2. 中国科学院大学, 北京 100049;
3. 同位素地球化学国家重点实验室, 中国科学院广州地球化学研究所, 广州 510640;
4. 中国科学院青藏高原地球科学卓越创新中心, 北京 100101;
5. 中国科学院海洋研究所, 深海研究中心, 青岛 266071;
6. 青岛海洋科学与技术试点国家实验室, 海洋矿产资源评价与探测技术功能实验室, 青岛 266237
2. University of Chinese Academy of Sciences, Beijing 100049, China;
3. State Key Laboratory of Isotope Geochemistry, Guangzhou Institute of Geochemistry, Chinese Academy of Sciences, Guangzhou 510640, China;
4. CAS Center for Excellence in Tibetan Plateau Earth Sciences, Chinese Academy of Sciences, Beijing 100101, China;
5. Center of Deep Sea Research, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China;
6. Laboratory for Marine Mineral Resources, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266237, China
铂族元素(Platinum-group elements,简称PGE)是指元素周期表第Ⅷ副族第五周期的钌(Ru)、铑(Rh)、钯(Pd)三种轻铂元素和第六周期的锇(Os)、铱(Ir)、铂(Pt)三种重铂元素。长期以来,PGE都作为重要的稀贵金属矿产资源进行探查研究(Naldrett, 2004)。PGE具有极强亲硫性,主要富集于硫化物中,能够随着岩浆作用从地幔向地壳迁移(Sun et al., 2003a, b, 2004),同时,PGE在地球化学性质上总体较为惰性,只有这些元素之间的熔点差异会导致其在岩浆演化过程中发生分异。因此,PGE通常被认为是不活动性元素,可以作为一种地球化学示踪剂,对地幔源区和镁铁-超镁铁质岩浆的演化提供重要信息(Naldrett, 2004; Price et al., 2004; Song et al., 2004; Wang et al., 2011, 2012, 2014; Barnes and Liu, 2012),比如可作为研究岩浆过程和岩浆矿床的岩石成因探针(Keays and Davison, 1976; Crocket, 1979; Zhang et al., 2017);可以用来制约硫化物从岩浆中分离的时间(Barnes et al., 1985; Hoatson and Keays, 1989; Keays, 1995);制约岩浆-硫化物质量平衡时间(Campbell and Naldrett, 1979; Campbell and Barnes, 1984)以及反映科马提岩的源区演化等(Puchtel et al., 2004; Maier et al., 2009; Fiorentini et al., 2011; Liu et al., 2019)。
然而,很多岩浆过程通常伴随着热液活动,或者后期受热液作用影响(Ding et al., 2018)。PGE的流体活动性在很大程度上影响了利用PGE研究镁铁-超镁铁质岩浆过程及源区示踪的准确性,如Schmidt et al. (2000)发现红海Zabargad岛橄榄岩中的斜方辉石和斜长异剥橄榄岩中的PGE含量以及分配型式不一致,认为部分橄榄岩中PGE遭受了地幔流体的影响,这种交代对依据PGE进行源区示踪造成了一定的困难。因此,明确PGE的流体活动性,不仅对镁铁-超镁铁质岩浆演化和岩石成因,也对PGE矿床的成矿模型和成矿理论具有重要的意义。有鉴于此,本文重点回顾了前人有关PGE流体活动性的实际观察和高温高压实验研究,总结归纳了PGE在不同成分热液中的赋存形式及活动能力,以期对PGE的流体活动性规律有一个初步的认识。在此基础上,进一部探讨当前研究存在的问题和未来的研究方向。
1 PGE的性质六个PGE元素有相似的地球化学性质,具有中等电负性、熔沸点高、电离势大、一般不容易失去电子而氧化等特点。PGE在自然界(包括各类矿床)中有多种赋存形式(刘英俊等, 1984; 刘少轻等, 2007):(1)自然金属,以单质金属形式存在,如自然Pt、Pd、Ir等;(2)金属互化物,常见的有Os-Ir-Ru-Pt、Pt-Pd等;(3)半金属互化物,Pt、Pd和Ir、Os等可与Bi、Te、Se、Sb形成金属键或者相当于金属键的共价键;(4)硫化物和砷化物,PGE以高价态离子与S、As、Sb、Bi构成离子键化合物;(5)类质同像形式,PGE离子代替Co2+、Fe2+进入尖晶石和橄榄石晶格中。
PGE在地球化学行为上表现出高度的亲铁和亲铜性(Sun et al., 2003b, 2004)。Borisov et al. (1994)和Borisov and Palme (1995)测定的PGE在铁金属/硅酸盐熔体相的分配系数高达107(Pd)和1012(Pt和Ir)。Mungall and Brenan (2014)测定PGE在硫化物/硅酸盐熔体相的分配系数为~4×105(Ru)和~2×106~3×106(Pt和Ir)。正因如此,PGE主要集中在地核中,少数在地幔中,而地壳的酸性岩浆岩中含量极低。PGE在球粒陨石、地球各圈层具体分布如表 1所示。由于地壳中PGE含量仅为n×10-12级别,幔源镁铁-超镁铁质岩浆对于地壳岩浆房中PGE的富集和成矿至关重要(刘英俊等, 1984)。
|
|
表 1 不同地质储库中PGE元素的丰度 Table 1 The abundance of PGE in varied geological reservoirs |
PGE在热液条件中也具有一定的活动性。Amosse et al. (1990)报道了由于受到d轨道的影响,PGE可以形成多种络合物,如PGE可以与Cl-结合形成[Pt-Sn4Cl4]4+这类稳定的络合物种型。而且,实验表明PGE无论在氧化性的热水卤水还是还原性的富硫热液中都可以溶解,并且以不同配位形式迁移(Vatin-Perignon et al., 2000)。PGE的配位形式多种多样,主要有Cl-、HS-、NH3、OH-等配位阴离子(Mountain and Wood, 1988)。
2 PGE流体活动性的地质记录虽然传统上认为PGE的活动性与镁铁-超镁铁质岩浆作用密切相关,但越来越多的实例也支持PGE具有一定的流体活动性。归纳起来,PGE流体活动性的地质记录包括:
(1) 岩浆后期热液叠加作用导致的PGE的富集以及成矿。岩浆作用是导致大多数铂金属矿床形成的必要因素,而岩浆演化后期的热液作用对PGE迁移、富集、沉淀以及成矿也有着极大的贡献。Wagner (1929)指出南非Waterberg地区铂族元素不同寻常的地质产状显示其有后期热液作用的影响;Mertie (1969)列举了热液成因铂族元素矿床的特征,包括石英-金-铂族元素矿脉,其寄主岩或是沉积岩、或是长英质火山岩和侵入岩。南非墨西拿的Artonvilla铜-铂矿床是著名的热液PGE矿床,其矿化产于蚀变石英岩和片麻岩的石英脉中,矿床成因与岩浆热液密切相关(Mihalik et al., 1974)。McCallum et al. (1976)研究发现美国怀俄明州的New Rambler-铜-铂矿床产于大剪切带之内,寄主岩为变闪长岩和变辉长岩,围岩蚀变明显,认为矿床为正常辉长岩遭热液淋滤而使PGE富集沉淀而成。王登红等(2002)发现四川杨柳坪铂金属矿床中热液矿石的PGE含量相对高于岩浆矿石,最高可达n×10-6级别,认为后期热液作用对矿床形成有重要贡献。世界著名的金川超大型矿床,通过研究发现其经历了早期岩浆喷发、三期岩浆上侵定位、含矿岩浆上侵就位后就地熔离和聚集以及后期的气化热液、热液叠加成矿作用和矿床的后期改造等,导致产生一个完整的、动态的成矿模式,其中热液富集PGE起到关键性作用(苏昌学, 2009)。王敏芳等(2010)通过总结分析斑岩铜矿中PGE的来源、富集及成矿过程,认为PGE的富集、成矿与成矿流体的组成、性质以及演化有密切的联系。在Sudbury地区同样发育着许多相互独立的热液型PGE矿床(Tuba et al., 2014)。李瑞鹏和颜建东(2013)认为世界上所有的铂族金属矿床都有热液流体活动的迹象,并且低温热液(< 500℃)中,PGE可能主要以氯络合物及氢硫基络合物的形式运移;高温热液(>500℃)中,PGE可能主要以氯络合物的形式运移。金宝山Pt-Pd型矿床的成矿模式为多期硫化物溶解-富集过程+滞后的硫化物饱和,深部岩浆房中形成的硫化物经历多期硫化物溶解-富集过程导致富PGE岩浆形成并进入浅部岩浆房形成富PGE层,之后,强烈的热液作用造成铂族元素从硫化物中释放再富集成矿(Wang et al., 2005, 2008, 2010)。Benkó et al. (2015)对Spruce Road deposit中的不同成分的流体包裹体进行分析,发现晚阶段的流体包裹体富含PGE的硫化物矿物沉淀,说明PGE在晚阶段热液作用中能随热液迁移并且可以选择性富集、沉淀。Holwell et al. (2017)对岩浆Ni-Cu-PGE矿床中的黄铁矿-黄铜矿-磁黄铁矿等一些矿物组合进行低温(< 200℃)热液蚀变实验模拟结果表明,在蚀变初期,磁黄铁矿损失一半以上的S和Fe,但是其中的Cu、Ni和PGE含量显著增加,认为这些矿物组合在蚀变过程中能选择性吸收流体中PGE。而Skouries矿床中的石英-黄铜矿-斑铜矿-磁铁矿等矿脉中也广泛分布PGE矿物,如碲钯矿、黄碲钯矿等,并且部分PGE矿物会以包裹体的形式出现在热液石英和硫化物中(McFall et al., 2018)。该现象说明PGE能在热液作用下显著活动,在地球化学障中进行富集、结晶而形成独立矿物。以上现象都表明热液活动为PGE迁移提供条件,也能促进PGE进行富集、结晶而形成富矿岩体、独立矿物以及成矿。
(2) 大洋或盆地热液活动区卤水或矿石矿物中PGE的富集。一些研究表明,PGE能在热液中活化、并通过淋滤、吸附等作用使得矿石矿物、卤水或沉积物的PGE富集。比如,加利福尼亚州Salton Sea卤水PGE含量为0.2×10-12~3×10-12,Pt浓度最高可达50×10-12,被认为是PGE随着流体迁移至表面而富集(Harrar and Stephens, 1984; McKibben et al., 1990)。Hekinian et al. (1980)通过对东太平洋21°N海底喷气块状硫化物矿床的研究发现,其中白铁矿和黄铜矿含Pt达1%,认为Pt的富集与热液活动有密切关系。在西南印度洋脊的49°39′E热液活动区中的硫化物,其PGE含量也可达到n×10-12级,这主要是热液对含矿围岩及其下伏基底物质淋滤作用或者是深部岩浆房挥发组分直接释放所致(黄威等, 2011)。在我国华南、加拿大、美国、捷克的裂谷和裂谷-火山环境海相黑色页岩中的PGE富集被认为与盆地卤水或火山热液有密切联系(pašava, 1993)。Kazachenko et al. (2006)通过实验发现,Sikhote Alin地区的金属沉积物中富含大量的Au、Ag、PGE、Sn、Zn、Pb等金属元素,认为其与三叠纪热液活动有关,这些金属元素被放射虫沉积物的粘性和有机物从热液中吸收。
3 PGE流体活动性的实验研究与模拟计算为了探讨热液中PGE的迁移和成矿作用,很多学者对PGE流体活动性以及相关制约条件进行了实验研究,其内容主要涉及溶解度和迁移种型。研究的方法主要分为:(1)实验研究,即直接测定PGE金属或矿物在不同温压条件下电解质溶液中的溶解度并确定其配位化合物形式,在此基础上对PGE在各种地质热液条件下的迁移量、迁移种型和沉淀机制进行探讨;(2)理论预测,即根据低温低压实验数据按照相关计算方法进行高温高压计算模拟,推测高温高压下PGE迁移量与迁移种型。
3.1 PGE溶解度PGE在富Cl-流体中的溶解度总体较高,但在不同实验中差异较大。Orlova et al. (1987)在600℃、150MPa和磁铁矿-赤铁矿(MH)氧逸度条件下,最早采用失重法测定了Pt金属在富Cl-流体中的溶解度最高可达500×10-6。Hsu et al. (1991)报道了在300~700℃、100MPa和近镍-氧化镍(NNO)氧逸度条件下的溶解度,发现Pd(Ⅱ)(Ⅱ表示离子价态,下文同)在0~3mol/L NaCl溶液中的溶解度随温度、NaCl浓度和pH变化而变化,其溶解度范围在0.5×10-6~73×10-6之间。而Gammons et al. (1992)采用Pt(Ⅱ)和Pd(Ⅱ)硫化物作为初始反应物,测定了300℃、NaCl/H2SO4溶液中Pt(Ⅱ)和Pd(Ⅱ)硫化物的溶解度,发现在中低温(< 350℃)、高氧逸度(赤铁矿-磁铁矿)、高NaCl浓度(>3mol Cl-)和低pH(< 4)条件下,Pt/Pd(Ⅱ)溶解度仅n×10-12级别。Xiong and Wood (2000)测定了Os和Pd在KCl溶液中不同pH、氧逸度、温度条件下的溶解度。Os(Ⅱ)实验结果为:(1)在1mol/L KCl溶液、NNO氧逸度和500℃条件下,Os溶解度为~3×10-12;(2)在1.5mol/L KCl溶液、NNO氧逸度和500℃条件下,Os溶解度为~130×10-12;(3)在1.5mol/L KCl溶液、RRO氧逸度和500℃条件下,Os溶解度为~1705×10-12。Pd(Ⅱ)实验结果为:(1)在0.1mol/L KCl溶液、RRO氧逸度和500℃下,Pd溶解度为~40×10-12;(2)在0.1mol/L KCl溶液、MMO氧逸度和400℃下,Pd溶解度为~15×10-12。由实验结果可知,Os(Ⅱ)在富Cl-流体中的溶解度与氧逸度和Cl-浓度成正比,最高可达n×10-6级别,Pd(Ⅱ)在富Cl-流体中的溶解度与氧逸度成正比,也可达10n×10-12。
相比于富Cl-流体,PGE在富HS-流体中的溶解度较低,仅达到n×10-12级别。但在不同实验研究中,实验结果仍存在较大差异。Pan and Wood (1994)测定了富HS-热液中Pd和Pt硫化物的溶解度,在25~350℃、压力为62~275大气压、pH为5.91~9.43、∑S为0.3~2.2mol/L、NaOH浓度在0.01~1.3mol/L条件下,PdS、PtS的溶解度为4×10-12~800×10-12、1×10-12~400×10-12,并且随着温度上升,PdS、PtS的溶解度逐渐增大,在~300℃达到最大值。Gammons and Bloom (1993)同样测定了PtS、PdS在30~300℃、∑S=0.01~1.0mol/L条件下的溶解度。其实验结果与上述实验结果相似,即PtS、PdS在富HS-流体中溶解度较低,只有n×10-12级别。但他们还发现,PtS、PdS溶解度随温度上升而下降,进一步的模拟计算表明,在黄铁矿稳定区间内,高硫浓度(∑S=0.1mol/L)时,Pt、Pd溶解度可达10×10-12以上,在低硫浓度(∑S=0.001mol/L)时则小于0.1×10-12。Mei et al. (2015)通过第一性原理方法模拟计算得出相似的结果,在富HS-热液体系中,Pd最大溶解度可达到n×10-12级别。
从以上可以看出,前人有关PGE溶解度的实验仅局限于Pd、Pt、Os元素,也仅涉及含Cl-和含HS-的流体成分。虽然前人研究结果有差异,但总体上PGE在富Cl-流体中的溶解度较大,可达10n×10-6~100n×10-6,主要受温度、压力、pH、氧逸度以及Cl-浓度等条件控制;而PGE在富HS-热液体系中溶解度相对较小,可达n×10-12级别,同样受诸多条件制约,如温度、压力、pH、氧逸度以及HS-浓度等。
3.2 PGE在热液中的络合物种型前人通过实验研究和模拟计算探讨PGE在热液中的赋存状态和迁移种型,主要涉及的无机配位阴离子包括Cl-、HS-、OH-、NH3(Mountain and Wood, 1988; Wood et al., 1989, 1992; Sassani and Shock, 1990; Hsu et al., 1991; Tait et al., 1991; Gammons et al., 1992; Gammons and Bloom, 1993; Pan and Wood, 1994; Gammons, 1995, 1996; Van Middlesworth and Wood, 1999; Xiong and Wood, 2000; Azaroual et al., 2001; Levitin and Schmuckler, 2003; Bazarkina et al., 2014; Mei et al., 2015)。PGE离子与配位阴离子结合时,其累积平衡常数除了受温度的影响,还受配位阴离子个数的控制(表 2),其反应方程式为:
|
|
表 2 在25~300℃条件下Pt、Pd与Cl-、HS-、OH-以及NH3络合反应的平衡常数 Table 2 Stability contants of Pt and Pd chloride, bisulfide, hydroxide and ammonia at 25~300℃ |
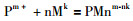
|
(1) |
其中P代表PGE离子,M代表配位阴离子,m、n、k分别代表PGE离子价态、配位阴离子个数以及配位离子价态。β代表累积平衡常数,其表达方式表示为:
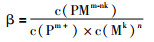
|
(2) |
其中c表示离子浓度。由表 2可知,PGE-Mn(M代表Cl-、OH-、NH3)配位形式的平衡常数受温度制约效果不显著,配位体个数的影响相对较大,其β与n成正相关性。而PGE-Mn(M代表HS-)受温度控制较为明显,其β与n成负相关性。除了受温度与配位体个数控制之外,PGE络合物累积平衡常数也间接受氧逸度影响。氧逸度对PGE络合物的配位阴离子的稳定性会产生一定影响,从而影响其累积平衡常数,如在高氧逸度条件下,S2-易被氧化,其形成的PGE络合物发生不稳定,因而影响PGE的活动性。对于不同PGE迁移种型的情况,下面将详细介绍。
3.2.1 Cl-配位体Cl-在PGE的热液迁移过程中有着重要的作用,而且PGE-Cl的络合物种型、迁移量明显受Cl-浓度、pH、氧逸度、温度等因素制约。Mountain and Wood (1988)和Wood et al. (1989)首先尝试利用理论计算来确定在热液低温条件下(≤300℃)Pt/Pd(Ⅱ)-Cl络合物的稳定常数,认为Pt/Pd(Ⅱ)-Cl络合物种型主要存在于相对氧化、酸性的条件。Wilde et al. (1989)通过对不整合型铀矿的研究,得出了相同的结论。同样,Pt/Pd(Ⅱ)-Cl络合物种型在高温热液中(≥400℃)也尤为重要(Wood, 1987; Mountain and Wood, 1988; Sassani and Shock, 1990)。实验模拟研究发现,Pd/Pt在富Cl-流体中的络合物种型及迁移量受温度、pH、氧逸度、Cl-浓度制约(Orlova et al., 1987; Tait et al., 1991)。进一步研究发现,在富Cl-、贫S2-、高氧逸度流体中,Pd(Ⅱ)-Cl-OH络合物种型变得尤为重要(Bazarkina et al., 2014)。可以看出,PGE-Cl络合物种型在中低温、高氧逸度、高Cl-浓度和低pH条件下较为稳定,其存在的种型以高配位离子数为主(Gammons et al., 1992, 1993; Wood et al., 1992; Gammons, 1995, 1996; Van Middlesworth and Wood, 1999; Likhoidov et al., 2000; Levitin and Schmuckler, 2003)。通过总结前人实验及理论计算结果,下面具体讨论不同因素对PGE-Cl的赋存状态的影响。
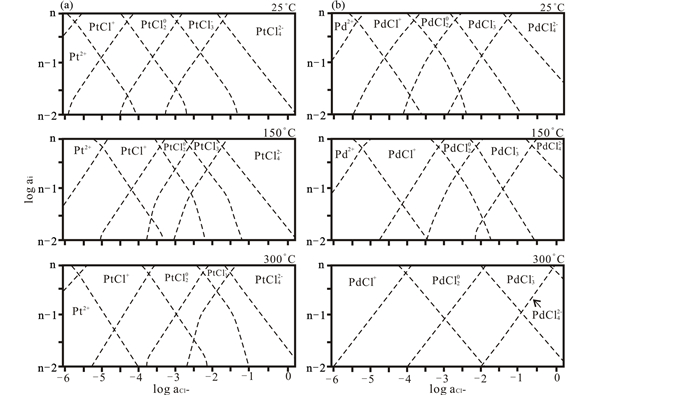
流体中Cl-的浓度对PGE的迁移种型有极其重要的影响。Pt/Pd(Ⅱ)-Cl在流体中的种型随着流体Cl-浓度变化而变化。随着流体中Cl-浓度增加,Pt/Pd(Ⅱ)-Cln中n增大,其种型也逐渐转变为Pt/Pd2+→Pt/PdCl+→Pt/PdCl20→Pt/PdCl3-→Pt/PdCl42-(图 1、图 2)(Wood et al., 1992; Levitin and Schmuckler, 2003)。由于流体中Cl-浓度增加,更多Cl-与Pt/Pd2+或低配位数Pt/Pd-Cl络合物相结合,从而形成高配位数的Pt/Pd-Cl络合物;并且在足够的Cl-条件下,高配位数的Pt/Pd-Cl络合物离子在流体中稳定区间变大,从而促进PGE在更长距离范围内稳定迁移。相对于还原态的PGE离子,氧化态的PGE离子(如Pt(Ⅳ))也表现出一致的实验结果。Pt(Ⅳ)-Cl络合物中的Cl-配位数也随着Cl-浓度增大而增多,并且在普遍的地质流体中,高配位离子数的PtCl5-、PtCl62-种型相对较为稳定,迁移能力较强(Gammons, 1995)。上述结果同样适用于Os(Ⅱ)和Rh(Ⅲ),Xiong and Wood (2000)通过实验研究表明,Os(Ⅱ)-Cl迁移量随着流体中Cl-浓度增加而增大,说明Cl-配位数也随着Cl-浓度增大而增多。Levitin and Schmuckler (2003)利用拉曼光谱测定不同Cl-浓度条件下,Rh(Ⅲ)-Cl在流体中种型的变化。结果表明随着Cl-浓度增大,其配位阴离子数增加,络合物种型从Rh3+→RhCl2+→RhCl2+→RhCl30→RhCl4-→RhCl52-→RhCl63-逐级转变,并且RhCl52-有相对较大的稳定区间(图 3)。
|
图 1 在25、150和300℃条件下Pt2+(a)和Pd2+(b)不同配位氯离子形式络合物与氯离子活度的关系(据Wood et al., 1992; Levitin and Schmuckler, 2003修改) 所有金属离子标准化到10-n单位,n为任何正整数,aCl-代表Cl-活度 Fig. 1 Distribution of chloride complexes of Pt2+(a) and Pd2+ (b) as a function of the activity of free chloride at 25, 150 and 300℃ (modified after Wood et al., 1992; Levitin and Schmuckler, 2003) The diagrams are generalized for total metal concentrations of 10-n molar, where n is any positive integer, the aCl- represent the activity of free chloride |
|
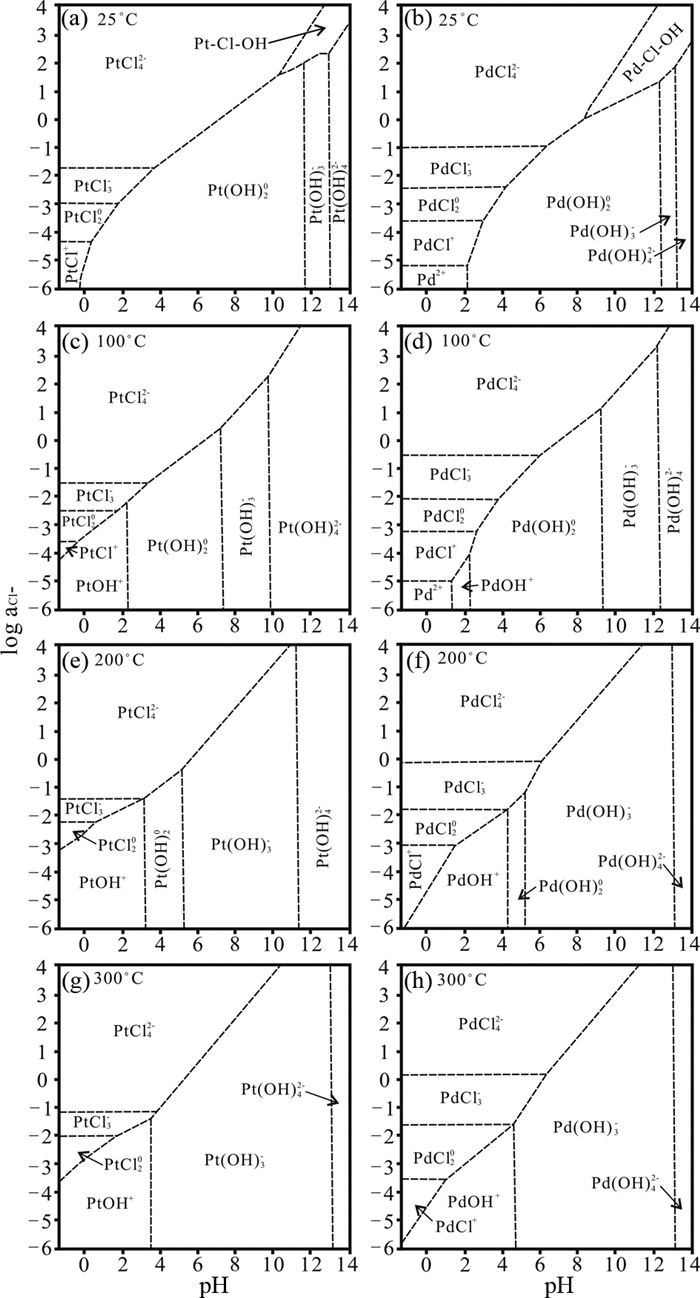
图 2 不同pH、Cl-活度、温度下,Pt2+和Pd2+的Cl-、OH-以及Cl-OH络合种型的稳定范围(据Wood et al., 1992修改) Fig. 2 Plot of log acl- vs. pH for Pt2+ and Pd2+ at 25℃, 100℃, 200℃, 300℃ showing the fields of predominance of the various chloride, hydroxide and mixed hydroxychloride complexes (modified after Wood et al., 1992) |
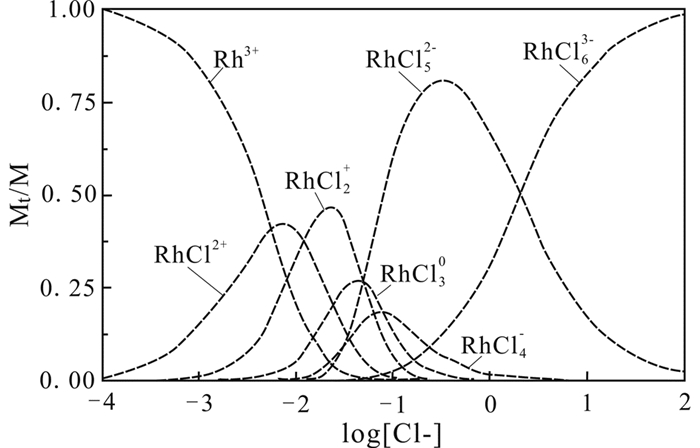
|
图 3 不同Cl-离子活度下Rh-Cl种型的分布图(据Levitin and Schmuckler, 2003修改) Mt为实时不同Rh迁移形式含量,M为流体中总Rh含量 Fig. 3 Distribution of Rh-Cl complexes as a function of free chloride concentration (modified after Levitin and Schmuckler, 2003) Mt/M is the concentration of different complexes of Rh/the total concentration of Rh in fluids |
相对于流体中Cl-浓度对PGE-Cl络合物种型的影响,温度的影响则正好相反,即Pt/Pd(Ⅱ)-Cl络合物种型中Cl-配位数随着温度增加而减少。如图 1所示,当温度从25℃逐渐升高到300℃时,低配位数的离子络合物,如Pt/PdCl+、Pt/PdCl20稳定区间逐渐增大,而高配位数的离子络合物,如Pt/PdCl3-、Pt/PdCl42-稳定区间逐渐缩小。并且随着温度的逐渐升高,Pt/Pd(Ⅱ)-Cl络合物种型逐渐向Pt/Pd(Ⅱ)-OH络合物种型转变(图 2)。同样的结果也在Pt(Ⅳ)-Cl络合物种型中发现,Gammons (1995)测定了不同温度下,Pt(Ⅳ)-Cl的络合物种型,并且发现当温度由100℃升高到300℃过程中,Pt(Ⅳ)-Cl迁移种型由PtCl62-向PtCl5-,甚至向PtCl42-转变(图 4)。在中低温范围内,PtCl5-、PtCl62-种型较为稳定。这主要是由于在高温条件下,流体中存在更多OH-,流体逐渐向碱性条件转变,使得OH-能取代络合物离子中的Cl-,造成Pt/Pd-Cl种型逐渐向Pt/Pd-OH种型转变。从阳离子水解角度来说,高温条件下水的极性增强,电离出更多的羟基OH-,能与高极化力的PGE离子进行优先络合,进而促使OH-逐级置换Cl-,使得PGE-Cl络合物转变为PGE-Cl-OH络合物,再完全转变为PGE-OH络合物,进而发生PGE的金属沉淀(Ding et al., 2018)。该过程类似于钛的氟酸基络合物水解过程(何俊杰等, 2015)。
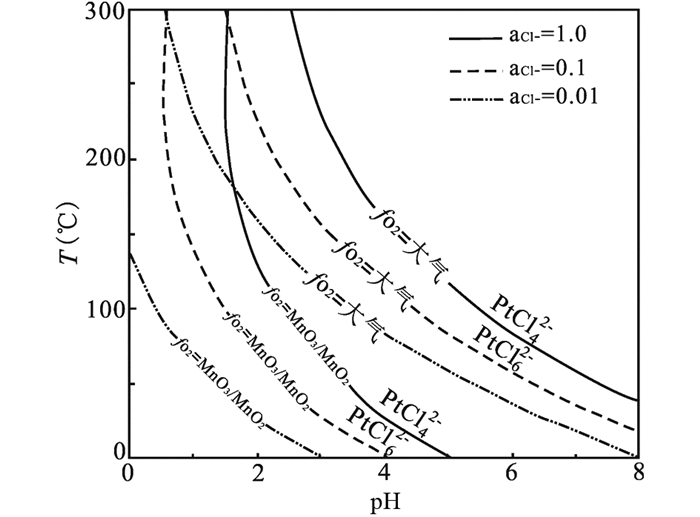
|
图 4 PtCl42-和PtCl62-稳定性随温度、pH变化图(据Gammons, 1995修改) Fig. 4 Diagram summarizing the relative stability of PtCl42- and PtCl62- as a function of temperature and pH (modified after Gammons, 1995) |
氧逸度对热液中PGE-Cl络合物种型的影响也很显著,与Cl-浓度有相似的效果。Pd(Ⅱ)/Pt(Ⅱ)/Pt(Ⅳ)-Cl络合物种型随着氧逸度的变化而变化(Gammons, 1995)。当流体中氧逸度从大气转变为Mn2O3/MnO2时,PtCl62-、PtCl42-稳定区间向酸性、低温条件迁移(图 4);对于Pd/Pt(Ⅱ)-Cl络合物也同样如此,在氧逸度升高时,Pd/Pt(Ⅱ)-Cl倾向以高配位数络合离子形式迁移,并且其迁移量随着氧逸度升高而增高(图 5)。在高氧逸度条件下,高配位数的PGE-Cl络合物种型在流体中占主导。因此,高的氧逸度一方面促进PGE阳离子被氧化至高价态,另一方面有利于PGE阳离子形成高配位数的PGE-Cl络合物,从而增强其流体活动性。
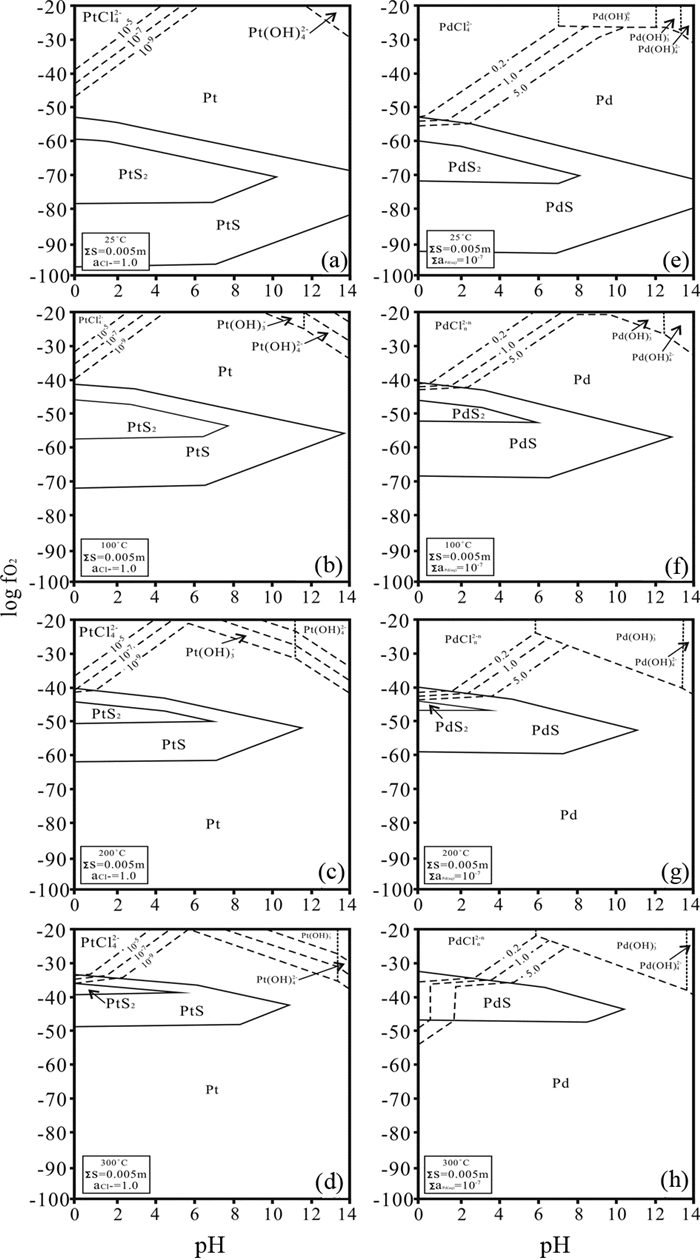
|
图 5 在Pd/Pt-H-O-S-Cl体系中,Pd/Pt-Cl/OH络合物形式迁移稳定性随着氧逸度、pH变化图(据Wood et al., 1992修改) Fig. 5 Log fO2-pH diagrams for the system Pd/Pt-H-O-S-Cl showing the stability of various Pd/Pt phases and their solubility as chloride and hydroxide complexes (modified after Wood et al., 1992) |
流体pH对PGE-Cl络合物种型的影响体现在对水的离解反应控制上。pH越小,其流体中H+浓度越大、OH-浓度越小,PGE-Cl络合物中的阴、阳离子的水解就被抑制,导致流体中的PGE-Cl络合物更为稳定。因此,pH越小,PGE趋向于以Pd(Ⅱ)/Pt(Ⅱ)/Pt(Ⅳ)-Cl络合物种型在热液中迁移(图 2、图 4、图 5)。相反地,一旦pH升高,Pt/Pd(Ⅱ)-Cl络合物种型逐渐向Pt/Pd(Ⅱ)-OH转变,其迁移量也逐渐减小(Wood et al., 1992)(图 2、图 5)。
综上可知,在中低温(< 300℃)、高盐度、高氧逸度和酸性条件下,PGE在富Cl-流体中主要以高配位体数的PGE-Cl络合物种型稳定存在,并可进行长距离迁移,如Pt/PdCl42-、Pt/PdCl3-、PtCl62-、PtCl5-、RhCl52-;PGE-Cl-OH种型对于PGE的迁移也具有一定贡献。随着流体中Cl-浓度、氧逸度增加以及pH、温度减小,其络合离子中配位Cl离子数也增加。
3.2.2 HS-配位体在热液流体中,PGE-HS对PGE的活动和迁移也有着一定的贡献,其迁移形式受流体中S2-浓度、温度、氧逸度以及pH控制。Mountain and Wood (1988)通过热力学计算发现,Pt/Pd(Ⅱ)-HS络合物形式对于Pt、Pd的热液迁移有重要作用。随后Wood et al. (1989)通过实验研究也发现,当温度在300℃、log(fO2)-pH-∑S在黄铁矿-磁黄铁矿-磁铁矿区间的条件下,Pt、Pd以HS-络合物种型迁移量仅有10×10-12。Wood et al. (1992)进一步研究认为,Pt/Pd(Ⅱ)-HS是PGE热液活动的有效迁移种型,其迁移能力受温度、氧逸度、pH等多因素制约。随后的实验结果证实了在低温(< 400℃)、富S2-流体中,Pt/Pd(Ⅱ)-HS络合物的溶解度和迁移量较低(n×10-12级别)(Gammons and Bloom, 1993; Pan and Wood, 1994)。Mei et al. (2015)通过第一性原理模拟计算的结果再一次肯定了之前的研究。
综合来看,PGE-HS络合物在热液中的稳定性同样受体系温度、S2-浓度、氧逸度和pH的影响。当温度由30℃逐渐升高至200℃时,富S2-流体中Pd的迁移量呈现逐渐下降的趋势(Gammons and Bloom, 1993)。如图 6所示,当流体中S2-浓度由0.001mol/L增加到0.1mol/L时,Pt/Pd(Ⅱ)-HS迁移能力增大了三到四个数量级。而低氧逸度则有利于Pt/Pd(Ⅱ)-HS络合物迁移。当log(fO2)从-60升至-30时,其Pt/Pd(Ⅱ)-HS迁移能力可从n×10-15级别增加到10n×10-12。pH不仅对Pt/Pd(Ⅱ)-HS迁移能力有显著影响,对其迁移形式或存在的种型也有重要影响。比如,酸性条件下,PGE在热液中以Pt/Pd(HS)20络合物种型为主,其迁移量 < 1×10-12;当pH由酸性转变为碱性时,其络合物种型也从Pt/Pd(HS)20转变为Pt/Pd(HS)3-,其迁移量可增高至10×10-12。因此,随着S2-浓度、氧逸度、pH升高或者温度的降低,PGE-HS迁移能力会逐步提高,可由n×10-15级别升到n×10-12级别;其存在的络合物种型的阴离子配位体数也会增加,由Pt/Pd(HS)20转变为Pt/Pd(HS)3-。也就是说,在还原、碱性、高S2-浓度和相对中低温条件下,PGE在流体中主要以PGE-HS络合物种型存在或迁移,如Pt/Pd(HS)20和Pt/Pd(HS)3-。
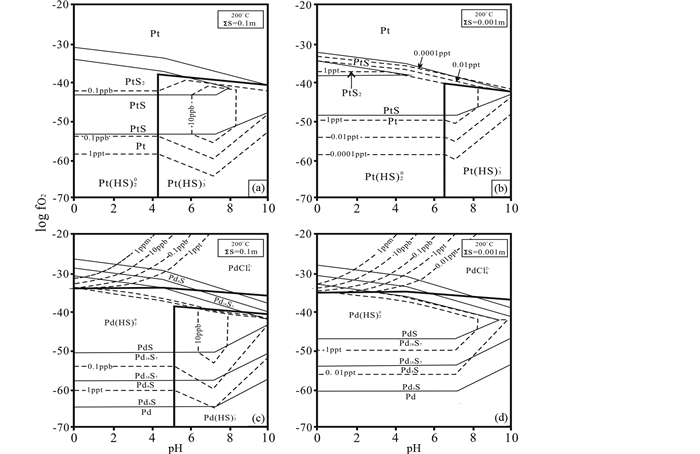
|
图 6 在200℃下0.1 m ∑S (a、c)和0.001 m ∑S (b、d)的Pt/Pd-S-H2O体系中,Pd/Pt络合物随氧逸度、pH变化图(据Gammons and Bloom, 1993修改) Fig. 6 Log fO2-pH diagrams for the Pt/Pd-S-H2O system 0.1m ∑S (a, c) and 0.001m ∑S (b, d) at 200℃ (solubility contours are given by the dashed lines modified after Gammons and Bloom, 1993) |
理论上而言,在纯水或低浓度溶剂阴离子体系中,PGE将以羟基络合物的形式存在。Mountain and Wood (1988)和Wood et al. (1989)通过热力学计算以及总结前人研究结果认为,在25℃、较大的pH范围内,Pt、Pd主要以OH-络合物种型迁移,其迁移种型主要为Pt/Pd(OH)20。Tait et al. (1991)通过实验研究发现,在低Cl-、HS-、NH3浓度和偏中性(pH=5~6)条件下,Pd(OH)20是Pd主要赋存种型,并认为Pd(OH)20是PGE在浅表海洋环境中的主要赋存种型,而氧逸度、pH对Pd(Ⅱ)-OH络合物的稳定性和迁移能力影响极大。Van Middlesworth and Wood (1999)通过实验研究也得出相同的结果。相似的结论适用于Pt,其在海水中的主要迁移种型也是PtOH+、Pt(OH)20(Bazarkina et al., 2014)。但Wood et al. (1992)却得出完全相反的结论,通过实验测试,他们认为PGE-OH迁移种型在淡水中尤为重要,但是在海水中的作用较弱。

|
图 7 Pt/Pd-H-O-N-S体系下,Pt-NH3络合物种型稳定性随fO2、pH变化图(据Wood et al., 1992修改) Fig. 7 Log fO2 vs. pH diagrams for Pt/Pd-H-O-N-S showing the solubility of Pt as ammonia complexes (modified after Wood et al., 1992) |
与其他阴离子一样,PGE-OH络合物的稳定性和迁移能力同样受温度、pH和氧逸度的影响;稍有不同的是,其络合物稳定性还受体系中存在的其他阴离子或阴离子团的浓度影响。在其他因素不变条件下,当温度由25℃逐渐升高到300℃时,流体中Pt/Pd(Ⅱ)-Cl迁移种型向Pt/Pd(Ⅱ)-OH迁移种型转变的转折点的Cl-浓度逐渐增大、而pH则逐渐减小,并且Pt/Pd(OH)3-迁移种型稳定区间也逐渐增大(图 2)。同样如图 2所示,当Cl-浓度降低时,Pt/Pd(Ⅱ)迁移种型会从Pt/Pd(Ⅱ)-Cl向Pt/Pd(Ⅱ)-OH转变,当pH逐渐升高时,Pt/Pd(Ⅱ)迁移种型也会从Pt/Pd(Ⅱ)-Cl转化为Pt/Pd(Ⅱ)-OH,并且OH-配位体数逐级增加,转变形式为Pt/PdOH+→Pt/Pd(OH)20→Pt/Pd(OH)3-→Pt/Pd(OH)42-。Pt/Pd(Ⅱ)-OH的迁移种型也随着氧逸度的变化而改变,随着氧逸度升高,其迁移种型从Pt/Pd(OH)3-向Pt/Pd(OH)42-转变,其迁移量也逐步增加(图 5)。
综上所述,PGE-OH络合物也是PGE在流体中常见的一种,其稳定的条件为高温、碱性和低的其他阴离子浓度,主要种型为PGE-(OH)20和PGE-(OH)3-。
3.2.4 NH3配位体Rasmussen and Jørgensen (1968)和Skibsted and Bjerrum (1975)利用平衡常数进行相关模拟得出,Pt和Pd在地质流体中可以Pt/Pd(NH3)42+络合物种型进行迁移。Wood et al. (1989)研究也发现,Pt、Pd可以与NH3结合形成稳定的络合物,并且温度升高时,PGE-NH3迁移能力会增强。Lechler and Hsu (1990)证明在浅表环境中,Pt、Pd在一定程度上对NH3有氧化作用。Wood et al. (1992)总结前人实验并进行模拟计算,认为PGE-NH3络合物种型对PGE热液迁移有重要作用,其稳定性受流体温度、pH、氧逸度等多因素制约。
PGE-NH3络合物稳定性的制约因素与PGE-OH相似。如图 7所示,当温度由25℃逐渐升高至300℃时,Pt/Pd(Ⅱ)-NH3络合物的稳定区间逐渐向酸性、高氧逸度的范围移动,其迁移量逐渐减少,并且其主要络合物种型(Pt/Pd(NH3)42+)的稳定区间也逐步缩小。在一定条件下,Pt/Pd(Ⅱ)-NH3迁移能力随着氧逸度的增大、pH升高,呈现先增强后降低的趋势,其最佳稳定区间随着温度、NH3浓度、S2-浓度、压力等因素而变化,如在25℃、1bar、0.005mol/L S2-、0.001mol/L NH3条件下,Pt/Pd(NH3)42+最稳定的区间为氧逸度(logfO2)为约70~55和pH为6.5~9.5。由上可知,PGE-NH3络合物稳定的最佳条件为相对中低温、中碱性、相对低氧逸度的地质环境。
4 结语与展望 4.1 PGE流体活动性的研究进展小结传统上认为PGE在地质过程中具有高度惰性,其活动性多与幔源岩浆作用息息相关;但越来越多的地质证据表明PGE也具有热液活动性,具体体现在不同构造环境下热液活动导致的卤水、矿石矿物或地质体中PGE的富集。
PGE的溶解度实验表明,PGE在富Cl-流体中具有较大的溶解度,最高可达100n×10-6;在富HS-流体中溶解度较小,最高仅为100n×10-12。高温高压实验和模拟计算表明,PGE,尤其是Pt(Ⅱ)和Pd(Ⅱ),通常以络合物形式在流体中进行迁移和富集。与PGE结合的无机配位阴离子或分子通常包括Cl-、HS-、OH-以及NH3;这些络合阴离子团的稳定性受流体的温度、氧逸度、离子浓度、pH等诸多因素制约。其中,PGE-Cl络合物种型在热液体系中对于PGE的活动性贡献极大,其迁移量最高可达100n×10-6。PGE-Cl种型稳定的条件为中低温(7#60;300℃)、高盐度、高氧逸度、酸性的地质环境,并且其络合离子团中配位离子数随着流体中Cl-浓度、氧逸度增加或pH、温度的减小而增加。PGE-HS络合物种型在热液体系中广泛存在,其迁移量可达n×10-12级别。在还原、碱性、高S2-浓度、相对中低温条件下,PGE-HS络合物种型在热液中占主导,主要以高配位数络合离子团形式稳定存在。PGE-OH种型的稳定条件为高温、碱性、低溶剂阴离子(如Cl-、S2-等)浓度环境。相对其他几种络合物形式,PGE-NH3对于PGE流体活动性的贡献较小,其最佳稳定条件为相对中低温、中碱性、相对低氧逸度的地质环境。结合已有的实验研究、模拟计算以及地质过程中已有的PGE富集的实例,本文认为酸性、氧化的、高盐度流体对于铂族元素的迁移富集有着至关重要的影响。相反,中低温、中碱性、相对低氧逸度的条件虽然适合PGE-NH3形式的络合物形成,但是在PGE的流体迁移中很可能仅发挥极有限的作用。
4.2 问题和展望PGE流体活动性研究在岩石成因、矿床形成演化、壳幔作用、俯冲交代等各方面有重要意义。如前所述,之前对于天然样品的研究主要集中在PGE的岩浆和成矿作用方面,而实验研究则主要集中在PGE溶解度和赋存方式方面。目前亟需解决的问题有:(1)PGE在矿物-流体-熔体之间的分配行为。虽然前人测定了PGE在金属相和硅酸盐熔体之间的分配系数(Borisov et al. 1994; Borisov and Palme, 1995)以及硫化物相和硅酸盐熔体之间的分配系数(Mungall and Brenan, 2014),但是PGE在不同成分硅酸盐熔体与不同矿物相之间的分配以及影响因素知之甚少,尤其是PGE在熔体和流体之间的分配行为研究极其缺乏。这严重制约了我们对岩浆房中岩浆-热液活动和PGE成矿过程的准确认识。(2)PGE的溶解度评估。在前人早期的溶解度实验中,多数采用PGE金属或金属合金作为初始反应物,以此来保证元素的充分供应。但自然界中PGE很少以单质金属形式存在;同时,以金属或金属合金作为初始反应物,实验过程易于发生氧化还原反应,导致实验限定条件发生变化,过程更加复杂,而且实验也很难达到平衡。另外,在实验技术上,多数溶解度实验采用失重法,难以避免实验过程中溶解-再沉淀以及淬火效应的发生,导致实验结果产生较大偏差。这些不足会严重影响PGE流体活动性程度以及PGE在流体赋存形式的准确评估。(3)PGE与其他阴离子络合能力评估。已查明的与PGE结合的无机配位阴离子或分子主要有Cl-、HS-、OH-以及NH3,然而,自然界流体中的阴离子成分众多(Ding et al., 2018; 何俊杰等, 2015),PGE与其他阴离子之间的络合能力以及形成的络合物稳定性如何也知之甚少,尤其是与成矿过程密切相关的流体组分如F-、CO32-、PO43-和NO32-等等,增加了对PGE流体活动程度以及定量评估的难度。
由于PGE在地壳及大部分矿石矿物中含量极低,PGE的分析测试技术对于PGE的元素性质研究至关重要。近十余年来,随着低检测限、高精度分析仪器和技术方法的改进,尤其是原位微区分析技术的应用,低浓度的PGE含量测试已经取得巨大的进步;另一方面,新的高温高压实验技术的革新,比如多层反应容器嵌套方式的应用(Migdisov et al., 2011)、高温高压条件下流体包裹体的合成和LA-ICPMS单个流体包裹体成分分析相结合、热液金刚石压腔和毛细硅管等可视腔与高分辨率共聚焦激光拉曼或同步辐射联用,大大增强了高温高压实验结果的准确性(Ding et al., 2018; Li et al., 2018)。因此,在以后的PGE流体活动性研究中,最新的高温高压实验技术和分析技术有望陆续得到运用,当前存在的不足逐渐得到填补,比如更宽泛的实验条件(如温度、压力,pH、氧逸度、硫逸度)和更接近自然的复杂流体成分,最终使得PGE流体活动性的规模以及控制机理得到更全面的认识,从而为相关矿床的形成演化和岩石成因提供强有力的理论制约。
致谢 感谢高剑峰研究员和袁顺达研究员对本文提出的建设性意见。
Amosse J, Allibert M, Fischer W and Piboule M. 1990. Experimental study of the solubility of platinum and iridium in basic silicate melts:Implications for the differentiation of platinum-group elements during magmatic processes. Chemical Geology, 81(1-2): 45-53 DOI:10.1016/0009-2541(90)90038-9 |
Anders E and Grevesse N. 1989. Abundances of the elements:Meteoritic and solar. Geochimica et Cosmochimica Acta, 53(1): 197-214 DOI:10.1016/0016-7037(89)90286-X |
Azaroual M, Romand B, Freyssinet P and Disnar JR. 2001. Solubility of platinum in aqueous solutions at 25℃ and pHs 4 to 10 under oxidizing conditions. Geochimica et Cosmochimica Acta, 65(24): 4453-4466 DOI:10.1016/S0016-7037(01)00752-9 |
Barnes SJ, Naldrett AJ and Gorton MP. 1985. The origin of the fractionation of platinum-group elements in terrestrial magmas. Chemical Geology, 53(3-4): 303-323 DOI:10.1016/0009-2541(85)90076-2 |
Barnes SJ, Boyd R, Korneliussen A, Nilsson LP, Often M, Pedersen RB and Robins B. 1988. The use of mantle normalization and metal ratios in discriminating between the effects of partial melting, crystal fractionation and sulphide segregation on platinum-group elements, gold, nickel and copper: Examples from norway. In: Prichard HM, Potts PJ, Bowles JFW and Cribb SJ (eds.). Geo-Platinum 87. Dordrecht: Springer, 113-143
|
Barnes SJ and Liu WH. 2012. Pt and Pd mobility in hydrothermal fluids:Evidence from komatiites and from thermodynamic modelling. Ore Geology Reviews, 44: 49-58 DOI:10.1016/j.oregeorev.2011.08.004 |
Bazarkina EF, Pokrovski GS and Hazemann JL. 2014. Structure, stability and geochemical role of palladium chloride complexes in hydrothermal fluids. Geochimica et Cosmochimica Acta, 146: 107-131 DOI:10.1016/j.gca.2014.09.024 |
Benkó Z, Mogessie A, Molnár F, Krenn K, Poulson SR, Hauck S, Severson M and Arehart GB. 2015. Hydrothermal alteration and Cu-Ni-PGE mobilization in the charnockitic rocks of the footwall of the South Kawishiwi intrusion, Duluth Complex, USA. Ore Geology Reviews, 67: 170-188 DOI:10.1016/j.oregeorev.2014.11.010 |
Borisov A, Palme H and Spettel B. 1994. Solubility of palladium in silicate melts:Implications for core formation in the Earth. Geochimica et Cosmochimica Acta, 58(2): 705-716 DOI:10.1016/0016-7037(94)90500-2 |
Borisov A and Palme H. 1995. The solubility of iridium in silicate melts:New data from experiments with Ir10Pt90 alloys. Geochimica et Cosmochimica Acta, 59(3): 481-485 DOI:10.1016/0016-7037(94)00358-S |
Campbell IH and Naldrett AJ. 1979. The influence of silicate:Sulfide ratios on the geochemistry of magmatic sulfides. Economic Geology, 74(6): 1503-1506 DOI:10.2113/gsecongeo.74.6.1503 |
Campbell IH and Barnes SJ. 1984. A model for the geochemistry of the platinum-group elements in magmatic sulfide deposits. Canadian Mineralogist, 22(2): 151-160 |
Crocket JH. 1979. Platinum-group elements in mafic and ultramafic rocks:A survey. Canadian Mineralogist, 17: 391-402 |
Ding X, Harlov DE, Chen B and Sun WD. 2018. Fluids, metals, and mineral/ore deposits. Geofluids, 2018: 1452409 |
Elding LI. 1972. Palladium(Ⅱ) halide complexes. I. Stabilities and spectra of palladium(Ⅱ) chloro and bromo aqua complexes. Inorganica Chimica Acta, 6: 647-651 DOI:10.1016/S0020-1693(00)91874-7 |
Elding LI. 1978. Stabilities of platinum(Ⅱ) chloro and bromo complexes and kinetics for anation of the tetraaquaplatinum(Ⅱ) ion by halides and thiocyanate. Inorganica Chimica Acta, 28: 255-262 DOI:10.1016/S0020-1693(00)87444-7 |
Elliott S. 1988. Linear free energy techniques for estimation of metal sulfide complexation constants. Marine Chemistry, 24(3-4): 203-213 DOI:10.1016/0304-4203(88)90032-1 |
Fiorentini ML, Barnes SJ, Maier WD, Burnham OM and Heggie G. 2011. Global variability in the platinum-group element contents of komatiites. Journal of Petrology, 52(1): 83-112 DOI:10.1093/petrology/egq074 |
Gammons CH, Bloom MS and Yu Y. 1992. Experimental investigation of the hydrothermal geochemistry of platinum and palladium:Ⅰ. Solubility of platinum and palladium sulfide minerals in NaCl/H2SO4 solutions at 300℃. Geochimica et Cosmochimica Acta, 56(11): 3881-3894 DOI:10.1016/0016-7037(92)90003-2 |
Gammons CH and Bloom MS. 1993. Experimental investigation of the hydrothermal geochemistry of platinum and palladium:Ⅱ. The solubility of PtS and PdS in aqueous sulfide solutions to 300℃. Geochimica et Cosmochimica Acta, 57(11): 2451-2467 DOI:10.1016/0016-7037(93)90409-P |
Gammons CH, Yu Y and Bloom MS. 1993. Experimental investigation of the hydrothermal geochemistry of platinum and palladium:Ⅲ. The solubility of Ag-Pd alloy+AgCl in NaCl/HCl solutions at 300℃. Geochimica et Cosmochimica Acta, 57(11): 2469-2479 DOI:10.1016/0016-7037(93)90410-X |
Gammons CH. 1995. Experimental investigations of the hydrothermal geochemistry of platinum and palladium:Ⅳ. The stoichiometry of Pt(Ⅳ) and Pd(Ⅱ) chloride complexes at 100 to 300℃. Geochimica et Cosmochimica Acta, 59(9): 1655-1667 DOI:10.1016/0016-7037(95)00074-A |
Gammons CH. 1996. Experimental investigations of the hydrothermal geochemistry of platinum and palladium:Ⅴ. Equilibria between platinum metal, Pt(Ⅱ), and Pt(Ⅳ) chloride complexes at 25 to 300℃. Geochimica et Cosmochimica Acta, 60(10): 1683-1694 DOI:10.1016/0016-7037(96)00048-8 |
Gao S, Luo TC, Zhang BR, Zhang HF, Han YW, Zhao ZD and Hu YK. 1998. Chemical composition of the continental crust as revealed by studies in East China. Geochimica et Cosmochimica Acta, 62(11): 1959-1975 DOI:10.1016/S0016-7037(98)00121-5 |
Grinberg AA and Gel'fman MI. 1961. The stability of complex compounds of Pt(Ⅱ) of the tetraamine type:Akad. Nauk SSSR Doklady, 137: 87-90 |
Harrar JE and Stephens FB. 1984. Recovery of platinum from concentrated sodium chloride brine by electrodeposition on vitreous carbon. CA, USA: Lawrence Livermore National Lab
|
He JJ, Ding X, Wang YR and Sun WD. 2015. The effects of precipitation-aging-re-dissolution and pressure on hydrolysis of fluorine-rich titanium complexes in hydrothermal fluids and its geological implications. Acta Petrologica Sinica, 31(7): 1870-1878 (in Chinese with English abstract) |
Hekinian R, Fevrier M, Bischoff JL, Picot P and Shanks WC. 1980. Sulfide deposits from the east pacific rise near 21°N. Science, 207(4438): 1433-1444 DOI:10.1126/science.207.4438.1433 |
Hoatson DM and Keays RR. 1989. Formation of platiniferous sulfide horizons by crystal fractionation and magma mixing in the Munni Munni Layered intrusion, West Pilbara Block, western Australia. Economic Geology, 84(7): 1775-1804 DOI:10.2113/gsecongeo.84.7.1775 |
Holwell DA, Adeyemi Z, Ward LA, Smith DJ, Graham SD, McDonald I and Smith JW. 2017. Low temperature alteration of magmatic Ni-Cu-PGE sulfides as a source for hydrothermal Ni and PGE ores:A quantitative approach using automated mineralogy. Ore Geology Reviews, 91: 718-740 DOI:10.1016/j.oregeorev.2017.08.025 |
Hsu LC, Lechler PJ and Nelson JH. 1991. Hydrothermal solubility of palladium in chloride solutions from 300 to 700℃:Preliminary experimental results. Economic Geology, 86(2): 422-427 DOI:10.2113/gsecongeo.86.2.422 |
Huang W, Li J, Tao CH, Sun ZL, He YJ and Cui NY. 2011. PGE characteristics of sulfide chimney bodies in hydrothermal regions, Southwest Indian ridge 49°39'E. Acta Mineralogica Sinica, 31(Suppl.1): 691 (in Chinese) |
Izatt RM, Eatough D and Christensen JJ. 1967. A study of Pd2+(aq) hydrolysis. Hydrolysis constants and the standard potential for the Pd, Pd2+ couple. Journal of the Chemical Society A:Inorganic, Physical, Theoretical, 52: 1301-1304 |
Jochum KP. 1996. Rhodium and other platinum-group elements in carbonaceous chondrites. Geochimica et Cosmochimica Acta, 60(17): 3353-3357 DOI:10.1016/0016-7037(96)00186-X |
Kazachenko VT, Miroshnichenko NV, Perevoznikova EV and Karabtsov AA. 2006. Sikhote Alin as a possible province of hydrothermal sedimentary gold, silver, PGE, tin, zinc, lead, and tungsten deposits. Doklady Earth Sciences, 410(1): 1007-1013 DOI:10.1134/S1028334X06070014 |
Keays RR and Davison RM. 1976. Palladium, iridium, and gold in the ores and host rocks of nickel sulfide deposits in Western Australia. Economic Geology, 71(7): 1214-1228 DOI:10.2113/gsecongeo.71.7.1214 |
Keays RR. 1995. The role of komatiitic and picritic magmatism and S-saturation in the formation of ore deposits. Lithos, 34(1-3): 1-18 DOI:10.1016/0024-4937(95)90003-9 |
Kragten J. 1980. An evaluation of the stability constants of the chloro-complexes of palladium(Ⅱ). Talanta, 27(4): 375-377 DOI:10.1016/0039-9140(80)80100-7 |
Lechler PJ and Hsu LC. 1990. Interpretation of soil nitrite-ammonium ratios with possible application to pedogeochemical exploration for platinum mineralization. Journal of Geochemical Exploration, 37(1): 37-50 DOI:10.1016/0375-6742(90)90081-K |
Levitin G and Schmuckler G. 2003. Solvent extraction of rhodium chloride from aqueous solutions and its separation from palladium and platinum. Reactive and Functional Polymers, 54(1-3): 149-154 DOI:10.1016/S1381-5148(02)00190-6 |
Li CY, Jiang YH, Zhao Y, Zhang CC, Ling MX, Ding X, Zhang H and Li J. 2018. Trace element analyses of fluid inclusions using laser ablation ICP-MS. Solid Earth Sciences, 3(1): 8-15 DOI:10.1016/j.sesci.2017.12.001 |
Li RP and Yan JD. 2013. Hydrothermal fluids and ore genesis of PGE. Contributions to Geology and Mineral Resources Research, 28(3): 335-343 (in Chinese with English abstract) |
Li T. 1976. Chemical element abundances in the earth and it's major shells. Geochimica, (3): 167-174 (in Chinese with English abstract) |
Likhoidov GG, Plyusnina LP, Scheka JA and Eva TBA. 2000. Experimental study of gold and platinum solubility in a complex fluid under hydrothermal conditions. Resource Geology, 50(2): 83-92 DOI:10.1111/j.1751-3928.2000.tb00058.x |
Liu H, Sun WD, Zartman R and Tang M. 2019. Continuous plate subduction marked by the rise of alkali magmatism 2.1 billion years ago. Nature Communications, 10(1): 3408 |
Liu SQ, Shi YZ and Zhu RH. 2007. Development of species analysis of platinum group element. Chinese Journal of Spectroscopy Laboratory, 24(2): 43-49 (in Chinese with English abstract) |
Liu YJ, Cao LM, Li ZL, Wang HN, Chu TQ and Zhang JR. 1984. Elemental Geochemistry. Beijing:Science Press: 1-548 (in Chinese) |
Maier WD, Barnes SJ, Campbell IH, Fiorentini ML, Peltonen P, Barnes SJ and Smithies RH. 2009. Progressive mixing of meteoritic veneer into the early Earth's deep mantle. Nature, 460(7255): 620-623 DOI:10.1038/nature08205 |
McCallum ME, Loucks RR, Carlson RR, Cooley EF and Doerge TA. 1976. Platinum metals associated with hydrothermal copper ores of the New Rambler Mine, Medicine Bow Mountains, Wyoming. Economic Geology, 71(7): 1429-1450 DOI:10.2113/gsecongeo.71.7.1429 |
McDonough WF and Sun SS. 1995. The composition of the Earth. Chemical Geology, 120(3-4): 223-253 DOI:10.1016/0009-2541(94)00140-4 |
McFall KA, Naden J, Roberts S, Baker T, Spratt J and McDonald I. 2018. Platinum-group minerals in the Skouries Cu-Au (Pd, Pt, Te) porphyry deposit. Ore Geology Reviews, 99: 344-364 DOI:10.1016/j.oregeorev.2018.06.014 |
McKibben MA, Williams AE and Hall EM. 1990. Solubility and transport of platinum-group elemants and Au in saline hydrothermal fluids:Constraints from geothermal brine data. Economic Geology, 85(8): 1926-1934 DOI:10.2113/gsecongeo.85.8.1926 |
Mei Y, Etschmann B, Liu WH, Sherman DM, Barnes SJ, Fiorentini ML, Seward TM, Testemale D and Brugger J. 2015. Palladium complexation in chloride-and bisulfide-rich fluids:Insights from ab initio molecular dynamics simulations and X-ray absorption spectroscopy. Geochimica et Cosmochimica Acta, 161: 128-145 DOI:10.1016/j.gca.2015.04.009 |
Mertie Jr JB. 1969. Economic geology of the platinum metals. Washington: USGS, 1-115
|
Migdisov AA, Williams-Jones AE, van Hinsberg V and Salvi S. 2011. An experimental study of the solubility of baddeleyite (ZrO2) in fluoride-bearing solutions at elevated temperature. Geochimica et Cosmochimica Acta, 75(23): 7426-7434 DOI:10.1016/j.gca.2011.09.043 |
Mihalik P, Jacobsen JBE and Hiemstra SA. 1974. Platinum-group minerals from a hydrothermal environment. Economic Geology, 69(2): 257-262 DOI:10.2113/gsecongeo.69.2.257 |
Mountain BW and Wood SA. 1988. Chemical controls on the solubility, transport and deposition of platinum and palladium in hydrothermal solutions:A thermodynamic approach. Economic Geology, 83(3): 492-510 DOI:10.2113/gsecongeo.83.3.492 |
Mungall JE and Brenan JM. 2014. Partitioning of platinum-group elements and Au between sulfide liquid and basalt and the origins of mantle-crust fractionation of the chalcophile elements. Geochimica et Cosmochimica Acta, 125: 265-289 DOI:10.1016/j.gca.2013.10.002 |
Nabivauets BI. 1970. State of palladium(Ⅱ) in perchlorate solutious. Russian Journal Inorganic Chemistry, 15(6): 818-821 |
Naldrett AJ, Hoffman EL, Green AH, Chou CL, Naldrett SR and Alcock RA. 1979. The composition of Ni-sulfide ores, with particular reference to their content of PGE and Au. Canadian Mineralogist, 17: 403-415 |
Naldrett AJ. 2004. Platinum group element (PGE) deposits. In: Naldrett AJ (ed.). Magmatic Sulfide Deposits: Geology, Geochemistry and Exploration. Berlin, Heidelberg: Springer, 481-612
|
Orlova GP, Ryabchikov ID, Distler VV and Gladyshev GD. 1987. Platinum migration in fluids during the formation of magmatic sulfides. International Geology Review, 29(3): 360-362 DOI:10.1080/00206818709466152 |
Palme H and Beer H. 1993. Abundances of the elements in the solar system. In: Landolt B (ed.). Numerical Data and Functional Relationship in Science and Technology. New York: Springer-Verlag
|
Pan P and Wood SA. 1994. Solubility of Pt and Pd sulfides and Au metal in aqueous bisulfide solutions. Mineralium Deposita, 29(5): 373-390 DOI:10.1007/BF01886955 |
Pašava J. 1993. Anoxic sediments:An important environment for PGE:An overview. Ore Geology Reviews, 8(5): 425-445 DOI:10.1016/0169-1368(93)90037-Y |
Price MM, Samson IM and Fryer BJ. 2004. Influence of hydrothermal fluids on PGE mineralization in the River Valley Intrusion, Ontario, Canada. Geochmica et Cosmochimica Acta, 72(12): A761 |
Puchtel IS, Humayun M, Campbell AJ, Sproule RA and Lesher CM. 2004. Platinum group element geochemistry of komatiites from the Alexo and Pyke Hill areas, Ontario, Canada. Geochimica et Cosmochimica Acta, 68(6): 1361-1383 DOI:10.1016/j.gca.2003.09.013 |
Rasmussen L and Jørgensen CK. 1968. Palladium(Ⅱ) Complexes. Ⅰ. Spectra and formation constants of ammonia and ethylenediamine complexes. Acta Chem. Scand, 22(7): 2313-2323 |
Rudnick RL and Gao S. 2003. Composition of the continental crust. Treatise on Geochemistry, 3: 1-64 |
Sassani DC and Shock EL. 1990. Speciation and solubility of palladium in aqueous magmatic-hydrothermal solutions. Geology, 18(10): 925-928 DOI:10.1130/0091-7613(1990)018<0925:SASOPI>2.3.CO;2 |
Schmidt G, Palme H, Kratz KL and Kurat G. 2000. Are highly siderophile elements (PGE, Re and Au) fractionated in the upper mantle of the earth? New results on peridotites from Zabargad. Chemical Geology, 163(1-4): 167-188 DOI:10.1016/S0009-2541(99)00136-9 |
Skibsted LH and Bjerrum J. 1974. Studies on gold complexes. Ⅱ. the equilibrium between gold(Ⅰ) and gold(Ⅲ) in the ammonia system and the standard potentials of the couples involving gold, diamminegold(Ⅰ), and tetramminegold(Ⅲ). Acta Chem. Scand, 28: 764-770 |
Song XY, Zhou MF and Cao ZM. 2004. Genetic relationships between base-metal sulfides and platinum-group minerals in the Yangliuping Ni-Cu-(PGE) sulfide deposit, southwestern China. The Canadian Mineralogist, 42(2): 469-483 DOI:10.2113/gscanmin.42.2.469 |
Su CX. 2009. Geochemical characteristics and mineralization of PGE in copper-nickel sulfide deposit, Jinchuan. Master Degree Thesis. Kunming: Kunming University of Science and Technology, 1-107 (in Chinese)
|
Sun WD, Arculus RJ, Bennett VC, Eggins SM and Binns RA. 2003a. Evidence for rhenium enrichment in the mantle wedge from submarine arc-like volcanic glasses (Papua New Guinea). Geology, 31(10): 845-848 DOI:10.1130/G19832.1 |
Sun WD, Bennett VC, Eggins SM, Kamenetsky VS and Arculus RJ. 2003b. Enhanced mantle-to-crust rhenium transfer in undegassed arc magmas. Nature, 422(6929): 294-297 DOI:10.1038/nature01482 |
Sun WD, Bennett VC and Kamenetsky VS. 2004. The mechanism of Re enrichment in arc magmas:Evidence from Lau Basin basaltic glasses and primitive melt inclusions. Earth and Planetary Science Letters, 222(1): 101-114 DOI:10.1016/j.epsl.2004.02.011 |
Tait CD, Janecky DR and Rogers PSZ. 1991. Speciation of aqueous palladium(Ⅱ) chloride solutions using optical spectroscopies. Geochimica et Cosmochimica Acta, 55(5): 1253-1264 DOI:10.1016/0016-7037(91)90304-N |
Taylor SR and McLennan MS. 1985. The Continental Crust: Its Composition and Evolution. Oxford: Blackwell Scientific Publications, 1-372
|
Taylor SR and McLennan SM. 1995. The geochemical evolution of the continental crust. Reviews of Geophysics, 33(2): 241-265 DOI:10.1029/95RG00262 |
Tuba G, Molnár F, Ames DE, Péntek A, Watkinson DH and Jones PC. 2014. Multi-stage hydrothermal processes involved in "low-sulfide" Cu(-Ni)-PGE mineralization in the footwall of the Sudbury Igneous Complex (Canada):Amy Lake PGE zone, East Range. Mineralium Deposita, 49(1): 7-47 DOI:10.1007/s00126-013-0468-1 |
Van Middlesworth JM and Wood SA. 1999. The stability of palladium(Ⅱ) hydroxide and hydroxy-chloride complexes:An experimental solubility study at 25~85℃ and 1bar. Geochimica et Cosmochimica Acta, 63(11-12): 1751-1765 DOI:10.1016/S0016-7037(99)00058-7 |
Vatin-Perignon N, Amossé J, Radelli L, Keller F and Leyva TC. 2000. Platinum group element behaviour and thermochemical constraints in the ultrabasic-basic complex of the Vizcaino Peninsula, Baja California Sur, Mexico. Lithos, 53(1): 59-80 DOI:10.1016/S0024-4937(00)00006-2 |
Wagner PA. 1929. The Platinum Deposits and Mines of South Africa. South Africa: Struik (C.) Pty Ltd, 1-338
|
Wang CY, Zhou MF and Zhao DG. 2005. Mineral chemistry of chromite from the Permian Jinbaoshan Pt-Pd-sulphide-bearing ultramafic intrusion in SW China with petrogenetic implications. Lithos, 83(1-2): 47-66 DOI:10.1016/j.lithos.2005.01.003 |
Wang CY, Prichard HM, Zhou MF and Fisher PC. 2008. Platinum-group minerals from the Jinbaoshan Pd-Pt deposit, SW China:Evidence for magmatic origin and hydrothermal alteration. Mineralium Deposita, 43(7): 791 DOI:10.1007/s00126-008-0196-0 |
Wang CY, Zhou MF and Qi L. 2010. Origin of extremely PGE-rich mafic magma system:An example from the Jinbaoshan ultramafic sill, Emeishan large igneous province, SW China. Lithos, 119(1-2): 147-161 DOI:10.1016/j.lithos.2010.07.022 |
Wang CY, Zhou MF and Qi L. 2011. Chalcophile element geochemistry and petrogenesis of high-Ti and low-Ti magmas in the Permian Emeishan large igneous province, SW China. Contributions to Mineralogy and Petrology, 161(2): 237-254 DOI:10.1007/s00410-010-0529-8 |
Wang CY, Zhou MF, Sun YL and Arndt NT. 2012. Differentiation, crustal contamination and emplacement of magmas in the formation of the Nantianwan mafic intrusion of the~260Ma Emeishan large igneous province, SW China. Contributions to Mineralogy and Petrology, 164(2): 281-301 DOI:10.1007/s00410-012-0738-4 |
Wang CY, Zhou MF, Yang SH, Qi L and Sun YL. 2014. Geochemistry of the Abulangdang intrusion:Cumulates of high-Ti picritic magmas in the Emeishan large igneous province, SW China. Chemical Geology, 378-379: 24-39 DOI:10.1016/j.chemgeo.2014.04.010 |
Wang DH, Liu FS, Chu YS and Luo FX. 2002. Discovery of PGE-rich hydrothermal ore in Yangliuping, Sichuan, and its significance. Geological Bulletin of China, 21(3): 158-162 (in Chinese with English abstract) |
Wang MF, Deng XD, Li ZK and Bi SJ. 2010. Current situation and existing problems in the study of platinum group elements in porphyry copper deposits. Acta Petrologica et Mineralogica, 29(1): 100-108 |
Wilde AR, Bloom MS and Wall VJ. 1989. Transport and deposition of gold, uranium and platinum-group elements in unconformity-related uranium deposits. Economic Geology and the Bulletin of the Society of Economic Geologists, 6: 637-650 |
Wood SA. 1987. Thermodynamic calculations of the volatility of the platinum group elements (PGE):The PGE content of fluids at magmatic temperatures. Geochimica et Cosmochimica Acta, 51(11): 3041-3050 DOI:10.1016/0016-7037(87)90377-2 |
Wood SA, Mountain BW and Fenlon BJ. 1989. Thermodynamic constraints on the solubility of platinum and palladium in hydrothermal solutions-reassessment of hydroxide, bisulfide, and ammonia complexing. Economic Geology, 84(7): 2020-2028 DOI:10.2113/gsecongeo.84.7.2020 |
Wood SA, Mountain BW and Pan P. 1992. The aqueous geochemistry of platinum, palladium and gold:Recent experimental constraints and a re-evaluation of theoretical predictions. The Canadian Mineralogist, 30: 955-982 |
Xiong Y and Wood SA. 2000. Experimental quantification of hydrothermal solubility of platinum-group elements with special reference to porphyry copper environments. Mineralogy and Petrology, 68(1-3): 1-28 DOI:10.1007/s007100050001 |
Zhang LP, Hu YB, Liang JL, Ireland T, Chen YL, Zhang RQ, Sun SJ and Sun WD. 2017. Adakitic rocks associated with the Shilu copper-molybdenum deposit in the Yangchun Basin, South China, and their tectonic implications. Acta Geochimica, 36(2): 132-150 DOI:10.1007/s11631-017-0146-6 |
何俊杰, 丁兴, 王玉荣, 孙卫东. 2015. 沉淀-陈化-返溶作用和压力对热液中氟钛络合物高温水解的影响及地质意义. 岩石学报, 31(7): 1870-1878. |
黄威, 李军, 陶春辉, 孙治雷, 何拥军, 崔汝勇. 2011. 西南印度洋脊49°39'E热液活动区硫化物烟囱体的铂族元素特征. 矿物学报, 31(增1): 691. |
李瑞鹏, 颜建东. 2013. 铂族元素矿床热液流体成矿模型探讨. 地质找矿论丛, 28(3): 335-343. |
黎彤. 1976. 化学元素的地球丰度. 地球化学, (3): 167-174. DOI:10.3321/j.issn:0379-1726.1976.03.004 |
刘少轻, 施燕支, 朱若华. 2007. 铂族金属及其形态分析进展. 光谱实验室, 24(2): 43-49. DOI:10.3969/j.issn.1004-8138.2007.02.001 |
刘英俊, 曹励明, 李兆麟, 王鹤年, 储同庆, 张景荣. 1984. 元素地球化学. 北京: 科学出版社, 1-548.
|
苏昌学. 2009.金川铜镍硫化物矿床铂族元素地球化学特征及成矿作用研究.硕士学位论文.昆明: 昆明理工大学, 1-107 http://cdmd.cnki.com.cn/Article/CDMD-10674-1011055761.htm
|
王登红, 刘凤山, 楚萤石, 罗辅勋. 2002. 四川杨柳坪热液型富铂族元素矿石的发现及其意义. 地质通报, 21(3): 158-162. DOI:10.3969/j.issn.1671-2552.2002.03.010 |
王敏芳, 邓晓东, 李占轲, 毕诗健. 2010. 斑岩型铜矿床中铂族元素的研究现状与存在问题. 岩石矿物学杂志, 29(1): 100-108. DOI:10.3969/j.issn.1000-6524.2010.01.012 |
 2020, Vol. 36
2020, Vol. 36


