2. 中国科学院大学地球与行星科学学院, 北京 100049
2. College of Earth and Planetary Sciences, University of Chinese Academy of Sciences, Beijing 100049, China
俯冲带-岛弧系统的碳循环模式可以概括这样一种图景:含碳岩石通过俯冲过程的变质作用形成含碳流体,含碳流体运移至上覆地幔楔交代其中的岩石导致部分熔融产生含碳的岛弧岩浆,岩浆上升到地表将碳以CO2形式释放到地表的物质圈层中去。岛弧火山作用释放的CO2在碳同位素组成上证明了这种过程的存在。岛弧岩浆的δ13C在-0.1‰~-11.6‰之间,这种成分的碳是俯冲带的碳酸盐(δ13C=0‰)、蚀变大洋玄武岩及其下覆地慢岩的碳(δ13C=-5‰)和俯冲岩石中的有机碳(δ13C=-30‰)混合的结果(Sano and Marty, 1995; Shaw et al., 2003; De Leeuw et al., 2007)。对几处环太平洋岛弧火山岩的碳同位素研究表明,80%以上的碳来自俯冲带的岩石(Sano and Williams, 1996; De Leeuw et al., 2007)。因此,在俯冲过程中,岩石变质反应生成的含碳流体控制了俯冲带-岛弧系统碳循环过程。
变质反应生成的含碳流体包括含碳水流体、富硅酸盐的超临界流体和含碳熔体。碳酸盐脱碳的变质反应通常生成COH流体,而碳酸盐矿物也可以溶解在水中生成含碳电解质流体,两种机制对碳转运中的作用是目前的热点研究问题。当含碳酸盐岩石达到部分熔融条件时,岩石能够生成含碳熔体或者形成第二临界端点之上的富硅酸盐超临界流体。无论何种含碳流体,其形成与P-T条件和岩石成分有关,其中岩石初始的流体成分特征对流体形成的P-T有重要的影响。因此,俯冲带P-T条件和初始岩石的成分特征控制了碳的释放。
岩石中金刚石和由流体形成的含碳酸盐的高压矿物组合是含碳流体在俯冲带深部活动的直接物质证据,这些证据从大洋俯冲带和大陆俯冲带的岩石中都能观察到。近年来,相应的实验岩石学研究也在增多,特别是含碳酸盐岩石的部分熔融的实验研究明显增加。
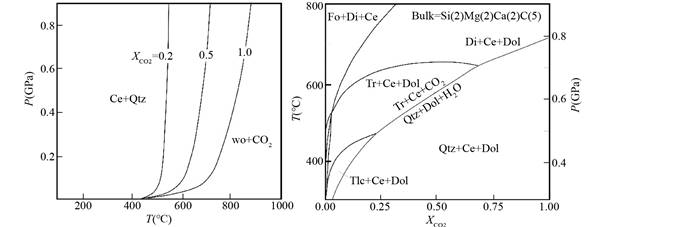
1 含碳的流体在俯冲带含碳酸盐岩石中,碳酸盐矿物的脱碳变质反应能够形成CO2流体,例如在含硅的方解石大理岩中,反应CaCO3+SiO2=CaSiO3+CO2,或者在含硅的白云石大理岩中,反应5CaMg(CO3)2+8SiO2+H2O=Ca2Mg5Si8O22(OH)2+3CaCO3+7CO2是两个常见的脱碳变质反应。两个反应的温压条件都与初始的流体成分有关。在第一个反应中,水是无关相,如果初始的岩石中含水,它能降低流体中CO2的活度,导致反应的温度降低(图 1)。第二个反应是混合挥发份的变质反应,初始流体的成分决定了反应的温压条件,CO2含量越高,反应的温度越高(图 1)。从两个简单的反应与流体成分的关系来看,在一个实际的岩石系统中,如果有外来水流体的加入,碳酸盐矿物能够在更低的温度下发生变质脱碳反应,形成含碳的流体。
|
图 1 两个脱碳反应的温度-流体成分图(据Bucher and Grapes, 2011) 左图:硅辉石生成的反应;右图:全岩成分为Si(2)Mg(2)Ca(2)C(5)的视剖面图.Wo-硅辉石; Qtz-石英; Cc-方解石; Fo-镁橄榄石; Di-透辉石; Dol-白云石; Tr-透闪石; Tlc-滑石 Fig. 1 Two decarbonation reactions showing the relationship between the composition of fluid and temperature (after Bucher and Grapes, 2011) |
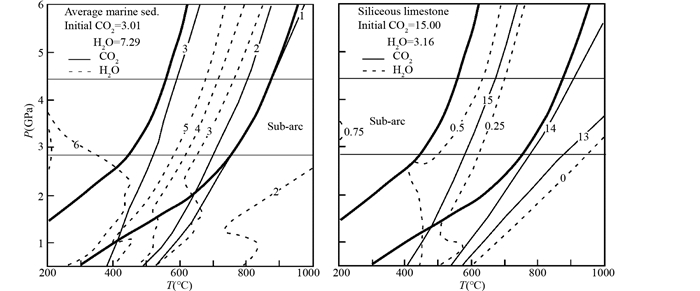
俯冲带含碳酸盐矿物的岩石通常是远洋沉积物,主要是泥灰岩和硅质碳酸盐岩。Kerrick and Connolly (2001)用马里亚纳和瓦努阿图海沟的硅质灰岩、安的列斯海沟的泥灰岩和远洋含碳酸盐矿物沉积物的平均成分,通过相平衡的方法模拟了温度在200~1000℃和压力达到6GPa的流体生成情况。从图 2可以看出,硅质碳酸盐岩类在俯冲的地温梯度下只有很少的脱碳,而远洋含沉积物和泥灰岩类会脱去大部分碳,形成交代地幔楔的COH流体。
|
图 2 远洋含碳酸盐矿物的沉积物(左)和硅质灰岩(右)的平衡变质矿物组合中的流体含量(据Kerrick and Connolly, 2001) 生成的流体成分和含量等于初始含量减去平衡矿物组合中的流体成分和含量.粗黑线为俯冲带的地温线,资料来自日本西北(低的地温线)和东南(高的地温线)的俯冲带. Sub-arc:岛弧岩浆形成区域 Fig. 2 Calculated fluid contents of mineral assemblages for average marine sediment (left) and siliceous limestone (right) by phase modeling (after Kerrick and Connolly, 2001) |
Kerrick and Connolly (2001)的相图模拟未涉及矿物在流体中的溶解问题,也未涉及岩石部分熔融问题。溶解度的实验研究表明,在低于3kb的压力下,方解石的溶解度随温度的上升而降低,而大于3kb的压力下,溶解度随温度上升而上升,并且压力越大,升幅越高(图 3, Caciagli and Manning, 2003)。因此,在俯冲带环境中,不能忽略碳酸盐矿物在流体溶解作用,其形成的含碳电解质流体能够携带大量的碳。方解石在高温高压下的溶解反应为CaCO3+2H+=Ca2++CO2, aq+H2O,因此,碳酸盐类矿物的溶解导致流体变为碱性。碳酸盐矿物的溶解度不仅与温压条件有密切关系,而且与流体的盐度也密切相关(图 3)。流体中盐组分导致矿物溶解度提高的现象称盐助溶效应(Salting-in effect, Newton and Manning, 2000, 2002)。对于方解石,盐助溶效应实际是盐和方解石反应的结果,即CaCO3+2NaClaq=CaCl2, aq+Na2CO3, aq,反应的生成物均可以溶于水中。方解石的盐助溶效应能够大大提高流体的含碳量,从而提高俯冲带含碳物质的转移能力。

|
图 3 方解石的溶解度与温压(左)和流体盐度(右)的关系 方解石的溶解度据Caciagli and Manning (2003);右图据Newton and Manning (2002). mCaCO3:每千克水中溶解方解石的摩尔数 Fig. 3 The relations between calcite solubility and P-T condition (left, after Caciagli and Manning, 2003), between calcite solubility and fluid salinity (right, after Newton and Manning, 2002) |
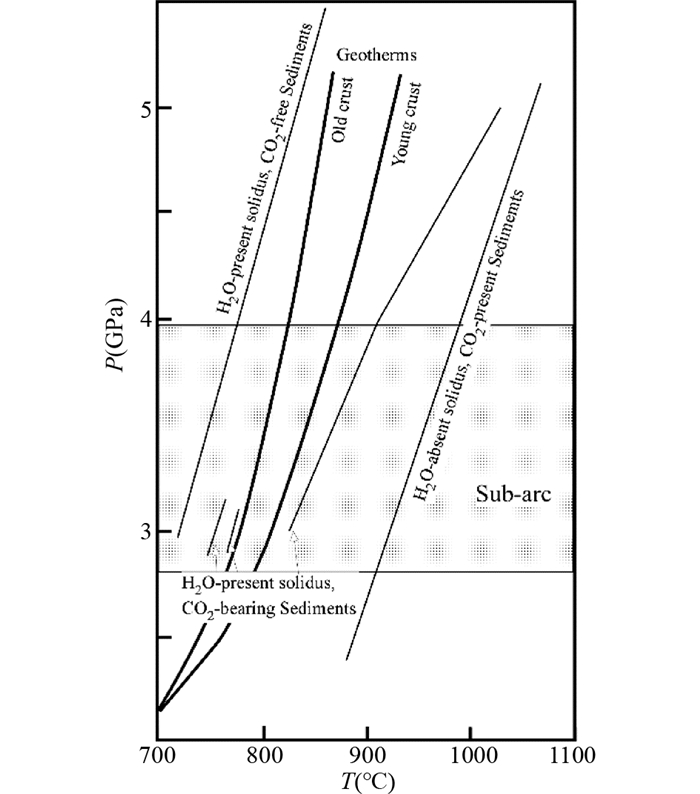
最近的一系列高温高压实验研究结果表明含碳酸盐矿物的岩石在700℃以上能够发生部分熔融(图 4)。从水存在的含碳酸盐矿物沉积岩的熔融实验来看,3~5GPa条件下,固相线的温度在740~1000℃ (Thomsen and Schmidt, 2008; Tsuno and Dasgupta, 2012; Tsuno et al., 2012; Mann and Schmidt, 2015; Skora et al., 2015)。对比Kerrick and Connolly (2001)远洋含碳酸盐矿物沉积物模拟的相图来看,如果岩石低温阶段脱出的水流体没有离开岩石体系,那么进入700℃以上的高温高压阶段,岩石应该会发生部分熔融,生成含碳的岩浆。
|
图 4 含碳酸盐矿物岩石的固相线(据Skora et al., 2015) Sub-arc:岛弧岩浆形成区域.粗黑线为俯冲带的地温线,老洋壳(Old crust)代表快速的冷俯冲;新洋壳(Young crust)代表慢速的热俯冲 Fig. 4 Solidus of carbonate-bearing rocks (after Skora et al., 2015) |
部分熔融实验研究了流纹质岩浆碳含量与温压的关系以及碳在岩浆中出现的组分形式(species)。Duncan and Dasgupta (2014)的实验表明,流纹质岩浆的碳含量正相关于压力和岩浆中的H2O含量,与温度无关。在岩浆中,碳以CO2和CO32-组分存在,并且当H2O含量高时以CO32-形式为主,反之以CO2形式为主。在3.0GPa条件下,以CO2计量的含量可达2%。
图 2和图 4中也显示了俯冲带的地温梯度,从俯冲带的温压环境看,脱碳的变质反应可以生成COH流体,流体的出现也会导致碳酸盐矿物在流体的溶解,同时在700℃以上俯冲带环境中可能出现含碳的熔体。
2 流体中的碳组分碳在流体中以什么样的组分(species)存在是另一个重要问题。含碳的组分类型和含量与温压条件、氧逸度、流体的pH值密切相关,是研究俯冲带岩石物理化学特征和流体携带碳的能力重要的方面。流体中的碳组分有中性的CO2和CH4分子、重碳酸根离子(HCO3-)、碳酸根离子(CO32-),它们稳定的氧逸度和pH值不同。甲烷稳定在低氧逸度环境中,而其它组分稳定在高氧逸度环境中。相比二氧化碳,重碳酸根离子和碳酸根离子在更高的pH值下稳定。近几年来,一个令人关注的方面是流体中的有机碳研究。利用Deep Earth Water模型(Sverjensky et al., 2014a),高压条件下碳饱和岩石-COH流体系统中,计算的与岩石矿物组合平衡流体中的碳组分包括CO2、CH4、HCO3-和有机碳离子组分。有机碳离子组分有甲酸根(HCOO-)、乙酸根(CH2COO-)和丙酸根(CH3CH2COO-) (Sverjensky et al., 2014b; Sverjensky and Huang, 2015)。碳饱和MgO-SiO2-COH系统的实验表明,Mg和Si在COH流体中的溶解度比相同温压条件下的MgO-SiO2-H2O系统高了5倍之多(Tumiati et al., 2017; Tiraboschi et al., 2018),Mg和Si的溶解度可以用Mg[OSi(OH)3][CH3CH2OO]+H+=Mg2++SiO2 aq+CH3CH2OO-+H2O来描述(Tiraboschi et al., 2018)。这个实验侧面证实有机的Mg-Si-C物质存在于实验的流体相中。有机碳在深部地幔条件稳定存在,不仅对俯冲带-岛弧系统碳循环打开了新的研究窗口,而且对早期生命起源也打开了新的研究窗口。当务之急不仅仅是理论计算和实验研究,在自然界高压、超高压岩石中寻找有机碳存在的证据也提上了日程。
3 岩石学观察俯冲带的岩石记录了含碳流体的活动情况。物质的记录包括两个方面:高压、超高压矿物中的包裹体和高压、超高压岩石中的脉体。在榴辉岩相矿物中,例如在石榴石、绿辉石、蓝晶石等矿物中,可以识别出三种类型流体包裹体:水流体包裹体/气体包裹体、多相固体包裹体和熔体包裹体(Frezzotti and Ferrando, 2015)。
3.1 流体包裹体水流体包裹体多数含盐,盐度从低盐到盐饱和(Scambelluri and Philippot, 2001; Frezzotti and Ferrando, 2015),液体水和气态水占流体包裹体体积的40%以上。一个典型的实例是阿尔卑斯Monviso地区Piemonte带榴辉岩所显示的情况:在榴辉岩相的脉体中,绿辉石中发育有大量流体包裹体(Philippot and Selverstone, 1991)。流体包裹体以高盐为特征,除了盐的子晶外,还有钠长石、榍石、方解石、白云石、石膏、硬石膏、重晶石、黄铁矿和氧化物等子晶矿物的存在。流体包裹体中沉淀的方解石和白云石指示高盐流体中存在溶解的碳酸盐矿物,因此,这种流体通过盐助溶机制可以携带大量的溶解的碳酸盐物质。对于不含盐的水流体,高压、超高压条件下碳酸盐的溶解度也会大幅提高(图 3)。在Lago di Cignana地区Piemonte带经历超高压变质的锰结核中,石榴石中就包裹了这种特征的流体包裹体(Frezzotti et al., 2011)。包裹体含金刚石、无定型碳、方解石等碳酸盐矿物、石英、钠云母、石膏、金红石,流体中还存在SO42-、CO32-、HCO3-和SiO44-单体等组分。这种流体包裹体的组成特征,反映出流体中的碳主要来源于碳酸盐矿物在水流体中的溶解,同时,水流体中也溶解了大量硅酸盐矿物。气体包裹体见于大别山、Dora Maira等地的超高压榴辉岩相岩石中(Castelli et al., 2007; Fu et al., 2001, 2002, 2003a, b; Xiao et al., 2000, 2002),包裹体包括N2、CH4、N2-CH4、N2-CH4-CO2-H2O±盐子晶,这种成分的流体与盐流体之间存在不混溶性(Heinrich, 2007),因此可以与盐流体共存。这类包裹体是否是变质脱碳反应生成的仍然未知,但目前观察的流体包裹体中,COH包裹体在高压、超高压岩石中明显偏少。
多相固体包裹体(Multiphase solid inclusion)是目前在超高压岩石中发现最广泛的一类包裹体(Frezzotti and Ferrando, 2015),出现在石榴石、绿辉石、蓝晶石等矿物中。一般的尺度在100μm以下,主要由多晶固体矿物组成,其中可以存在空隙或有少量流体。形态上从负晶形到不规则状,普遍发育爆裂结构(offshoot)。这种特征指示包裹体未结晶前是一种富硅酸盐的流体物质。最好的实例是Erzgebirge和Kokchetav超高压片麻岩石榴石等矿物中的含金刚石多相固体包裹体(Stöckhert et al., 2001, 2009; Dobrzhinetskaya et al., 2003; Hwang et al., 2003)。含金刚石多相固体包裹体除金刚石外,还包含黑云母、钠云母、多硅白云母、绿泥石、绿帘石、角闪石等的一些含水的铝硅酸盐矿物和无水的石英、钾长石、蓝晶石、辉石等矿物,碳酸盐、磷灰石、氧化物也是常见矿物。从矿物组成上看,多相固体包裹体的成分明显不同于岩浆的花岗质成分(Frezzotti and Ferrando, 2015)。这类包裹体含的碳物质有金刚石、石墨和碳酸盐矿物,显示流体可以携带大量碳,是重要的碳迁移介质。
近年来高压、超高压岩石中石榴石中陆续有熔体包裹体的报道(Hwang et al., 2001; Korsakov et al., 2004; Korsakov and Hermann, 2006; Zeng et al., 2009; Gao et al., 2012, 2013, 2014; Liu et al., 2013; Cesare et al., 2015; Ferrero et al., 2015, 2016)。熔体包裹体形态上从负晶形到不规则状,尺度在几个到几十微米,也常见发育爆裂结构。熔体包裹体的成分是花岗质的,主要由石英/方英石、长石、少量云母、花岗质玻璃等组成。长石类矿物包括钾长石、钠长石、kokchetavite和kumdykolite,其中钾长石和kokchetavite(Hwang et al., 2004)、钠长石和kumdykolite(Hwang et al., 2009)分别是同质多相矿物。方英石作为亚稳定矿物常常出现在熔体包裹体中(Ferrero et al., 2016)。玻璃在熔融包裹体非常常见,包裹体的结晶程度变化很大,从完全的玻璃到完全结晶体(Cesare et al., 2015)。玻璃的存在一方面可能与快速的折返有关,但包裹体尺度可能对岩浆的矿物成核和生长有重要影响,小的包裹体孔径会抑制成核作用而导致亚稳态的玻璃被保存下来(Putnis et al., 1995; Holness and Sawyer, 2008; Cesare et al., 2009; Ferrero et al., 2012)。熔体包裹体中,含碳的矿物包括金刚石和方解石(Korsakov et al.,2004; Korsakov and Hermann, 2006; Gao et al., 2014)。因此,从天然岩石的观察到实验研究的结果,均反映出熔体可以携带大量碳。此外,实验研究还表明,含碳酸盐矿物的岩石体系,发生部分熔融时,还存在不混溶的COH流体(Mann and Schmidt, 2015)。
三种类型包裹体形成的条件是另一个重要研究问题。矿物中有水流体包裹体/气体包裹体的高压、超高压岩石,通常形成的温度不高于650℃,岩石未达到部分熔融的温度。从Erzgebirge和Kokchetav超高压片麻岩经历的温压条件来看,多相固体包裹体和熔体包裹体出现在高温、高压条件下。这两个地区岩石经历的温度均达到或超过了1000℃,压力在4~8GPa之间(Massonne, 2003; Korsakov and Hermann, 2006; Dobrzhinetskaya et al., 2013)。就熔融包裹体而言,只要达到岩石部分熔融的温度,就可能在转融矿物中出现熔融包裹体(Cesare et al., 2015)。然而,多相固体包裹体作为一种流体包裹体并未得到很好的解释。从矿物溶解度方面来看,包裹体中存在的流体很少,矿物的溶解度很低,不能够解释为什么包裹体有这么多的硅酸盐溶质。目前倾向性的解释是这种流体可能是第二临界端点之上的超临界流体(图 5)。压力在岩石-流体系统的第二临界端点之上,岩石的部分熔融的熔体成分与岩石平衡的流体成分变为一条连续的曲线(图 5c),熔体成分和溶解的流体成分之间缺乏可以定义的界线,中间部分(图 5c)形成了介于熔体和水流体之间的富硅酸盐的流体(silicate-rich fluid)。然而,这种富硅酸盐流体中的水含量至少超过20%以上(图 5c),从多相固体包裹体含水矿物的水含量来看,包裹体中的流体含量远远少于这样的数量。可能的解释是该类包裹体在形成后,在降压过程中包裹体中的流体通过扩散或在包裹体的爆裂过程中逃逸了(Frezzotti and Ferrando, 2015)。另一方面的问题是多相固体包裹体的成分问题。如果多相固体包裹体是富硅酸盐的流体形成的,那么从已知的实验来看,溶质的成分应该也是花岗岩质成分的(Kessel et al., 2005a, b),这与观察的包裹体成分不符。一种可能的解释是主晶矿物与多相固体包裹体代表的流体在后来的温压条件变化下发生了变质反应,多相固体包裹体并不代表流体中原始的溶质成分(Perchuk et al., 2005; Frezzotti and Ferrando, 2015)。

|
图 5 岩石-H2O系统中岩石部分熔融、岩石溶解度与温压之间的相关系 (a)岩石熔融的P-T-H2O相关系,Critical point:水-熔体之间的临界点;Critical curve:临界线(临界点的连线);2nd Critical endpoint:第二临界端点;(b)图a中P1位置的等压切面熔融的相关系,Eutectic point:共熔点.低压下,相图中存在熔体(melt)+流体(fluid)不混溶区.压力增高,混溶区变小并最终消失.这导致临界线和共熔点组成的湿固相线(wet solidus)交于一点,该点即为第二临界端点.压力在第二临界端点之上的P2位置,等压切面相关系为如图c所示:岩石的熔体成分与平衡的流体成分变为一条连续的曲线,两者之间缺乏可以定义界线。中间部分形成富硅酸盐的流体(silicate-rich fluid). (d)岩石熔融和临界线以及流体中溶质含量在P-T切面上的示意投图. (a-c)据Stalder et al. (2000)修改,也见Manning (2004);(d)据Hermann et al. (2006)修改 Fig. 5 P-T phase relations showing partial melting and solubility of a rock in rock-H2O system (a) sketch P-T-H2O phase relation of rock melting; (b) sketch T-H2O phase relation of rock melting at constant pressure (P1 at Fig. 5a). (c) sketch T-H2O phase relation of rock melting at constant pressure above the second critical endpoint (P2 at Fig. 5a). (d) sketch P-T diagram showing solidus, critical curve, the second critical endpoint and solute contents in fluids. (a-c) from Stalder et al. (2000), Manning (2004), and (d) is simplified from Hermann et al. (2006) |
俯冲带含碳岩石的部分熔融与岩石成分和俯冲带的地温梯度相关。就图 2和图 4给出的热俯冲的地温线而言,合适的岩石成分完全可以发生部分熔融。另一个可以考虑的过程是俯冲带岩石的底辟作用。由于俯冲带含较多的蛇纹岩等粘度较低的岩石,这些岩石可能发生底辟作用进入上覆地幔楔的高温环境,从而产生部分熔融,形成含碳的岩浆(Duncan and Dasgupta, 2014)。
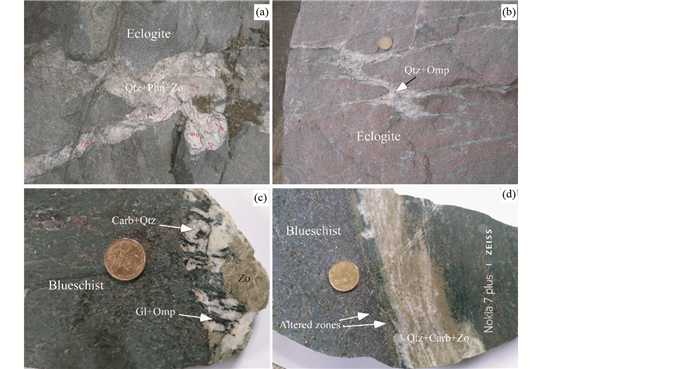
3.2 高压、超高压岩石中含碳酸盐矿物的脉体俯冲带内含碳流体活动的另一个物质记录是高压、超高压岩石发育的脉体(Ague and Nicolescu, 2014; Piccoli et al., 2016)。脉体在变质岩石中广泛发育,是流体中的溶质在岩石裂隙沉淀而成。高压、超高压岩石中脉体种类很多(如图 6),从单一矿物构成的石英脉、碳酸盐脉到有几种矿物构成复杂成分的脉,而且矿物在脉体中可以形成分带现象。图 6显示了大别山-苏鲁超高压带榴辉岩和天山蓝片岩中发育的脉体,这些脉体在矿物组成和整体成分上是多样性的。天山蓝片岩的脉体除含蓝闪石、绿辉石、黝帘石、石英等硅酸盐矿物外(图 6c, d),还含大量碳酸盐矿物,反映出流体是一种含硅酸盐和碳酸盐溶质的流体。碳酸盐矿物在脉体的结晶表明,这种流体中的碳主要来自碳酸盐的溶解,而不是变质反应形成的COH流体。一些形成脉体的流体可以与围岩反应形成蚀变带(如图 6d),而另一些则没有这样的蚀变带(如图 6a, b)。
|
图 6 榴辉岩和蓝片岩中发育的脉体 (a)大别山朱家冲榴辉岩(eclogites)中发育的石英(Qtz)+黝帘石(Zo)+多硅白云母(Phn)脉体,多硅白云母和石英显示了分带性;(b)苏鲁东海榴辉岩中发育的绿辉石(Omp)石英(Qtz)脉. (a、b)中的脉体周围的围岩中没有蚀变带; (c、d)天山石榴石蓝片岩(blueschist)发育含碳酸盐矿物(Carb)的脉体, Gl-蓝闪石. (d)中脉体两侧发育蚀变带(altered zones),为流体与蓝片岩之间的反应带 Fig. 6 Photographs showing the veins in eclogites and blueschist (a) from the Dabie UHP metamorphic belt; (b) from the Sulu UHP metamorphic belt; (c, d) from the Tianshan subduction complex |
Ague and Nicolescu (2014)研究了希腊基克拉迪群岛(Cycladic)俯冲混杂岩带碳酸岩块中发育的脉体。形成脉体的流体起源于混杂岩带蛇纹岩脱水,当流体沿裂隙进入碳酸岩块时,形成了脉体。脉体主要由石英、绿帘石组成,并含少量蓝闪石和绿辉石,指示流体中含大量硅酸盐溶质。脉体与大理岩的接触带形成了一个贫碳酸盐矿物的蚀变带。通过质量平衡、氧和碳同位素、矿物的流体包裹体和相平衡模拟等方面的研究,发现蚀变带部分有60%~90%以CO2计量的脱碳量,而以变质反应为主的脱碳量仅有5%~15%。质量平衡确定出蚀变带的脱碳是通过碳酸盐矿物在流体中的溶解实现的,并且大量碳酸盐矿物的溶解导致了流体中水活度的降低,致使硅酸盐矿物沉淀形成了脉体。可以推测与碳酸岩平衡之后的流体应该是含大量碳酸盐溶质的新流体,它们迁移到地幔楔能够产生含碳的岛弧岩浆,从而完成碳从深部到浅部的循环过程。
4 展望远洋或半远洋沉积物是俯冲带的主要含碳岩石,在俯冲过程这些岩石的变质作用导致含碳流体的形成。通过对大洋俯冲带和大陆俯冲带岩石的观察,可以确定出三类流体,即含碳水流体、富硅酸盐的超临界流体和熔体。
含碳水流体通常都有一定的含盐量,盐流体与含CO2流体之间在变质的温压条件下是不混溶的(Spear, 1993; Heinrich, 2007; Manning et al., 2013)。对含碳水流体包裹体的观察发现包裹体中存在硅酸盐矿物的子晶,表明这样的流体中存在一定量的硅酸盐溶质,因此,它们实际上是成分复杂的含碳水流体。在高压条件下,多组分溶质的流体中,组分存在的形式、各组分之间的关系和流体的P-T-V特征目前仍然需要更多实验和理论上的研究。从含碳水流体包裹体岩石经历的温压条件看,它们形成在相对低温的条件下,岩石没有达到部分熔融的温压条件。在这样的温压条件下,岩石变质作用以含流体矿物的脱水和碳酸盐矿物脱CO2的反应为特征。碳酸盐矿物脱CO2反应的温度与岩石初始的流体成分相关,岩石中水活度越高,碳酸盐矿物脱CO2的反应温度越低。其岩石学意义在于如果一个含碳酸盐矿物岩石有外来水流体的加入时,该岩石会同步产生碳酸盐矿物脱CO2的反应。同时,近来的研究表明碳酸盐矿物在水流体的溶解作用形成的含碳流体对岩石中的碳迁移不可忽视,其作用可能远远超过碳酸盐矿物脱CO2的反应造成的碳迁移。因此,外来流体流入含碳酸盐矿物的岩石,不仅促进了碳酸盐矿物脱CO2的反应,而且造成了大量碳酸盐矿物在流体中的溶解。
富硅酸盐的超临界流体和熔体包裹体形成的温压条件非常高,这些包裹体主要出现在大陆俯冲带的岩石中。正如前文中指出对多相固体包裹体代表的流体还缺乏实验的研究,其偏离花岗质成分的特征要求对每一种不同成分的包裹体开展相应的实验研究,以确定它们形成的条件;不足的水含量需要开展对包裹体水逃逸的具体研究,同时也需要进一步确定包裹体与主晶矿物之间的反应关系。熔融包裹体存在表明俯冲带可以存在部分熔融,如图 2和图 4给出的俯冲带的地温线那样,一些俯冲带在岛弧岩浆产生的深度上,其温度已经超过了700℃以上。在这种深度上,如果岩石中含水矿物脱水或者有外来流体的加入,都可以造成俯冲带岩石的部分熔融。最近Syracuse et al. (2010)对全球俯冲带的热模拟表明,在2.5~5GPa的深度范围内,俯冲带的顶面温度多在750~850℃之间。这样的温度范围能够引起俯冲带岩石的部分熔融,从而形成含碳岩浆。岩石部分熔融不仅会以熔融包裹体形式记录下来,也会形成混合岩。最典型的地区是苏鲁超高压变质带中的威海地区,虽然已有的研究认为威海地区的混合岩形成在岩石的折返阶段(Liu et al., 2010, 2012; Xu et al., 2013),然而对混合岩浅色体的定年并不能确定部分熔融的时间。超高压阶段的部分熔融形成的熔体可以在折返后发生低压结晶,造成折返的部分熔融的假象。因此,超高压岩石部分熔融温压条件和部分熔融的时间仍然是重要的课题。进一步研究的方面也包括,部分熔融形成的岩浆与碳酸岩之间的反应关系,正像花岗岩与大理岩之间能造成矽卡岩而导致大量含碳流体的形成一样,高压、超高压条件下片麻岩的部分熔融产生的岩浆侵入到大理岩也应该有相同的结果。
目前的理论计算和实验证实有机碳在深部地幔条件下可以稳定存在,这对俯冲带-岛弧系统的碳循环和生命起源都打开了新的研究窗口。进一步的理论计算和实验研究是必须的,同时在自然界高压、超高压岩石中寻找有机碳存在的证据也是当务之急。
俯冲带热结构的差异决定了岩石生成流体的特征,不同类型流体在全球碳循环中的地位目前尚不清楚。粗略估计俯冲带的变质作用导致岩石总脱碳量约占到了俯冲岩石携带量的三分之一(Marty and Tolstikhin, 1998; Hayes and Waldbauer, 2006; Dasgupta and Hirschmann, 2010; Ague and Nicolescu, 2014),其它以碳酸盐矿物形式随岩石折返到地表或进入了更深的地幔中去。这些含碳的流体不仅记录了深部岩石变质作用的重要信息,而且碳循环回到地表也将对地球的气候等地表地质过程产生重要影响。因此,俯冲带-岛弧系统的碳循环在未来一段时间内仍然是地球科学研究的热点问题之一。
致谢 谨以此文祝贺叶大年院士八十华诞。借此感谢叶大年院士多年来在专业上对我的帮助与支持!
Ague JJ and Nicolescu S. 2014. Carbon dioxide released from subduction zones by fluid-mediated reactions. Nature Geoscience, 7(5): 355-360. DOI:10.1038/ngeo2143 |
Bucher K and Grapes R. 2011. Petrogenesis of Metamorphic Rocks. 8th Edition. Heidelberg: Springer.
|
Caciagli NC and Manning CE. 2003. The solubility of calcite in water at 6~16kbar and 500~800℃. Contributions to Mineralogy and Petrology, 146(3): 275-285. DOI:10.1007/s00410-003-0501-y |
Castelli D, Rolfo F, Groppo C and Compagnoni R. 2007. Impure marbles from the UHP Brossasco-Isasca Unit (Dora-Maira Massif, western Alps):Evidence for Alpine equilibration in the diamond stability field and evaluation of the X (CO2) fluid evolution. Journal of Metamorphic Geology, 25(6): 587-603. DOI:10.1111/jmg.2007.25.issue-6 |
Cesare B, Ferrero S, Salvioli-Mariani E, Pedron D and Cavallo A. 2009. Nanogranite and glassy inclusions:The anatectic melt in migmatites and granulites. Geology, 37(7): 627-630. DOI:10.1130/G25759A.1 |
Cesare B, Acosta-Vigil A, Bartoli O and Ferrero S. 2015. What can we learn from melt inclusions in migmatites and granulites?. Lithos, 239: 186-216. DOI:10.1016/j.lithos.2015.09.028 |
Dasgupta R and Hirschmann MM. 2010. The deep carbon cycle and melting in Earth's interior. Earth and Planetary Science Letters, 298(1-2): 1-13. DOI:10.1016/j.epsl.2010.06.039 |
De Leeuw GAM, Hilton DR, Fischer TP and Walker JA. 2007. The He-CO2 isotope and relative abundance characteristics of geothermal fluids in El Salvador and Honduras:New constraints on volatile mass balance of the Central American Volcanic Arc. Earth and Planetary Science Letters, 258(1-2): 132-146. DOI:10.1016/j.epsl.2007.03.028 |
Dobrzhinetskaya LF, Green HW, Bozhilov NK, Mitchell TE and Dickerson RM. 2003. Crystallization environment of Kazakhstan microdiamond:Evidence from nanometric inclusions and mineral associations. Journal of Metamorphic Geology, 21(5): 425-437. DOI:10.1046/j.1525-1314.2003.00452.x |
Dobrzhinetskaya LF, Wirth R, Green HW, Schreiber A and O'Bannon E. 2013. First find of polycrystalline diamond in ultrahigh-pressure metamorphic terrane of Erzgebirge, Germany. Journal of Metamorphic Geology, 31(1): 5-18. DOI:10.1111/jmg.12010 |
Duncan MS and Dasgupta R. 2014. CO2 solubility and speciation in rhyolitic sediment partial melts at 1.5~3.0GPa:Implications for carbon flux in subduction zones. Geochimica et Cosmochimica Acta, 124: 328-347. DOI:10.1016/j.gca.2013.09.026 |
Ferrero S, Bartoli O, Cesare B, Salvioli-Mariani E, Acosta-Vigil A, Cavallo A, Groppo C and Battiston S. 2012. Microstructures of melt inclusions in anatectic metasedimentary rocks. Journal of Metamorphic Geology, 30(3): 303-322. DOI:10.1111/jmg.2012.30.issue-3 |
Ferrero S, Wunder B, Walczak K, O'Brien PJ and Ziemann MA.2015.Preserved near ultrahigh-pressure melt from continental crust subducted to mantle depths.Geology, 43(5): 447-450
|
Ferrero S, Ziemann MA, Angel RJ, O'Brien PJ and Wunder B. 2016. Kumdykolite, kokchetavite, and cristobalite crystallized in nanogranites from felsic granulites, Orlica-Snieznik Dome (Bohemian Massif):Not evidence for ultrahigh-pressure conditions. Contributions to Mineralogy and Petrology, 171: 3. DOI:10.1007/s00410-015-1220-x |
Frezzotti ML, Selverstone J, Sharp ZD and Compagnoni R. 2011. Carbonate dissolution during subduction revealed by diamond-bearing rocks from the Alps. Nature Geoscience, 4(10): 703-706. DOI:10.1038/ngeo1246 |
Frezzotti ML and Ferrando S. 2015. The chemical behavior of fluids released during deep subduction based on fluid inclusions. American Mineralogist, 100(2-3): 352-377. DOI:10.2138/am-2015-4933 |
Fu B, Touret JLR and Zheng YF. 2001. Remnants of pre-metamorphic fluid and oxygen-isotopic signatures in eclogites and garnet clinopyroxenite from the Dabie-Sulu terranes, eastern China. Journal of Metamorphic Geology, 21: 561-578. |
Fu B, Zheng YF and Touret JLR. 2002. Petrological, isotopic and fluid inclusion studies of eclogites from Sujiahe, NW Dabie Shan (China). Chemical Geology, 187(1-2): 107-128. DOI:10.1016/S0009-2541(02)00014-1 |
Fu B, Touret JLR and Zheng YF. 2003a. Remnants of premetamorphic fluid and oxygen isotopic signatures in eclogites and garnet clinopyroxenite from the Dabie-Sulu terranes, eastern China. Journal of Metamorphic Geology, 21(6): 561-578. DOI:10.1046/j.1525-1314.2003.00464.x |
Fu B, Touret JLR, Zheng YF and Jahn BM. 2003b. Fluid inclusions in granulites, granulitized eclogites and garnet clinopyroxenites from the Dabie-Sulu terranes, eastern China. Lithos, 70(3-4): 293-319. DOI:10.1016/S0024-4937(03)00103-8 |
Gao XY, Zheng YF and Chen YX. 2012. Dehydration melting of ultrahigh-pressure eclogite in the Dabie orogen:Evidence from multiphase solid inclusions in garnet. Journal of Metamorphic Geology, 30(2): 193-212. DOI:10.1111/jmg.2012.30.issue-2 |
Gao XY, Zheng YF, Chen YX and Hu Z. 2013. Trace element composition of continentally subducted slab-derived melt:Insight from multiphase solid inclusions in ultrahigh-pressure eclogite in the Dabie orogen. Journal of Metamorphic Geology, 31(4): 453-468. DOI:10.1111/jmg.2013.31.issue-4 |
Gao XY, Zheng YF, Chen YX and Hu Z. 2014. Composite carbonate and silicate multiphase solid inclusions in metamorphic garnet from ultrahigh-P eclogite in the Dabie orogeny. Journal of Metamorphic Geology, 32(9): 961-980. DOI:10.1111/jmg.12102 |
Hayes JM and Waldbauer JR. 2006. The carbon cycle and associated redox processes through time. Philosophical Transactions of the Royal Society B:Biological Sciences, 361(1470): 931-950. DOI:10.1098/rstb.2006.1840 |
Heinrich W. 2007. Fluid immiscibility in metamorphic rocks. Reviews in Mineralogy and Geochemistry, 65(1): 389-430. DOI:10.2138/rmg.2007.65.12 |
Hermann J, Spandler C, Hack A and Korsakov AV. 2006. Aqueous fluids and hydrous melts in high-pressure and ultra-high pressure rocks:Implications for element transfer in subduction zones. Lithos, 92(3-4): 399-417. DOI:10.1016/j.lithos.2006.03.055 |
Holness MB and Sawyer EW. 2008. On the pseudomorphing of melt-filled pores during the crystallization of migmatites. Journal of Petrology, 49(7): 1343-1363. DOI:10.1093/petrology/egn028 |
Hwang SL, Shen PY, Chu HT, Yui TF and Lin CC. 2001. Genesis of microdiamonds from melt and associated multiphase inclusions in garnet of ultrahigh-pressure gneiss from Erzgebirge, Germany. Earth and Planetary Science Letters, 188(1-2): 9-15. DOI:10.1016/S0012-821X(01)00314-4 |
Hwang SL, Shen PY, Yui TF and Chu HT. 2003. Metal-sulfur-COH-silicate fluid mediated diamond nucleation in Kokchetav ultrahigh-pressure gneiss. European Journal of Mineralogy, 15(3): 503-511. DOI:10.1127/0935-1221/2003/0015-0503 |
Hwang SL, Shen PY, Chu HT, Yui TF, Liou JG, Sobolev NV, Zhang RY, Shatsky VS and Zayachkovsky AA. 2004. Kokchetavite:A new potassium-feldspar polymorph from the Kokchetav ultrahigh-pressure terrane. Contributions to Mineralogy and Petrology, 148(3): 380-389. DOI:10.1007/s00410-004-0610-2 |
Hwang SL, Shen PY, Chu HT, Yui TF, Liou JG and Sobolev NV. 2009. Kumdykolite, an orthorhombic polymorph of albite, from the Kokchetav ultrahigh-pressure massif, Kazakhstan. European Journal of Mineralogy, 21(6): 1325-1334. DOI:10.1127/0935-1221/2009/0021-1970 |
Kerrick DM and Connolly JAD. 2001. Metamorphic devolatilization of subducted marine sediments and the transport of volatiles into the Earth's mantle. Nature, 411(6835): 293-296. DOI:10.1038/35077056 |
Kessel R, Ulmer P, Pettke T, Schmidt MW and Thompson AB. 2005a. The water-basalt system at 4 to 6GPa:Phase relations and second critical endpoint in a K-free eclogite at 700 to 1400℃. Earth and Planetary Science Letters, 273(3-4): 873-892. |
Kessel R, Schmidt MW, Ulmer P and Pettke T. 2005b. Trace element signature of subduction-zone fluids, melts and supercritical liquids at 120~180km depth. Nature, 437(7059): 724-727. DOI:10.1038/nature03971 |
Korsakov AV, Theunissen K and Smirnova LV. 2004. Intergranular diamonds derived from partial melting of crustal rocks at ultrahigh-pressure metamorphic conditions. Terra Nova, 16(3): 146-151. DOI:10.1111/ter.2004.16.issue-3 |
Korsakov AV and Hermann J. 2006. Silicate and carbonate melt inclusions associated with diamonds in deeply subducted carbonate rocks. Earth and Planetary Science Letters, 241(1-2): 104-118. DOI:10.1016/j.epsl.2005.10.037 |
Liu FL, Robinson PT, Gerdes A, Xue HM, Liu PH and Liou JG. 2010. Zircon U-Pb ages, REE concentrations and Hf isotope compositions of granitic leucosome and pegmatite from the north Sulu UHP terrane in China:Constraints on the timing and nature of partial melting. Lithos, 117(1-4): 247-268. DOI:10.1016/j.lithos.2010.03.002 |
Liu FL, Robinson PT and Liu PH. 2012. Multiple partial melting events in the Sulu UHP terrane:Zircon U-Pb dating of granitic leucosomes within amphibolite and gneiss. Journal of Metamorphic Geology, 30(8): 887-906. DOI:10.1111/jmg.2012.30.issue-8 |
Liu Q, Hermann J and Zhang JF. 2013. Polyphase inclusions in the Shuanghe UHP eclogites formed by subsolidus transformation and incipient melting during exhumation of deeply subducted crust. Lithos, 177: 91-109. DOI:10.1016/j.lithos.2013.06.010 |
Mann U and Schmidt MW. 2015. Melting of pelitic sediments at subarc depths:1.Flux vs.fluid-absent melting and a parameterization of melt productivity. Chemical Geology, 404: 150-167. DOI:10.1016/j.chemgeo.2015.02.032 |
Manning CE. 2004. The chemistry of subduction-zone fluids. Earth and Planetary Science Letters, 223(1-2): 1-16. DOI:10.1016/j.epsl.2004.04.030 |
Manning CE, Shock EL and Sverjensky DA. 2013. The chemistry of carbon in aqueous fluids at crustal and upper-mantle conditions:Experimental and theoretical constraints. Reviews in Mineralogy and Geochemistry, 75(1): 109-148. DOI:10.2138/rmg.2013.75.5 |
Marty B and Tolstikhin IN. 1998. CO2 fluxes from mid-ocean ridges, arcs and plumes. Chemical Geology, 145(3-4): 233-248. DOI:10.1016/S0009-2541(97)00145-9 |
Massonne HJ. 2003. A comparison of the evolution of diamondiferous quartz-rich rocks from the Saxonian Erzgebirge and the Kokchetav Massif:Are so-called diamondiferous gneisses magmatic rocks?. Earth and Planetary Science Letters, 216(3): 347-364. DOI:10.1016/S0012-821X(03)00512-0 |
Newton RC and Manning CE. 2000. Quartz solubility in H2O-NaCl and H2O-CO2 solutions at deep crust-upper mantle pressures and temperatures:2~15kbar and 500~900℃. Geochimica et Cosmochimica Acta, 64(17): 2993-3005. DOI:10.1016/S0016-7037(00)00402-6 |
Newton RC and Manning CE. 2002. Experimental determination of calcite solubility in H2O-NaCl solutions at deep crust/upper mantle pressures and temperatures:Implications for metasomatic processes in shear zones. American Mineralogist, 87(10): 1401-1409. DOI:10.2138/am-2002-1016 |
Perchuk AL, Burchard M, Maresch WV and Schertl HP. 2005. Fluid-mediated modification of garnet interiors under ultrahigh-pressure conditions. Terra Nova, 17(6): 545-553. DOI:10.1111/ter.2005.17.issue-6 |
Philippot P and Selverstone J. 1991. Trace-element-rich brines in eclogitic veins:Implications for fluid composition and transport during subduction. Contributions to Mineralogy and Petrology, 106(4): 417-430. DOI:10.1007/BF00321985 |
Piccoli F, Brovarone AV, Beyssac O, Martinez I, Ague JJ and Chaduteau C. 2016. Carbonation by fluid-rock interactions at high-pressure conditions:Implications for carbon cycling in subduction zones. Earth and Planetary Science Letters, 445: 146-159. DOI:10.1016/j.epsl.2016.03.045 |
Putnis A, Prieto M and Fernandez-Diaz L. 1995. Fluid supersaturation and crystallization in porous media. Geological Magazine, 132(1): 1-13. DOI:10.1017/S0016756800011389 |
Sano Y and Marty B. 1995. Origin of carbon in fumarolic gas from island arcs. Chemical Geology, 119(1-4): 265-274. DOI:10.1016/0009-2541(94)00097-R |
Sano Y and Williams SN. 1996. Fluxes of mantle and subducted carbon along convergent plate boundaries. Geophysical Research Letters, 23(20): 2749-2752. DOI:10.1029/96GL02260 |
Scambelluri M and Philippot P. 2001. Deep fluids in subduction zones. Lithos, 55(1-4): 213-227. DOI:10.1016/S0024-4937(00)00046-3 |
Shaw AM, Hilton DR, Fischer TP, Walker JA and Alvarado GE. 2003. Contrasting He-C relationships in Nicaragua and Costa Rica:Insights into C cycling through subduction zones. Earth and Planetary Science Letters, 214(3-4): 499-513. DOI:10.1016/S0012-821X(03)00401-1 |
Skora S, Blundy J, Brooker R, Green E, de Hoog JCM and Connolly JAD. 2015. Hydrous phase relations and trace element partitioning behaviour in calcareous sediments at subduction-zone conditions. Journal of Petrology, 56(5): 953-980. DOI:10.1093/petrology/egv024 |
Spear FS. 1993. Metamorphic Phase Equilibria and Pressure-Temperature-Time Paths. Washington: Mineralogical Society of America.
|
Stalder R, Ulmer P, Thompson AB and Günther D. 2000. Experimental approach to constrain second critical end points in fluid/silicate systems:Near-solidus fluids and melts in the system albite-H2O. American Mineralogist, 85(1): 68-77. DOI:10.2138/am-2000-0108 |
Stöckhert B, Duyster J, Trepmann C and Massonne HJ. 2001. Microdiamond daughter crystals precipitated from supercritical COH+silicate fluids included in garnet, Erzgebirge, Germany. Geology, 29(5): 391-394. DOI:10.1130/0091-7613(2001)029<0391:MDCPFS>2.0.CO;2 |
Stöckhert B, Trepmann CA and Massonne HJ. 2009. Decrepitated UHP fluid inclusions:About diverse phase assemblages and extreme decompression rates (Erzgebirge, Germany). Journal of Metamorphic Geology, 27(9): 673-684. DOI:10.1111/jmg.2009.27.issue-9 |
Sverjensky DA, Stagno V and Huang F. 2014a. Important role for organic carbon in subduction-zone fluids in the deep carbon cycle. Nature Geoscience, 7(12): 909-913. DOI:10.1038/ngeo2291 |
Sverjensky DA, Harrison B and Azzolini D. 2014b. Water in the deep Earth:The dielectric constant and the solubilities of quartz and corundum to 60kb and 1200℃. Geochimica et Cosmochimica Acta, 129: 125-145. DOI:10.1016/j.gca.2013.12.019 |
Sverjensky DA and Huang F. 2015. Diamond formation due to a pH drop during fluid-rock interactions. Nature Communications, 6: 8702. DOI:10.1038/ncomms9702 |
Syracuse EM, van Keken PE and Abers GA. 2010. The global range of subduction zone thermal models. Physics of the Earth and Planetary Interiors, 183(1-2): 73-90. DOI:10.1016/j.pepi.2010.02.004 |
Thomsen TB and Schmidt MW. 2008. Melting of carbonated pelites at 2. 5~5.0GPa, silicate-carbonatite liquid immiscibility, and potassium-carbon metasomatism of the mantle.Earth and Planetary Science Letters, 267(1-2): 17-31. |
Tiraboschi C, Tumiati S, Sverjensky D, Pettke T, Ulmer P and Poli S. 2018. Experimental determination of magnesia and silica solubilities in graphite-saturated and redox-buffered high-pressure COH fluids in equilibrium with forsterite+enstatite and magnesite+enstatite. Contributions to Mineralogy and Petrology, 173(1): 2. DOI:10.1007/s00410-017-1427-0 |
Tsuno K and Dasgupta R. 2012. The effect of carbonates on near-solidus melting of pelite at 3GPa:Relative efficiency of H2O and CO2 subduction. Earth and Planetary Science Letters, 319-320: 185-196. DOI:10.1016/j.epsl.2011.12.007 |
Tsuno K, Dasgupta R, Danielson L and Righter K. 2012. Flux of carbonate melt from deeply subducted pelitic sediments:Geophysical and geochemical implications for the source of Central American volcanic arc. Geophysical Research Letters, 39(16): L16307. |
Tumiati S, Tiraboschi C, Sverjensky D, Pettke T, Recchia S, Ulmer P, Miozzi F and Poli S. 2017. Silicate dissolution boosts the CO2 concentrations in subduction fluids. Nature Communications, 8(1): 616. DOI:10.1038/s41467-017-00562-z |
Xiao YL, Hoefs J, van den Kerkhof AM, Fiebig J and Zheng YF. 2000. Fluid history of UHP metamorphism in Dabie Shan, China:A fluid inclusion and oxygen isotope study on the coesite-bearing eclogite from Bixiling. Contributions to Mineralogy and Petrology, 139(1): 1-16. DOI:10.1007/s004100050570 |
Xiao YL, Hoefs J, van den Kerkhof AM, Simon K, Fiebig J and Zheng YF. 2002. Fluid evolution during HP and UHP metamorphism in Dabie Shan, China:Constraints from mineral chemistry, fluid inclusions and stable isotopes. Journal of Petrology, 43(8): 1505-1527. DOI:10.1093/petrology/43.8.1505 |
Xu HJ, Ye K, Song YR, Chen Y, Zhang JF, Liu Q and Guo S. 2013. Prograde metamorphism, decompressional partial melting and subsequent melt fractional crystallization in the Weihai migmatitic gneisses, Sulu UHP terrane, eastern China. Chemical Geology, 341: 16-37. DOI:10.1016/j.chemgeo.2013.01.002 |
Zeng LS, Liang FH, Asimow P, Chen FY and Chen J. 2009. Partial melting of deeply subducted continental crust and the formation of quartzofeldspathic polyphase inclusions in the Sulu UHP eclogites. Chinese Science Bulletin, 54(15): 2580-2594. |
 2019, Vol. 35
2019, Vol. 35

