2. Department of Master's Program in Neurology, Kaohsiung Medical University, Kaohsiung 807, Taiwan, China;
3. Department of Neurology, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung 807, Taiwan, China;
4. Department of Medical Laboratory Science and Biotechnology, Kaohsiung Medical University, Kaohsiung 807, Taiwan, China;
5. Department of Neurology, Linkou Chang Gung Memorial Hospital, Taoyuan City 333, Taiwan, China;
6. Faculty of Medicine, "National Yang-Ming University Schools of Medicine", Taipei 112, Taiwan, China;
7. Department of Neurology, Neurological Institute, Taipei Veterans General Hospital, Taipei 112, Taiwan, China
Ching-Kuan Liu, E-mail:ckliu@cc.kmu.edu.tw.
The angiotensin-converting enzyme (ACE) gene has been carefully studied in the context of late-onset Alzheimer's disease (AD), but the results have been inconsistent[1-3], especially in subjects of different races[4, 5]. We have consistently identified an association between ACE insertion/deletion (I/D) polymorphisms and Alzheimer's disease (AD) in the population in Taiwan[6]. The function of the ACE protein is thought to be related to cardiovascular events[7-9] that contribute to AD, inflammatory processes, oxidative stress, cell growth, matrix deposition, and the interference of acetylcholine release in neurons involved in cognitive processing[10]. In addition, the ACE protein has been shown to degrade beta-amyloid, which is one of the pathological hallmarks of AD[11], in a cell culture study[12]. These results have suggested that the ACE protein might be beneficial as a treatment for patients with AD[13]. Other cell culture studies have shown that the degradation of beta-amyloid by the ACE protein can be inhibited by captopril, an ACE inhibitor[12, 14]. However, in contrast to these cell culture results, clinical observations have shown that the use of ACE inhibitors decreases the prevalence of AD and improves cognitive function[15, 16].
In an earlier study, we found that an ACE insertion/ insertion (I/I) genotype was less likely to be observed in patients who had been diagnosed with AD[6]. This finding might be related to a report of lower levels of ACE protein in the renin-angiotensin system in subjects with this genotype[6], in whom the ACE protein was less likely to promote cardiovascular effects or increase blood pressure[17]. However, the Aβ-degrading effects of the ACE protein might also be reduced by decreased ACE protein levels in the subjects with the ACE I/I genotype. Consequently, the ACE I/I genotype would then contribute to an increased risk for the development of AD. However, the relationship between the ACE I/D genotype and AD remains unclear. The mechanisms that link the ACE gene to AD have been hypothesized to involve an interaction between the renin-angiotensin system and the metabolism of the amyloid precursor protein[17].
The apolipoprotein E (APOE) gene, which is localized on chromosome 19, consists of three major alleles (ε2, ε3, and ε4). Of these alleles, ε4 has been identified as a genetic risk factor for AD[18]. Carriers of two APOE ε4 alleles (ε4/ε4) have a higher risk of AD[19]. APOE ε4 is considered as a biomarker for the preclinical stage of AD[20]. In 2002, Lange et al. found that a patient's APOE genotype had little effect on their rate of memory decline[21]. However, the results of most research studies have suggested that APOE ε4 is associated with age-related cognitive decline[22]. Although APOE ε4 is generally thought to play a role in the onset of AD, the mechanisms through which it exerts this effect remain unclear.
Two possible mechanisms that might underlie the link between the ACE gene and AD have been proposed. ACE I/D polymorphisms are considered biomarkers of AD because the ACE protein, in association with the ACE I/D genotype, directly degrades beta-amyloid and thereby aids cognitive function in patients with AD. In contrast, ACE activates the renin-angiotensin-aldosterone system (RASS), which leads to hypertension and cardiovascular events and worsens cognition in patients with AD. In this study, we examined the bidirectional association between ACE and AD by assessing the role of ACE I/D polymorphisms in patients with AD with and without hypertension.
2 Methods 2.1 SubjectsIn 2014, patients who were clinically diagnosed with AD and enrolled in longitudinal studies being conducted by several neurological departments at the Taipei Veterans General Hospital, Taipei Chang Gung Memorial Hospital, Kaohsiung Municipal Ta-Tung Hospital, and Kaohsiung Medical University Hospital were selected for the study. The Kaohsiung Municipal Ta-Tung Hospital and Kaohsiung Medical University Hospital are part of the Kaohsiung Medical University Group Hospitals in Kaohsiung City. All procedures were approved by the ethics committee of each hospital. This study was conducted in accordance with the Helsinki Declaration and approved by the Institutional Review Board of Kaohsiung Medical University Hospital (KMUHIRB-20140002).
All patients received comprehensive medical evaluations, including clinical history, physical and neurological examinations, brain computed tomography, and blood chemistry examinations, in order to exclude other causes of their current cognitive statuses. The diagnosis of AD was based on the criteria of the "National Institute of Neurological and Communicative Disorders and Stroke" and the Alzheimer's Disease and Related Disorders Association[23] that involve a series of comprehensive neuropsychological tests, including the Mini-Mental State Examination[24] and Clinical Dementia Rating[25]. In the current study, we included patients with Mini-Mental State Examination scores between 10 and 23. Patients with other conditions that might affect their diagnosis of AD were excluded. A self-reported history of hypertension or previous treatment for hypertension were used to diagnose hypertension.
2.2 Methods for genotypingAPOE genotyping was performed according to a modification of a previous protocol (Biotage AG, Uppsala, Sweden). Briefly, 10 ng of DNA was amplified in a reaction volume of 20 μL in which deoxyguanosine triphosphate was replaced by a mixture of 25% deoxyguanosine triphosphate and 75% deoxyinosine triphosphate to facilitate the analysis of the GC-rich fragment. A 276-bp fragment was generated with the forward primer AGA CGC GGG CAC GGC TGT and reverse biotinylated primer CTC GCG GAT GGC GCT GAG. Single-stranded DNA was prepared with streptavidin-coated beads, and the APOE gene variants at codons 112 and 158 were pyrosequenced with the primers (SNP112) GAC ATG GAG GAC GTG and (SNP158) CCG ATG ACC TGC AGA with a dispensation order of GCTGAGCTAGCGT.
The detection of I/D polymorphisms of the human ACE gene was performed as described previously[26]. To confirm the genotypes, especially the D/I and D/D genotypes, we performed a second analysis with a protocol described by Lindpainter et al.[27]. Briefly, this method produces 319-or 597-bp polymerase chain reaction fragments in the presence of the D or I alleles, respectively. The polymerase chain reaction fragments were amplified from ~20 ng of each DNA sample that was used as a template in the master mix (Thermo Fisher Scientific, Waltham, MA, USA) and 10 pmol of each of the following primers: CGCGGTACCCTGG AGACCACTCCCATCCTTTCT (ACE sense) and CCGCTCGAGGACGTGGCCATCACATTCGTCAGA (ACE antisense). The thermocycling procedure consisted of denaturation at 94 ℃ for 30 s, annealing at 56 ℃ for 45 s, and extension at 72 ℃ for 2 min, which was repeated for 35 cycles and which was followed by a final extension at 72 ℃ for 7 min.
2.3 StatisticsThe data analysis was performed with SPSS (version 12.0.1 for Windows, IBM Corporation, Armonk, NY, USA). All statistical tests were two-tailed, and an alpha of 0.05 was used to indicate significance.
Chi-squared tests were used to examine whether the unadjusted likelihood of AD with hypertension (vs. without hypertension) was associated with the frequency of the three ACE genotypes, at least one APOE ε4 allele, gender, and ACE allele status. T-tests were conducted on the differences in the mean ages and education between the groups. A logistic regression was used to examine whether the ACE genotypes (D/D as the reference) were significantly associated with the presence or absence of hypertension in the patients with AD after adjusting for the effects of gender, age, education, and the presence of at least one APOE ε4 allele [APOE ε4 (+)]. A second logistic regression model was conducted to examine whether the ACE allele (I vs. D) was significantly associated with hypertension status after adjusting for the covariables.
The age at the time of the diagnosis of AD was recorded for all participants as they entered the study. Age and education were treated as continuous variables that increased in 1-year increments. Gender, APOE ε4 (+), and the ACE genotypes were treated as categorical variables.
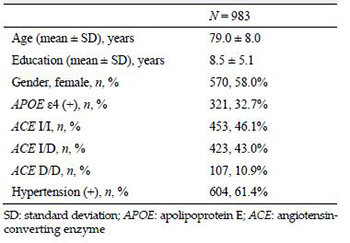
3 ResultsAD, 478 from the Taipei Veterans General Hospital, 42 from the Taipei Chang Gung Memorial Hospital, and 463 from the Kaohsiung Medical University Group Hospitals were selected from previous longitudinal studies for inclusion in these statistical analyses. Most these patients, which had a mean ± standard deviation age of 79.0 ± 8.0 years, were female (58%), and most had been diagnosed with hypertension (61.4%).
For the APOE ε4 carriers, 32.7% of the patients with AD had at least one APOE ε4. In the analysis of the ACE I/D genotype, most of the patients with AD were ACE I/I carriers (46.1%) compared to all the other polymorphisms (Table 1).
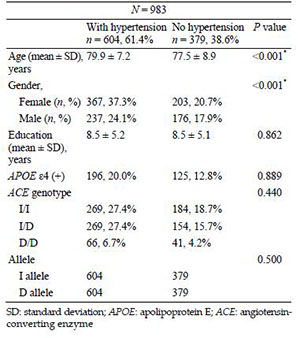
The groups of patients with AD who also had or did not have a diagnosis of hypertension significantly differed in age (P < 0.001) and gender (P < 0.001). Compared to the patients with AD who did not have hypertension, the hypertensive patients with AD were older (79.9 ± 7.2 vs. 77.5 ± 8.9 years) and predominantly female (37.3%) (Table 2). The prevalence of the ACE I/D genotype (P = 0.440) and I allele (P = 0.500) or the number of APOE ε4 carriers (P = 0.889) did not significantly differ between the groups (Table 2).
|
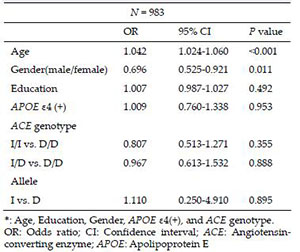
The results of the logistic regression after adjusting for the effects of age, education, gender, APOE ε4 carrier, and ACE genotype or I allele status in the patients with AD with and without hypertension showed that increased age [odds ratio = 1.042, 95% confidence interval = 1.024-1.060, P < 0.001] was significantly associated with hypertension. Compared to female patients, male patients with AD had a lower risk of a diagnosis of hypertension (odds ratio = 0.696, 95% confidence interval I= 0.525-0.921, P = 0.011). The distributions of the ACE I/D genotype (P = 0.355 for I/I vs. D/D; P = 0.888 for I/D vs. D/D) and I allele (P = 0.895) did not significantly differ between the patients with AD with or without hypertension (Table 3).
|
Our results indicated that the effects of the ACE gene on AD were not directly related to hypertension because the effects of the ACE genotypes (P = 0.355 for I/I vs. D/D; P = 0.888 for I/D vs. D/D) and the I allele (P = 0.895) did not significantly differ between the patients with AD according to the presence of hypertension. These findings suggested that the contribution of ACE to the mechanisms underlying AD might not be related to the mechanisms underlying hypertension. In addition, these findings might explain our negative results in the analysis of the association between the ACE genotype and the patients with AD according to their hypertension status. In other words, we have clarified the functional link between ACE and AD in the current study.
However, other mechanisms might account for the contribution of the ACE gene to the development of AD. A cell culture study showed that the ACE protein degrades beta-amyloid[14], which is one of the pathological hallmarks of AD[11]. The degradation of beta-amyloid by the ACE protein is related to the ACE I/D polymorphism[28]. Another cell culture study showed that the insertion (I) allele increases the function of the ACE gene promoter, which thereby enhances the function of the ACE gene and the ACE protein in neuronal cells[28]. The increased function of the ACE protein in neuronal cells might then increase the clearance of beta-amyloid, thereby reducing the risk for AD. The results of these studies have consistently suggested that one possible explanation for the relationship of ACE and the development of AD is through its functions related to beta-amyloid and not through hypertension or cardiovascular effects.
Because hypertension does not completely depend on the ACE gene, these interpretations of our results are not absolute. An alternative explanation is that the link between the ACE gene and AD depends mostly on beta-amyloid and only partly on hypertension.
Activation of the RAAS by the ACE gene and ACE protein is one of the established pathogeneses of hypertension that can lead to cardiovascular complications in patients who do not achieve their blood pressure goals. Although hypertension itself has a multifactorial pathogenesis[29], ACE, as part of the RAAS system, has shown the strongest association with hypertension after adjusting for age, sex, and weight in a genome-wide association study that was conducted in Korea and other countries[30, 31]. A recent genome-wide association study investigating ACE2 polymorphisms among Chinese and Caucasian participants has reported significant associations in women with hypertension or increased diastolic blood pressure[32].
The significant associations (P < 0.001) between AD and hypertension as well as AD and age that were found in our study also highlight the important issue that the contribution of hypertension to AD could be related to the age period during which the hypertension occurs. This idea has been suggested before. Similarly, midlife hypertension[32], not late-life hypertension[33], has been suggested as a risk factor for the development of AD.
Our study had several specific strengths. First, we used 983 patients who were clinically diagnosed with AD, and each participant went through a series of examinations, blood tests, neuropsychological assessments, and brain imaging to ensure diagnostic accuracy and exclude other factors that might affect the diagnosis. Second, clinical observations have found that the ACE gene is associated with AD. Unlike the association studies that examines the association between ACE and AD, we went one step further and examined the possible functions of the ACE gene and its relationship with hypertension and cardiovascular issues in patients with AD. One limitation of this study was that, because many factors contribute to hypertension, the association between AD and hypertension cannot be completely accounted for by the ACE gene. However, the ACE gene is considered a strong risk factor for hypertension[31-33]. Because the association exists, it can be used as a model to examine the functional link between the ACE gene and AD.
5 ConclusionWe used a large and well-defined clinical sample to examine and test the association of possible mechanisms that link ACE to AD and provide new findings and considerations. In contrast to established notions, we found that the contribution of the ACE gene to AD was unlikely to be associated with hypertension.
Conflicts of interest
None of the contributing authors have conflicts of interest.
| [1] | Jochemsen HM, Teunissen CE, Ashby EL, van der Flier WM, Jones RE, Geerlings MI, Scheltens P, Kehoe PG, Muller M. The association of angiotensin-converting enzyme with biomarkers for Alzheimer's disease. Alzheimers Res Ther, 2014, 6(3): 27. DOI:10.1186/alzrt257 |
| [2] | Kehoe PG, Russ C, McIlory S, Williams H, Holmans P, Holmes C, Liolitsa D, Vahidassr D, Powell J, McGleenon B, Liddell M, Plomin R, Dynan K, Williams N, Neal J, Cairns NJ, Wilcock G, Passmore P, Lovestone S, Williams J, Owen MJ. Variation in DCP1, encoding ACE, is associated with susceptibility to Alzheimer disease. Nat Genet, 1999, 21(1): 71–72. DOI:10.1038/5009 |
| [3] | Wang XB, Cui NH, Gao JJ, Qiu XP, Yang N, Zheng F. Angiotensin-converting enzyme gene polymorphisms and risk for sporadic Alzheimer's disease:A meta-analysis. J Neural Transm (Vienna), 2015, 122(2): 211–224. DOI:10.1007/s00702-014-1235-x |
| [4] | Lehmann DJ, Cortina-Borja M, Warden DR, Smith AD, Sleegers K, Prince JA, van Duijn CM, Kehoe PG. Large meta-analysis establishes the ACE insertion-deletion polymorphism as a marker of Alzheimer's disease. Am J Epidemiol, 2005, 162(4): 305–317. DOI:10.1093/aje/kwi202 |
| [5] | Meng Y, Baldwin CT, Bowirrat A, Waraska K, Inzelberg R, Friedland RP, Farrer LA. Association of polymorphisms in the Angiotensin-converting enzyme gene with Alzheimer disease in an Israeli Arab community. Am J Hum Genet, 2006, 78(5): 871–877. DOI:10.1086/503687 |
| [6] | Yang YH, Lai CL, Tyan YC, Chou MC, Wang LC, Yang MH, Liu CK. Angiotensin-converting enzyme gene and plasma protein level in Alzheimer's disease in Taiwanese. Age Ageing, 2011, 40(2): 238–242. DOI:10.1093/ageing/afq179 |
| [7] | Cosenso-Martin LN, Vaz-de-Melo RO, Pereira LR, Cesarino CB, Yugar-Toledo JC, Cipullo JP, de Souza Pinhel MA, Souza DR, Vilela-Martin JF. Angiotensin-converting enzyme insertion/deletion polymorphism, 24-h blood pressure profile and left ventricular hypertrophy in hypertensive individuals:a cross-sectional study. Eur J Med Res, 2015: 20–74. |
| [8] | Ma R, Li X, Su G, Hong Y, Wu X, Wang J, Zhao Z, Song Y, Ma S. Angiotensin-converting enzyme insertion/deletion gene polymorphisms associated with risk of atrial fibrillation:A meta-analysis of 23 case-control studies. J Renin Angiotensin Aldosterone Syst, 2015, 16(4): 793–800. DOI:10.1177/1470320315587179 |
| [9] | Sahin S, Ceyhan K, Benli I, Ozyurt H, Naseri E, Tumuklu MM, Aydogan L, Elalmis AO, Ozugurlu AF, Onalan O. Traditional risk factors and angiotensin-converting enzyme insertion/deletion gene polymorphism in coronary artery disease. Genet Mol Res, 2015, 14(1): 2063–2068. DOI:10.4238/2015.March.20.16 |
| [10] | Bartus RT, Dean RLRL, 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science, 1982, 217(4558): 408–414. DOI:10.1126/science.7046051 |
| [11] | Wang DS, Dickson DW, Malter JS. beta-Amyloid degradation and Alzheimer's disease. J Biomed Biotechnol, 2006, 2006(3): 58406. |
| [12] | Oba R, Igarashi A, Kamata M, Nagata K, Takano S, Nakagawa H. The N-terminal active centre of human angiotensinconverting enzyme degrades Alzheimer amyloid beta-peptide. Eur J Neurosci, 2005, 21(3): 733–740. DOI:10.1111/ejn.2005.21.issue-3 |
| [13] | Hu J, Igarashi A, Kamata M, Nakagawa H. Angiotensinconverting enzyme degrades Alzheimer amyloid beta-peptide (A beta); retards A beta aggregation, deposition, fibril formation; and inhibits cytotoxicity. J Biol Chem, 2001, 276(51): 47863–47868. DOI:10.1074/jbc.M104068200 |
| [14] | Hemming ML, Selkoe DJ. Amyloid beta-protein is degraded by cellular angiotensin-converting enzyme (ACE) and elevated by an ACE inhibitor. J Biol Chem, 2005, 280(45): 37644–37650. DOI:10.1074/jbc.M508460200 |
| [15] | Hanon O, Forette F. Prevention of dementia:Lessons from SYST-EUR and PROGRESS. J Neurol Sci, 2004, 226(1-2): 71–74. DOI:10.1016/j.jns.2004.09.015 |
| [16] | Tzourio C, Anderson C, Chapman N, Woodward M, Neal B, MacMahon S, Chalmers J. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med, 2003, 163(9): 1069–1075. DOI:10.1001/archinte.163.9.1069 |
| [17] | Kehoe PG, Wilcock GK. Is inhibition of the renin-angiotensin system a new treatment option for Alzheimer's disease?. Lancet Neurol, 2007, 6(4): 373–378. DOI:10.1016/S1474-4422(07)70077-7 |
| [18] | Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. JAMA, 1997, 278(16): 1349–1356. DOI:10.1001/jama.1997.03550160069041 |
| [19] | Davidson Y, Gibbons L, Pritchard A, Hardicre J, Wren J, Stopford C, Julien C, Thompson J, Payton A, PickeringBrown SM, Pendleton N, Horan MA, Burns A, Purandare N, Lendon CL, Neary D, Snowden JS, Mann DMA. Apolipoprotein E ε4 allele frequency and age at onset of Alzheimer's disease. Dement Geriatr Cogn Disord, 2007, 23(1): 60–66. |
| [20] | Resnick SM, Bilgel M, Moghekar A, An Y, Cai Q, Wang MC, Thambisetty M, Prince JL, Zhou Y, Soldan A, Wong DF, O'Brien RJ, Ferrucci L, Albert MS. Changes in Aβbiomarkers and associations with APOE genotype in 2 longitudinal cohorts. Neurobiol Aging, 2015, 36(8): 2333–2339. DOI:10.1016/j.neurobiolaging.2015.04.001 |
| [21] | Lange KL, Bondi MW, Salmon DP, Galasko D, Delis DC, Thomas RG, Thal LJ. Decline in verbal memory during preclinical Alzheimer's disease:Examination of the effect of APOE genotype. J Int NeuropsycholSoc, 2002, 8(7): 943–955. |
| [22] | Wisdom NM, Callahan JL, Hawkins KA. The effects of apolipoprotein E on non-impaired cognitive functioning:A meta-analysis. Neurobiol Aging, 2011, 32(1): 63–74. DOI:10.1016/j.neurobiolaging.2009.02.003 |
| [23] | McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease:Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology, 1984, 34(7): 939–944. DOI:10.1212/WNL.34.7.939 |
| [24] | Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res, 1975, 12(3): 189–198. DOI:10.1016/0022-3956(75)90026-6 |
| [25] | Morris JC. The Clinical Dementia Rating (CDR):Current version and scoring rules. Neurology, 1993, 43(11): 2412–2414. |
| [26] | Rigat B, Hubert C, Corvol P, Soubrier F. PCR detection of the insertion/deletion polymorphism of the human angiotensin converting enzyme gene (DCP1) (dipeptidyl carboxypeptidase 1). Nucleic Acids Res, 1992, 20(6): 1433. |
| [27] | Lindpaintner K, Pfeffer MA, Kreutz R, Stampfer MJ, Grodstein F, LaMotte F, Buring J, Hennekens CH. A prospective evaluation of an angiotensin-converting-enzyme gene polymorphism and the risk of ischemic heart disease. N Engl J Med, 1995, 332(11): 706–711. DOI:10.1056/NEJM199503163321103 |
| [28] | Wu SJ, Hsieh TJ, Kuo MC, Tsai ML, Tsai KL, Chen CH, Yang YH. Functional regulation of Alu element of human angiotensin-converting enzyme gene in neuron cells. Neurobiol Aging, 2013, 34(7): e1921–1927. |
| [29] | Sayed-Tabatabaei FA, Oostra BA, Isaacs A, van Duijn CM, Witteman JC. ACE polymorphisms. Circ Res, 2006, 98(9): 1123–1133. DOI:10.1161/01.RES.0000223145.74217.e7 |
| [30] | Song SB, Jin HS, Hong KW, Lim JE, Moon JY, Jeong KH, Ihm CG, Lee TW, Oh B, Lee SH. Association between renin-angiotensin-aldosterone system-related genes and blood pressure in a Korean population. Blood Press, 2011, 20(4): 204–210. DOI:10.3109/08037051.2011.555074 |
| [31] | Burrell LM, Harrap SB, Velkoska E, Patel SK. The ACE2 gene:Its potential as a functional candidate for cardiovascular disease. Clin Sci (Lond), 2013, 124(2): 65–76. DOI:10.1042/CS20120269 |
| [32] | Launer LJ, Ross GW, Petrovitch H, Masaki K, Foley D, White LR, Havlik RJ. Midlife blood pressure and dementia:the Honolulu-Asia aging study. Neurobiol Aging, 2000, 21(1): 49–55. DOI:10.1016/S0197-4580(00)00096-8 |
| [33] | Yang YH, Roe CM, Morris JC. Relationship between latelife hypertension, blood pressure, and Alzheimer's disease. Am J Alzheimers Dis Other Demen, 2011, 26(6): 457–462. DOI:10.1177/1533317511421779 |