温度计时,对于同样的实验数据,由于采用了不同的活度模型(表 1),得出的温度计模式反应的焓、熵、体积的变化量之间有很大的差别,也都与热力学数据库(Holland and Powell, 2011)的数据也有明显差别(表 1)。换句话说,建立温度计时得到的这些热力学数据,误差显然都很大。但是,除活度组合(c)之外(图 1c),不同版本的GB温度计回算实验温度的效果近似(图 1a, b, d),标准差介于±22~31℃(表 1)。从“实用”的角度看,这几个版本的GB温度计效果相近。
无论是将石榴子石、黑云母同时作为理想固溶体,还是同时都作为非理想固溶体,活度模型的巨大差异(表 1),并没有造成GB温度计过大的误差(图 1),说明活度误差可以被模式反应的焓、熵、体积变化量的误差所“吸收”,由此建立起“实用”的GB温度计。实际上,大多数矿物温度计、压力计都是采用此类方式建立的,这也就是为什么同一种矿物在不同温度计、压力计中的活度差别那么大,但却没有造成各个温度计、压力计误差过大的原因。
此外,从图 1可以看出,活度组合(c)的效果相对最差(图 1c),计算温度与实验温度的标准差为±37℃(表 1)。该模型组合已经充分考虑黑云母的非理想混合性质,但却将石榴子石作为理想固溶体处理,由此造成GB温度计误差过大。在实际应用中,将石榴子石、黑云母同时作为理想固溶体的GB温度计,计算温度总是系统性偏低(图 2、图 3、图 4),说明石榴子石、黑云母的确属于非理想固溶体。
一般说来,在建立矿物温度计或压力计过程中,不同矿物的活度模型如果是同时确定的,它们的活度误差会被“掩埋”,各种矿物活度的准确性是难以分别评判的。
4.2 可以逐个检验活度作用的情况在非超高温、非超高压的一般变质作用条件下,石榴子石、斜长石均表现出明显的非理想混合性质,其活度的准确刻画就显得非常必要。由于GASP压力计中的石榴子石、斜长石活度模型,不是在标定压力计的同时建立的,因此可以通过实际应用检验其准确性。
将石榴子石、斜长石同时作为理想固溶体(表 2)所建立的GASP压力计,计算压力严重偏高,将含有红柱石、夕线石的岩石全部错误地投到了蓝晶石稳定域(图 5a)。将石榴子石作为理想固溶体,采用不同的斜长石非理想活度模型(Fuhrman and Lindsley, 1988; Benisek et al. , 2010)所建立的GASP压力计,已能将大部分样品投入合适的Al2SiO5矿物稳定域(图 5b, d),只是采用Elkins and Grove (1990)斜长石活度模型的GASP压力计效果较差(图 5c)。与此不同的是,无论采用什么样的石榴子石非理想固溶体活度模型,只要将斜长石作为理想固溶体,那么得到的GASP压力计往往会过高估算压力(图 5e-g),说明斜长石是明显偏离理想溶液性质的非理想固溶体。
如果同时将石榴子石(Berman and Aranovich, 1996; Ganguly et al. , 1996; Mukhopadhyay et al. , 1997)、斜长石(Fuhrman and Lindsley, 1988; Elkins and Grove, 1990)作为非理想固溶体,那么无论采用什么样的活度模型组合(表 2),所得到的不同版本的GASP压力计,均能将含蓝晶石、红柱石、夕线石的变质泥质岩,准确投进各自的稳定域(图 5h-m)。这些石榴子石活度模型、斜长石活度模型中,对应的热力学参数分别都有很大区别;尽管活度模型差别这么大,但将它们“组装”入GASP压力计(表 2)之后,不同版本压力计的计算效果却几乎一致(图 5)。这说明,这些活度模型都有类似的可靠的准确度。
4.3 缺少通用型活度模型不同岩性中的同一种矿物,其化学成分一般有明显差异。例如,变质泥质岩中的石榴子石富FeO而贫MgO,离子混合发生在六面体结点上;变质基性岩(角闪岩、基性麻粒岩)中的石榴子石既富FeO也富MgO,离子混合发生在六面体结点上;变质超基性岩中的石榴子石贫FeO而富MgO,离子混合既发生在六面体结点上,也发生在八面体结点上;超高压变质的榴辉岩中的石榴子石,离子混合既发生在六面体结点上,还发生在四面体结点上。这些情况导致了适用于变质泥质岩的石榴子石-黑云母温度计、适用于斜长角闪岩的石榴子石-角闪石温度计、适用于基性麻粒岩的石榴子石-单斜辉石温度计、适用于变质超基性岩的石榴子石-橄榄石温度计,其中的石榴子石活度都不同。目前,还没有广泛适用的通用型石榴子石活度模型。
从变质演化角度看,石榴子石中不仅可以保存早期变质阶段的矿物组合,还易于保存退变质阶段的矿物组合,石榴子石本身还可以用于定年,因此石榴子石是变质地质学研究中最为看重的研究对象。从结晶学角度看,石榴子石是对称性最高的等轴晶系矿物。从化学角度看,其晶体化学规律早已查明。但是,就是这样一个应该标定好活度的矿物,其活度描述还很不理想。此外,角闪石既是广泛见于角闪岩相和麻粒岩相变质岩的矿物,还是晶体化学结构最复杂的常见矿物,其活度模型研究更是远不理想。其它矿物活度模型的研究,当然谈不上完善。由于人们开展的岩石学相平衡实验还严重不足,导致高质量的平衡矿物组合数据很是缺乏,这也直接导致通用型活度模型的匮乏。开发通用型矿物活度模型,应该是今后重点开展的研究方向。
4.4 矿物温度计、压力计计算结果并不受流体的影响既然矿物温度计、压力计未考虑流体,一个自然而然的疑问是,它们是否真实反映了客观地质事实?计算结果是否反映了含有流体的矿物组合的平衡P-T条件?
众所周知,在变质作用、岩浆作用过程中,如果存在流体,流体会参与岩石中的许多种类的反应。此处以图 8为例简单说明。图 8表示任意变质岩、岩浆岩中的矿物+流体组合演化到热力学平衡状态的过程。在给定温度、压力、流体条件下,岩石达到热力学平衡之前,涉及固相-固相、固相-流体相的各种反应,各自独立,反应线并未“聚合”在一起(图 8a)。岩石达到完全的热力学平衡时,所有反应线交汇于一点,这些平衡反应线组成了平衡状态的“全集”(图 8b)。从“全集”中抽提出来的不涉及流体相的温度计与压力计平衡反应线(图 8c),即全部平衡反应线的“子集”,肯定与“全集”的平衡压力与温度(P1, T1)条件完全一致。当流体条件发生改变时(图 8d-f),从未平衡演化到完全热力学平衡,规律仍然和图 8a-c类似,最终达到新的平衡压力与温度(P2, T2)条件。因此可以说,只要矿物组合达到了完全的热力学平衡,矿物温度计与压力计的计算结果,的确能反映客观地质事实,并不受是否考虑流体的影响。

|
图 8 矿物温度计-压力计计算结果与流体无关 假想的变质岩或岩浆岩中的矿物组合.接近平直的线条代表全部由固相矿物参与的反应(其中彩色线条代表矿物温度计与压力计),曲线代表有流体参与的反应. (a-c)含流体的矿物组合中的反应: (a)达到热力学平衡前的各种反应;(b)该流体条件下,矿物组合中各种反应达到完全热力学平衡,构成平衡反应的“全集”;(c)仅由平缓斜率(压力计)、陡倾斜率(温度计)的固相平衡反应线构成的平衡反应集合的一部分,即平衡反应集合的“子集”;(d-f): 流体成分改变后矿物组合中的各种反应: (d)达到热力学平衡前的各种反应;(e)新的流体条件下,矿物组合中各种反应达到完全热力学平衡,构成新的平衡反应新的“全集”;(f)仅由温度计与压力计组成的新的平衡反应“子集”. 注意(b)与(e)、(c)与(f)中的温度与压力条件不同 Fig. 8 P-T conditions determined by fluid-absent geothermobarometers are independent of fluid Take hypothetical metamorphic or magmatic phase assemblages as examples. Near straight curves stand for fluid-absent reactions, among which the color curves are geothermobarometers, whereas the curved lines are reactions involving fluid phases. (a-c) Fluid-related reactions of the assemblage: (a) reactions prior to thermodynamic equilibria; (b) at stable composition of the fluid, all the reactions reach thermodynamic equilibrium and constitute the full cluster; (c) the geobarometer (shallow curves) and geothermometer (steep curves) constitute the partial thermodynamic cluster; (d-f) The reactions under new composition of fluid: (d) reactions prior to new thermodynamic equilibrium; (e) new full thermodynamic cluster under new fluid composition; (f) new partial thermodynamic cluster consisted by geothermobarometers. The P-T conditions of (b) and (e) or (c) and (f) are different |
类似地,如果岩石中还存在熔体的话,那么,采用只考虑固相矿物的矿物温度计与压力计,也同样能准确反映客观地质事实,仍然不受“撇开”熔体、流体的影响。
需要说明的是,图 8中所示的与流体和熔体无关的、只由固相矿物构成的平衡反应中,那些P/T斜率 < 10bar/℃的平衡反应受温度影响很小,才是理想的矿物压力计;P/T斜率>80bar/℃的平衡反应受压力影响很小,才是理想的矿物温度计。其余的固相平衡反应,既不能作为压力计,也不能作为温度计。这也就是矿物温度计、压力计数量有限的原因。此外,由于微量元素、稳定同位素、稀土元素等在固相矿物之间的分配受到压力的影响较小,因此至少目前还难以采用这些元素建立矿物压力计。
5 结论(1) 将石榴子石、黑云母、斜长石当作理想固溶体,得到的石榴子石-黑云母温度计往往会过低估算温度条件,得到的GASP压力计往往会过高估算压力条件,它们都存在较大的系统误差;
(2) 一般变质作用条件下,固溶体矿物属于非理想固态溶液。如果其活度刻画不准确,将直接导致温度计、压力计的系统误差;
(3) 比较接近真实“有效浓度”的各种矿物活度模型,尽管其中热力学参数有所不同,但其实用性通常可以满足;
(4) 建立适用于多种岩石类型、温度与压力适用范围大的通用型矿物活度模型,是值得开展的重要研究任务;
(5) 虽然矿物温度计、压力计不涉及流体、熔体,但对于达到并保持了热力学平衡状态条件下化学成分的矿物组合而言,其计算结果的确能准确反映客观地质事实,并不受是否考虑流体、熔体的影响。
致谢 陈意教授和向华博士提出了宝贵的修改建议,提高了本文的学术水平。作者向他们致以真挚的感谢。
谨以拙文祝贺著名前寒武纪地质学家、岩石学家沈其韩院士百岁大寿,敬祝恩师健康长寿!
Applegate JDR and Hodges KV. 1994. Empirical evaluation of solution models for pelitic minerals and their application to thermobarometry. Contributions to Mineralogy and Petrology, 117(1): 56-65 DOI:10.1007/BF00307729 |
Aranovich LY and Berman RG. 1997. A new garnet-orthopyroxene thermometer based on reversed Al2O3 solubility in FeO-Al2O3-SiO2 orthopyroxene. American Mineralogist, 82(3-4): 345-353 DOI:10.2138/am-1997-3-413 |
Barth TFW. 1934. Temperatures in lavas and magmas and a new geological thermometer. Nature, 58(6): 187-192 |
Benisek A, Dachs E and Kroll H. 2010. A ternary feldspar-mixing model based on calorimetric data: Development and application. Contributions to Mineralogy and Petrology, 160(3): 327-337 DOI:10.1007/s00410-009-0480-8 |
Berman RG and Aranovich LY. 1996. Optimized standard state and solution properties of minerals. I. Model calibration for olivine, orthopyroxene, cordierite, garnet, and ilmenite in the system FeO-MgO-CaO-Al2O3-TiO2-SiO2. Contributions to Mineralogy and Petrology, 126(1-2): 1-24 DOI:10.1007/s004100050232 |
Bhowmik SK and Spiering B. 2004. Constraining the prograde and retrograde P-T paths of granulites using decomposition of initially zoned garnets: An example from the Central Indian Tectonic Zone. Contributions to Mineralogy and Petrology, 147(5): 581-603 DOI:10.1007/s00410-004-0573-3 |
Bohlen SR, Wall VJ and Boettcher AL. 1983. Experimental investigations and geological applications of equilibria in the system FeO-TiO2-Al2O3-SiO2-H2O. American Mineralogist, 68(11-12): 1049-1058 |
Bohlen SR and Liotta JJ. 1986. A barometer for garnet amphibolites and garnet granulites. Journal of Petrology, 27(5): 1025-1034 DOI:10.1093/petrology/27.5.1025 |
Briggs W and Foster CT. 1992. Pressuretemperature conditions of Early Proterozoic metamorphism during the Trans-Hudson Orogen as determined from rocks straddling the Flin Flon Kisseynew boundary at Niblock and File lakes, Manitoba. Canadian Journal of Earth Sciences, 29(11): 2497-2507 DOI:10.1139/e92-196 |
Bucher-Nurminen K. 1987. A recalibration of the chlorite-biotite-muscovite geobarometer. Contributions to Mineralogy and Petrology, 96(4): 519-522 DOI:10.1007/BF01166696 |
Cressey G, Schmid R and Wood BJ. 1978. Thermodynamic properties of almandine-grossular garnet solid solutions. Contributions to Mineralogy and Petrology, 67(4): 397-404 DOI:10.1007/BF00383299 |
Dachs E. 1994. Uncertainties in the activities of garnets and their propagation into geothermobarometry. European Journal of Mineralogy, 6(2): 291-296 DOI:10.1127/ejm/6/2/0291 |
Dasgupta S, Ganguly J and Neogi S. 2004. Inverted metamorphic sequence in the Sikkim Himalayas: Crystallization history, P-T gradient and implications. Journal of Metamorphic Geology, 22(5): 395-412 DOI:10.1111/j.1525-1314.2004.00522.x |
Delor CP, Burg JP and Leyreloup AF. 1984. Staurolite producing reactions and geothermobarometry of a high pressure thermal aureole in the French Massif Central. Journal of Metamorphic Geology, 2(1): 55-72 DOI:10.1111/j.1525-1314.1984.tb00285.x |
Ding L and Zhong DL. 1999. Metamorphic characteristics and geotectonic implications of the high-pressure granulites from Namjagbarwa, eastern Tibet. Science in China (Series D), 42(5): 491-505 DOI:10.1007/BF02875243 |
Dwivedi SB, Mohan A and Lal RK. 1998. Recalibration of the Fe-Mg exchange reaction between garnet and cordierite as a thermometer. European Journal of Mineralogy, 10(2): 281-290 DOI:10.1127/ejm/10/2/0281 |
Eckert JO Jr, Hatcher RD Jr and Mohr DW. 1989. The Wayah granulite-facies metamorphic core, southwestern North Carolina: High-grade culmination of Taconic metamorphism in the southern Blue Ridge. GSA Bulletin, 101(11): 1434-1447 DOI:10.1130/0016-7606(1989)101<1434:TWGFMC>2.3.CO;2 |
Elkins LT and Grove TL. 1990. Ternary feldspar experiments and thermodynamic models. American Mineralogist, 75(5-6): 544-559 |
Estrada-Carmona J, Weber B, Hecht L and Martens U. 2009. P-T-t trajectory of metamorphic rocks from the central Chiapas Massif Complex: The Custepec Unit, Chiapas, Mexico. Revista Mexicana de Ciencias Geologicas, 26(1): 243-259 |
Ferry JM and Spear FS. 1978. Experimental calibration of the partitioning of Fe and Mg between biotite and garnet. Contributions to Mineralogy and Petrology, 66(2): 113-117 DOI:10.1007/BF00372150 |
Foster G, Vance D, Argles T and Harris N. 2002. The Tertiary collision-related thermal history of the NW Himalaya. Journal of Metamorphic Geology, 20(9): 827-843 DOI:10.1046/j.1525-1314.2002.00410.x |
Fraser G, Worley B and Sandiford M. 2000. High-precision geothermobarometry across the High Himalayan metamorphic sequence, Langtang Valley, Nepal. Journal of Metamorphic Geology, 18(6): 665-681 DOI:10.1046/j.1525-1314.2000.00283.x |
Fuhrman ML and Lindsley DH. 1988. Ternary-feldspar modeling and thermometry. American Mineralogist, 73(3-4): 201-215 |
Ganguly J, Cheng WJ and Tirone M. 1996. Thermodynamics of aluminosilicate garnet solid solution: New experimental data, an optimized model, and thermometric applications. Contributions to Mineralogy and Petrology, 126(1-2): 137-151 DOI:10.1007/s004100050240 |
Gasparik T. 1984. Experimental study of subsolidus phase relations and mixing properties of pyroxene in the system CaO-Al2O3-SiO2. Geochimica et Cosmochimica Acta, 48(12): 2537-2545 DOI:10.1016/0016-7037(84)90304-1 |
Gessmann CK, Spiering B and Raith M. 1997. Experimental study of the Fe-Mg exchange between garnet and biotite: Constraints on the mixing behavior and analysis of the cation-exchange mechanisms. American Mineralogist, 82(11-12): 1225-1240 DOI:10.2138/am-1997-11-1218 |
Ghebreab W. 1999. Tectono-metamorphic history of Neoproterozoic rocks in eastern Eritrea. Precambrian Research, 98(1-2): 83-105 DOI:10.1016/S0301-9268(99)00040-6 |
Ghent E. 1975. Temperature, pressure, and mixed-volatile equilibria attending metamorphism of staurolite-kyanite-bearing assemblages, Esplanade Range, British Columbia. Geological Society of America Bulletin, 86(12): 1654-1660 DOI:10.1130/0016-7606(1975)86<1654:TPAMEA>2.0.CO;2 |
Ghent ED. 1976. Plagioclase-garnet-Al2SiO5-quartz: A potential geobarometer-geothermometer. American Mineralogist, 61(7-8): 710-714 |
Ghent ED and Stout MZ. 1981. Geobarometry and geothermometry of plagioclase-biotite-garnet-muscovite assemblages. Contributions to Mineralogy and Petrology, 76(1): 92-97 DOI:10.1007/BF00373688 |
Goldsmith JR. 1980. The melting and breakdown reactions of anorthite at high pressures and temperatures. American Mineralogist, 65(3-4): 272-284 |
Green NL and Usdansky SI. 1986. Toward a practical plagioclase-muscovite thermometer. American Mineralogist, 71(9-10): 1109-1117 |
Grew ES. 1981. Granulite-facies metamorphism at Molodezhnaya Station, East Antarctica. Journal of Petrology, 22(3): 297-336 DOI:10.1093/petrology/22.3.297 |
Guo JH, Peng P, Chen Y, Jiao SJ and Windley BF. 2012. UHT sapphirine granulite metamorphism at 1.93~1.92Ga caused by gabbronorite intrusions: Implications for tectonic evolution of the northern margin of the North China Craton. Precambrian Research, 222-223: 124-142 DOI:10.1016/j.precamres.2011.07.020 |
Hacker BR and Gans PB. 2005. Continental collisions and the creation of ultrahigh-pressure terranes: Petrology and thermochronology of nappes in the central Scandinavian Caledonides. GSA Bulletin, 117(1-2): 117-134 |
Hallett BW and Spear FS. 2014. The P-T history of anatectic pelites of the northern east Humboldt Range, Nevada: Evidence for tectonic loading, decompression, and anatexis. Journal of Petrology, 55(1): 3-36 DOI:10.1093/petrology/egt057 |
Hariya Y and Kennedy GC. 1968. Equilibrium study of anorthite under high pressure and high temperature. American Journal of Science, 266(3): 193-203 DOI:10.2475/ajs.266.3.193 |
Hays JF. 1966. Lime-alumina-silica. Carnegie Institution of Washington Yearbook, 65: 234-239 |
Hensen BJ, Schmid R and Wood BJ. 1975. Activity-composition relationships for pyrope-grossular garnet. Contributions to Mineralogy and Petrology, 51(13): 161-166 |
Himmelberg GR, Brew DA and Ford AB. 1991. Development of inverted metamorphic isograds in the western metamorphic belt, Juneau, Alaska. Journal of Metamorphic Geology, 9(2): 165-180 DOI:10.1111/j.1525-1314.1991.tb00512.x |
Hodges KV and Crowley PD. 1985. Error estimation and empirical geothermobarometry for pelitic systems. American Mineralogist, 70(7-8): 702-709 |
Hoisch TD. 1990. Empirical calibration of six geobarometers for the mineral assemblage quartz + muscovite + biotite + plagioclase + garnet. Contributions to Mineralogy and Petrology, 104(2): 225-234 DOI:10.1007/BF00306445 |
Hoisch TD. 1991. Equilibria within the mineral assemblage quartz + muscovite + biotite + garnet + plagioclase, and implications for the mixing properties of octahedrally-coordinated cations in muscovite and biotite. Contributions to Mineralogy and Petrology, 108(1-2): 43-54 DOI:10.1007/BF00307325 |
Holdaway MJ, Dutrow BL and Hinton RW. 1988. Devonian and carboniferous metamorphism in west-central Maine: The muscovite-almandine geobarometer and the staurolite problem revisited. American Mineralogist, 73(1-2): 20-47 |
Holdaway MJ and Mukhopadhyay B. 1993. A reevaluation of the stability relations of andalusite: Thermochemical data and phase diagram for the aluminum silicates. American Mineralogist, 78(3-4): 298-315 |
Holdaway MJ. 2000. Application of new experimental and garnet Margules data to the garnet-biotite geothermometer. American Mineralogist, 85(7-8): 881-892 DOI:10.2138/am-2000-0701 |
Holdaway MJ. 2001. Recalibration of the GASP geobarometer in light of recent garnet and plagioclase activity models and versions of the garnet-biotite geothermometer. American Mineralogist, 86(10): 1117-1129 DOI:10.2138/am-2001-1001 |
Holland TJB and Powell R. 2011. An improved and extended internally consistent thermodynamic dataset for phases of petrological interest, involving a new equation of state for solids. Journal of Metamorphic Geology, 29(3): 333-383 DOI:10.1111/j.1525-1314.2010.00923.x |
Homam SM. 2005. Geothermobarometry of Al2SiO5-bearing metapelites in the Ardara aureole, NW Ireland: An implication for P-T stability field of aluminium silicate polymorphs. Iranian Journal of Science and Technology Transaction A: Science, 29(1): 163-179 |
Huang MH, Buick IS and Hou LW. 2003. Tectonometamorphic evolution of the eastern Tibet Plateau: Evidence from the central Songpan-Garzê Orogenic Belt, western China. Journal of Petrology, 44(2): 255-278 DOI:10.1093/petrology/44.2.255 |
Imayama T, Takeshita T and Arita K. 2010. Metamorphic P-T profile and P-T path discontinuity across the far-eastern Nepal Himalaya: Investigation of channel flow models. Journal of Metamorphic Geology, 28(5): 527-549 DOI:10.1111/j.1525-1314.2010.00879.x |
Indares A and Martignole J. 1990. Metamorphic constraints on the tectonic evolution of the allochthonous monocyclic belt of the Grenville Province, western Quebec. Canadian Journal of Earth Sciences, 27(3): 371-386 DOI:10.1139/e90-034 |
Koziol AM and Newton RC. 1988. Redetermination of the anorthite breakdown reaction and improvement of the plagioclase-garnet-Al2SiO5-quartz geobarometer. American Mineralogist, 73(3-4): 216-223 |
Koziol AM and Newton RC. 1989. Grossular activity-composition relationships in ternary garnets determined by reversed displaced-equilibrium experiments. Contributions to Mineralogy and Petrology, 103(4): 423-433 DOI:10.1007/BF01041750 |
Koziol AM. 1990. Activity-composition relationships of binary Ca-Fe and Ca-Mn garnets determined by reversed, displaced equilibrium experiments. American Mineralogist, 75(3-4): 319-327 |
Koziol AM and Bohlen SR. 1992. Solution properties of almandine-pyrope garnet as determined by phase equilibrium experiments. American Mineralogist, 77(7-8): 765-773 |
Koziol AM. 1996. Quaternary (Ca-Fe-Mg-Mn) garnet: Displaced equilibrium experiments and implications for current garnet mixing models. European Journal of Mineralogy, 8(2): 453-460 DOI:10.1127/ejm/8/2/0453 |
Lang HM and Rice JM. 1985. Regression modelling of metamorphic reactions in metapelites, Snow Peak, Northern Idaho. Journal of Petrology, 26(4): 857-887 DOI:10.1093/petrology/26.4.857 |
Lemennicier Y, Le Fort P, Lombardo B, Pêcher A and Rolfo F. 1996. Tectonometamorphic evolution of the central Karakorum (Baltistan, northern Pakistan). Tectonophysics, 260(1-3): 119-143 DOI:10.1016/0040-1951(96)00080-7 |
Liu Y and Zhong DL. 1997. Petrology of high-pressure granulites from the eastern Himalayan syntaxis. Journal of Metamorphic Geology, 15(4): 451-466 DOI:10.1111/j.1525-1314.1997.00033.x |
McClelland WC, Anovitz LM and Gehrels GE. 1991. Thermobarometric constraints on the structural evolution of the Coast Mountains batholith, central southeastern Alaska. Canadian Journal of Earth Sciences, 28(6): 912-928 DOI:10.1139/e91-083 |
McKenna LW and Hodges KV. 1988. Accuracy versus precision in locating reaction boundaries: Implications for the garnet-plagioclase-aluminum silicate-quartz geobarometer. American Mineralogist, 73(9-10): 1205-1208 |
McLellan E. 1985. Metamorphic reactions in the kyanite and sillimanite zones of the Barrovian type area. Journal of Petrology, 26(4): 789-818 DOI:10.1093/petrology/26.4.789 |
Millonig L, Zeh A, Gerdes A and Klemd R. 2008. Neoarchaean high-grade metamorphism in the Central Zone of the Limpopo Belt (South Africa): Combined petrological and geochronological evidence from the Bulai pluton. Lithos, 103(3-4): 333-351 DOI:10.1016/j.lithos.2007.10.001 |
Mohan A and Windley BF. 1993. Crustal trajectory of sapphirine-bearing granulites from Ganguvarpatti, South India: Evidence for an isothermal decompression path. Journal of Metamorphic Geology, 11(6): 867-878 DOI:10.1111/j.1525-1314.1993.tb00196.x |
Mukhopadhyay B, Holdaway MJ and Koziol AM. 1997. A statistical model of thermodynamic mixing properties of Ca-Mg-Fe2+ garnets. American Mineralogist, 82(1-2): 165-181 DOI:10.2138/am-1997-1-219 |
Nichols GT, Berry RF and Green DH. 1992. Internally consistent gahnitic spinel-cordierite-garnet equilibria in the FMASHZn system: Geothermobarometry and applications. Contributions to Mineralogy and Petrology, 111(3): 362-377 DOI:10.1007/BF00311197 |
Owona S, Schulz B, Ratschbacher L, Ondoa JM, Ekodeck GE, Tchoua FM and Affaton P. 2011. Pan-African metamorphic evolution in the southern Yaounde Group (Oubanguide Complex, Cameroon) as revealed by EMP-monazite dating and thermobarometry of garnet metapelites. Journal of African Earth Sciences, 59(1): 125-139 DOI:10.1016/j.jafrearsci.2010.09.003 |
Patiño Douce AE, Johnston AD and Rice JM. 1993. Octahedral excess mixing properties in biotite: A working model with applications to geobarometry and geothermometry. American Mineralogist, 78: 113-131 |
Pattison DRM. 1992. Stability of andalusite and sillimanite and the Al2SiO5 triple point: Constraints from the Ballachulish aureole, Scotland. The Journal of Geology, 100(4): 423-446 DOI:10.1086/629596 |
Pattison DRM and Vogl JJ. 2005. Contrasting sequences of metapelitic mineral-assemblages in the aureole of the tilted Nelson Batholith, British Columbia: Implications for phase equilibria and pressure determination in andalusite-sillimanite-type settings. The Canadian Mineralogist, 43(1): 51-88 DOI:10.2113/gscanmin.43.1.51 |
Perchuk LL and Lavrent'eva IV. 1983. Experimental investigation of exchange equilibria in the system cordierite-garnet-biotite. In: Saxena SK (ed.) Kinetics and Equilibrium in Mineral Reactions. New York: Springer-Verlag, 199-239
|
Percival JA and Skulski T. 2000. Tectonothermal evolution of the northern Minto Block, Superior Province, Quebec, Canada. The Canadian Mineralogist, 38(2): 345-378 DOI:10.2113/gscanmin.38.2.345 |
Pham VT, Wang H, Zhang Q, Liu JH, Shi MY, Li Z and Wu CM. 2018. Metamorphic evolution and geochronology of the Changshanzi area, Dunhuang Orogenic Belt, Northwest China. Acta Petrologica Sinica, 34(9): 2773-2792 (in Chinese with English abstract) |
Putirka KD. 2008. Thermometers and barometers for volcanic systems. Reviews in Mineralogy and Geochemistry, 69(1): 61-120 DOI:10.2138/rmg.2008.69.3 |
Raase P and Schenk V. 1994. Petrology of granulite-facies metapelites of the Highland Complex, Sri Lanka: Implications for the metamorphic zonation and the P-T path. Precambrian Research, 66(1-4): 265-294 DOI:10.1016/0301-9268(94)90054-X |
Ríos C, García C and Takasu A. 2003. Tectono-metamorphic evolution of the Silgará Formation metamorphic rocks in the southwestern Santander Massif, Colombian Andes. Journal of South American Earth Sciences, 16(2): 133-154 DOI:10.1016/S0895-9811(03)00025-7 |
Robinson GR. 1983. Calibration of the muscovite-biotite-quartz-garnet-aluminosilicate geothermobarometer. EOS, 64: 351 |
Rütti R. 2001. Tectono-metamorphic evolution of the Simano-Adula nappe boundary, Central Alps, Switzerland. Schweizerische Mineralogische und Petrographische Mitteilungen, 81: 115-129 |
Schmid R, Cressey G and Wood BJ. 1978. Experimental determination of univariant equilibria using divariant solid-solution assemblages. American Mineralogist, 63: 511-515 |
Shen QH. 2009. The recommendation of a systematic list of mineral abbreviations. Acta Petrologica et Mineralogica, 28(5): 495-500 (in Chinese with English abstract) |
Stäubli A. 1989. Polyphase metamorphism and the development of the Main Central Thrust. Journal of Metamorphic Geology, 7(1): 73-93 DOI:10.1111/j.1525-1314.1989.tb00576.x |
St-Onge MR and Davis WJ. 2017. Wopmay orogen revisited: Phase equilibria modeling, detrital zircon geochronology, and U-Pb monazite dating of a regional Buchan-type metamorphic sequence. GSA Bulletin, 130(3-4): 678-704 |
Tropper P, Konzett J and Finger F. 2005. Experimental constraints on the formation of high-P/high-T granulites in the Southern Bohemian Massif. European Journal of Mineralogy, 17(2): 343-356 DOI:10.1127/0935-1221/2005/0017-0343 |
Vidal O, Parra T and Vieillard P. 2005. Thermodynamic properties of the Tschermak solid solution in Fe-chlorite: Application to natural examples and possible role of oxidation. American Mineralogist, 90(2-3): 347-358 DOI:10.2138/am.2005.1554 |
Vrána S, Šteědrá V and Fišera M. 2005. Petrology and geochemistry of the Běstvina granulite body metamorphosed at eclogite facies conditions, Bohemian Massif. Journal of the Czech Geological Society, 50(3-4): 95-106 |
Vrána S, Janoušek V and Franěk J. 2013. Contrasting mafic to felsic HP-HT granulites of the Blansky les Massif (Moldanubian Zone of southern Bohemia): Complexity of mineral assemblages and metamorphic reactions. Journal of Geosciences, 58(4): 347-378 |
Whitney DL, Mechum TA, Kuehner SM and Dilek YR. 1996. Progressive metamorphism of pelitic rocks from protolith to granulite facies, Dutchess County, New York, USA: Constraints on the timing of fluid infiltration during regional metamorphism. Journal of Metamorphic Geology, 14(2): 163-181 DOI:10.1046/j.1525-1314.1996.05836.x |
Whitney DL and Evans BW. 2010. Abbreviations for names of rock-forming minerals. American Mineralogist, 95(1): 185-187 DOI:10.2138/am.2010.3371 |
Wood BJ. 1978. Reactions involving anorthite and CaAl2Si2O6 pyroxene at high pressures and temperatures. American Journal of Science, 278(7): 930-942 DOI:10.2475/ajs.278.7.930 |
Wood BJ. 1988. Activity measurements and excess entropy-volume relationships for pyrope-grossular garnets. The Journal of Geology, 96(6): 721-729 DOI:10.1086/629273 |
Wu CM, Zhang J and Ren LD. 2004a. Empirical garnet-biotite-plagioclase-quartz (GBPQ) geobarometry in medium-to high-grade metapelites. Journal of Petrology, 45(9): 1907-1921 DOI:10.1093/petrology/egh038 |
Wu CM, Zhang J and Ren LD. 2004b. Empirical garnet-muscovite-plagioclase-quartz geobarometry in medium-to high-grade metapelites. Lithos, 78(4): 319-332 DOI:10.1016/j.lithos.2004.06.008 |
Wu CM and Cheng BH. 2006. Valid garnet-biotite (GB) geothermometry and garnet-aluminum silicate-plagioclase-quartz (GASP) geobarometry in metapelitic rocks. Lithos, 89(1-2): 1-23 DOI:10.1016/j.lithos.2005.09.002 |
Wu CM and Zhao GC. 2006a. The applicability of the GRIPS geobarometry in metapelitic assemblages. Journal of Metamorphic Geology, 24(4): 297-307 DOI:10.1111/j.1525-1314.2006.00638.x |
Wu CM and Zhao GC. 2006b. Recalibration of the garnet-muscovite (GM) geothermometer and the garnet-muscovite-plagioclase-quartz (GMPQ) geobarometer for metapelitic assemblages. Journal of Petrology, 47(12): 2357-2368 DOI:10.1093/petrology/egl047 |
Wu CM and Zhao GC. 2007a. A recalibration of the garnet-olivine geothermometer and a new geobarometer for garnet peridotites and garnet-olivine-plagioclase-bearing granulites. Journal of Metamorphic Geology, 25(5): 497-505 DOI:10.1111/j.1525-1314.2007.00706.x |
Wu CM and Zhao GC. 2007b. The metapelitic garnet-biotite-muscovite-aluminosilicate-quartz (GBMAQ) geobarometer. Lithos, 97(3-4): 365-372 DOI:10.1016/j.lithos.2007.01.003 |
Wu CM and Zhao GC. 2011. The applicability of garnet-orthopyroxene geobarometry in mantle xenoliths. Lithos, 125(1-2): 1-9 DOI:10.1016/j.lithos.2011.02.018 |
Wu CM. 2015. Revised empirical garnet-biotite-muscovite-plagioclase geobarometer in metapelites. Journal of Metamorphic Geology, 33(2): 167-176 DOI:10.1111/jmg.12115 |
Wu CM and Chen HX. 2015a. Calibration of a Ti-in-muscovite geothermometer for ilmenite- and Al2SiO5-bearing metapelites. Lithos, 212 |
Wu CM and Chen HX. 2015b. Revised Ti-in-biotite geothermometer for ilmenite-or rutile-bearing crustal metapelites. Science Bulletin, 60(1): 116-121 DOI:10.1007/s11434-014-0674-y |
Wu CM. 2017. Calibration of the garnet-biotite-Al2SiO5-quartz geobarometer for metapelites. Journal of Metamorphic Geology, 35(9): 983-998 DOI:10.1111/jmg.12264 |
Wu CM. 2018. Metapelitic garnet-muscovite-Al2SiO5-quartz (GMAQ) geothermobarometry. Journal of Earth Science, 29(5): 977-988 DOI:10.1007/s12583-018-0851-z |
Wu CM. 2019. Original calibration of a garnet geobarometer in metapelite. Minerals, 9(9): 540 DOI:10.3390/min9090540 |
Wu CM. 2020. Calibration of the biotite-muscovite geobarometer for metapelitic assemblages devoid of garnet or plagioclase. Lithos, 372-373: 105668 DOI:10.1016/j.lithos.2020.105668 |
Yin CQ, Zhao GC, Wei CJ, Sun M, Guo JH and Zhou XW. 2014. Metamorphism and partial melting of high-pressure pelitic granulites from the Qianlishan Complex: Constraints on the tectonic evolution of the Khondalite Belt in the North China Craton. Precambrian Research, 242: 172-186 DOI:10.1016/j.precamres.2013.12.025 |
Zeh A and Holness MB. 2003. The effect of reaction overstep on garnet microtextures in metapelitic rocks of the Ilesha Schist Belt, SW Nigeria. Journal of Petrology, 44(6): 967-994 DOI:10.1093/petrology/44.6.967 |
Zeh A, Klemd R, Buhlmann S and Barton JM. 2004. Pro- and retrograde P-T evolution of granulites of the Beit Bridge Complex (Limpopo Belt, South Africa): Constraints from quantitative phase diagrams and geotectonic implications. Journal of Metamorphic Geology, 22(2): 79-95 DOI:10.1111/j.1525-1314.2004.00501.x |
Zhang AL, Wei CJ, Tian W and Zhang CG. 2004. Low-pressure metamorphism of Erlangping Group in North Qinling Mountains. Acta Petrologica et Mineralogica, 23(1): 26-36 (in Chinese with English abstract) |
范文寿, 王浩, 张谦, 刘嘉惠, 石梦岩, 李真, 吴春明. 2018. 敦煌造山带长山子地区变质演化及年代学研究. 岩石学报, 34(9): 2773-2792. |
沈其韩. 2009. 推荐一个系统的矿物缩写表. 岩石矿物学杂志, 28(5): 495-500. DOI:10.3969/j.issn.1000-6524.2009.05.011 |
张阿利, 魏春景, 田伟, 张翠光. 2004. 北秦岭二郎坪群低压变质作用研究. 岩石矿物学杂志, 23(1): 26-36. DOI:10.3969/j.issn.1000-6524.2004.01.004 |
 2021, Vol. 37
2021, Vol. 37

