文章信息
- 傅健飞, 金霞云, 徐锡枫, 朱京京, 王庆华, 杜金林, 郑树
- Fu Jianfei, Jin Xiayun, Xu Xifeng, Zhu Jingjing, Wang Qinghua, Du Jinlin, Zheng Shu
- miR494对结直肠癌细胞迁移能力的影响及其分子机制
- Effect of miR494 on migration of colorectal cancer cells and its molecular mechanism
- 实用肿瘤杂志, 2019, 34(6): 503-507
- Journal of Practical Oncology, 2019, 34(6): 503-507
基金项目
- 浙江省自然科学基金项目(LY19H160020);金华市社会发展类重点项目(2019-3-013);金华市社会发展类重点(2018-3-001d)
-
作者简介
- 傅健飞(1980-), 男, 浙江金华人, 副主任医师, 博士, 从事结直肠癌基础与临床研究.
-
文章历史
- 收稿日期:2019-09-24
2. 浙江大学医学院附属第二医院肿瘤内科, 浙江 杭州 310009
2. Department of Oncology, the Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou 310009, China
结直肠癌发病率在中国逐年增加,是最常见的消化道肿瘤。其中15%~25%的患者表现为同时性肝转移,另有近50%的患者结直肠癌原发灶切除后2年出现异时性肝转移[1-2]。估计有50%~60%的结直肠癌患者会出现肝转移[3-5]。因此,减少患者复发转移发生率以及提高患者无瘤生存期是后肿瘤时代的研究重点。
microRNA(miRNA)通过作用于靶基因在肿瘤的发生中发挥相关生物调节作用,但其机制尚不明确[6]。Strubberg等[7]总结分析与结直肠癌发生和发展相关的miRNA,提出miRNA可作为结直肠癌新的生物标志物。miRNA对结直肠癌肝转移具有一定的调节作用[8-9]。Huang等[10]认为,miRNA是预测肝转移发生的标志物。芯片筛选发现,神经母细胞瘤中miR494表达升高[11]。随后在脑胶质瘤[12]、肝癌[13]和乳腺癌[14]中都有发现mir494促进肿瘤的发生和发展。而miR494在子宫颈癌[15]、胃癌[16]、胆管癌[17]和胰腺癌[18]中发现miRNA494发挥抑癌作用。在肺癌中,有研究认为miR494是促癌的[19-20],也有研究认为其发挥抑癌作用[21]。结直肠癌相关研究认为miR494是促癌的,但只通过miR494瞬转SW480和SW620细胞后采用transwell实验明确其促进结直肠癌细胞侵袭,并没进一步深入的研究[22]。
本课题组既往通过芯片筛选显示,miR494在结直肠癌肝转移组表达升高;同时石蜡组织验证表明,其高表达与肝转移相关,并且与预后相关。本研究拟通过建立miR494稳定高表达的Lovo-miR494细胞株,通过体外细胞实验验证miR494引起肝转移的分子机制。
1 材料与方法 1.1 细胞与试剂人结肠癌细胞株Lovo、SW480、SW620、RKO和HT-26细胞株购于美国标准细胞培养库ATCC。Trizol试剂购自北京天根生化科技有限公司。F-12、1640和DMEM培养液购自美国GIBCO公司。Transwell室购自美国BD Biosciences公司。结晶紫粉末购自美国Sigma公司。
1.2 qRT-PCR检测miR494及上皮间质转化相关指标的表达根据Trizol试剂说明书提取结肠癌细胞株细胞中的总RNA,用核酸蛋白分析仪检测RNA浓度和纯度,使用SYBR预混试剂进行反转录和PCR反应。扩增引物序列如下: miR494正向引物为5′CAGCAAAAGGCTCGAGCAGTGCCATGTAGATTCGG-3′, 反向引物为5′ATTCTGATCAGGATCCGCATGGCACGCTGTCAG-3′;N-cadherin正向引物为5′CATCCTGCTTATCCTTGTG 3′, 反向引物为5′TAGTCCTGGTCTTCTTCTC 3′;E-cadherin正向引物为5′GAGAACGCATTGCCACATACAC 3′,反向引物为5′AAGAGCACCTTCCATGACAGAC 3′;Vimentin(VIM)正向引物为5′GCGTGAAATGGAAGAGAAC 3′,反向引物为5′TGGAAGAGGCAGAGAAATC 3′;Matrix metallopeptidase 2(MMP2)正向引物为5′TTGACGGTAAGGACGGACTC 3′,反向引物为5′GGCGTTCCCATACTTCACAC 3′;Matrix metallopeptidase 9(MMP9)正向引物为5′AAGGGCGTCGTGGTTCCAACTC 3′,反向引物为5′AGCATTGCCGTCCTGGGTGTAG 3′;Snail family zinc finger 1 (SNAI1)正向引物为5′TTCCTGAGCTGGCCTGTCTG 3′,反向引物为5′TGGCCTGAGGGTTCCTTGTG3′;Glyceraldehyde-3-phosphate dehydrogenase (GAPDH)作为内参,正向引物为5′CACCCACTCCTCCACCTTTG 3′,反向引物为5′CCACCACCCTGTTGCTGTAG 3′。基因扩增反应条件均为95℃预变性10 min, 95℃变性10 s, 60℃退火20 s, 72℃延伸34 s,共35个循环。每个样品3个重复,以GAPDH为内参。
1.3 慢病毒载体稳转细胞株的建立合成pre-miR494序列,采用pCMV-G&RN-U6-shRNA载体构建miR494前体载体(北京天根生化科技有限公司,中国)。通过慢病毒包装系统分别包装含miR494前体载体和对照载体的慢病毒颗粒,侵染Lovo细胞株后分别建立得到miR494-Lovo细胞株和NC-Lovo细胞株[23]。
1.4 划痕试验检测结直肠癌细胞迁移能力用记号笔画线作为标记, 将细胞用胰酶消化计数后取8×105个分入35 mm2培养皿中培养。吸去培养液,用200 μL枪头垂直于记号笔标记划线,用磷酸缓冲盐溶液(phosphate buffer saline, PBS)反复冲洗去除划下的细胞,加入无血清培养液继续培养。在0 h、24 h和48 h分别拍照,选取记号笔画的线和细胞划痕的交点作为观察点,以定点观察。
1.5 Transwell试验检测结直肠癌细胞迁移能力实验前24 h将细胞培养液换成无血清1640培养液,1×PBS浸泡24孔板及transwell小室(8 μm)5 min,胰酶消化细胞,用无血清培养液洗涤细胞,接种0.5 mL细胞悬液(3×104细胞/mL)到transwell小室内,下层的24孔板中加入0.75 mL含10%胎牛血清的1640培养液,每组3复孔,48 h后用棉棒轻轻拭去上层细胞,迁移至下层的细胞用1 mL 4%甲醛溶液,用1 mL 0.5%结晶紫溶液染色,置于200倍显微镜下观察,计数每个视野中的细胞数。
1.6 统计学分析采用SPSS 20.0软件分析数据。采用方差分析分析两组以上的数据,独立t检验分析两组间数据。以P<0.05为差异具有统计学意义。
2 结果 2.1 miR494在结直肠癌细胞株中的表达SW460、SW260、RKO、HT-29和Lovo细胞qRT-PCR检测miR494的相对表达量分别为(10.120± 0.389)、(2.747±0.208)、(4.968±0.311)、(2.069± 0.0868)和(1.186±0.108),其中Lovo细胞表达最低,SW260细胞表达最高(图 1)。本研究选取Lovo细胞株用于后续研究。
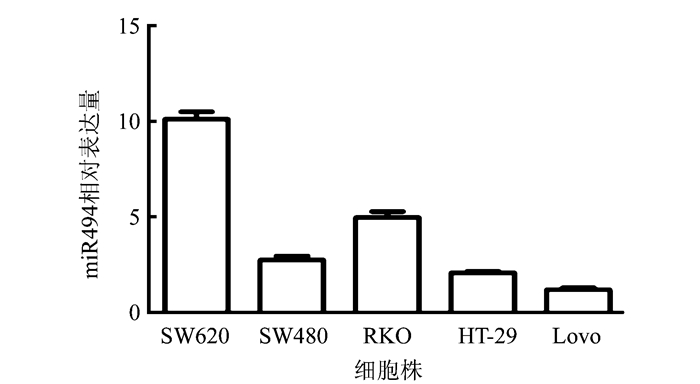
|
| 图 1 不同结直肠癌细胞株中miR494的相对表达量 Fig.1 Relative expression of miR494 in different colorectal cancer cell lines |
通过慢病毒包装系统建立miR494-Lovo细胞株和NC-Lovo细胞株。转染24 h后,qRT-PCR检测Lovo、NC-Lovo和miR494-Lovo细胞中miR494的相对表达量分别为(1.216±0.482)、(1.110±0.062)和(12.670±0.838),miR494-Lovo细胞中miR494表达量高于Lovo细胞与NC-Lovo细胞(均P < 0.05,图 2)。

|
| *与Lovo细胞和NC-Lovo细胞比较,均P < 0.05 图 2 Lovo、NC-Lovo和miR494-Lovo细胞株中miR494的相对表达量 Fig.2 Relative expression of miR494 in Lovo, NC-Lovo and miR494-Lovo cell lines |
通过划痕实验和transwell检测细胞的迁移能力,在24 h及48 h时, miR494-Lovo细胞迁移能力较NC-Lovo细胞和Lovo细胞增强(图 3~4)。

|
| 图 3 划痕试验光学显微镜下结果(×100) Fig.3 Scratch test results under light microscope (×100) |
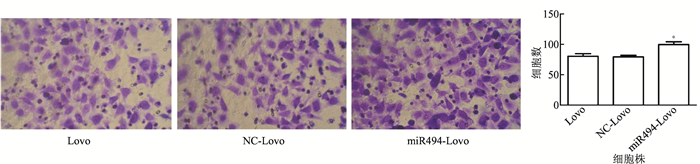
|
| 图 4 转染48 h后transwell试验检测Lovo、NC-Lovo和miR494-Lovo细胞迁移能力(×400) Fig.4 Transwell assay detecting the cell migration of Lovo, NC-Lovo and miR494-Lovo after 48 h transfection |
转染48 h后,RT-PCR检测显示,与NC-Lovo细胞比较,miR494-Lovo细胞E-cadherin表达升高,N-cadherin、MMP2、MMP9、vimentin和snail表达下降(均P < 0.05, 图 5)。

|
| *与 NC-Lovo 细胞比较,P < 0. 05 图 5 转染48 h后RT-PCR检测NC-Lovo和miR494-Lovo细胞上皮间质转化标志蛋白表达情况 Fig.5 RT-PCR results of the expression of epithelial-mesenchymal transition related proteins in NC-Lovo and miR494-Lovo cells after 48 h transfection |
近年来,miRNAs作为表观遗传修饰的重要调控分子,在结直肠癌中起着重要作用。研究表明,miR494可以通过靶向于P190B Rho GTPase Activating Protein 5影响表皮生长因子受体的稳定性,增加肿瘤的侵袭能力[12]。在敲除SMAD3裸鼠模型中,乳腺癌细胞4T1通过作用转化生长因子受体,募集肿瘤微环境中的骨髓来源的抑制性细胞(myeloid derived suppressor cells,MDSCs)[14]。MDSC细胞内miR494水平升高,可以通过靶向人类第十号染色体缺失与磷酸酶和张力蛋白同源基因(gene of phosphate and tension homology deleted on chromsome ten, PTEN),让MDSC释放更多MMP,进一步促进肿瘤细胞侵袭转移。前列腺癌的相关研究表明,miR494作用于人趋化因子受体4(chemokine receptor,CXCR4),抑制前列腺癌细胞的迁移能力[24]。miR494可引起SW480和SW620肠癌细胞株侵袭性增加[22]。本研究也表明,miR494多表达的Lovo细胞迁移能力增加。
研究显示,miR494在结直肠癌肝转移患者中表达高于无肝转移患者,提示miR494与结直肠癌肝转移的发生有密切关系,PEA15蛋白可通过激活ERK/MAPK通路影响结直肠癌上皮间质转化的发生从而促进结直肠癌肝转移的发生[25]。EMT的发生与肿瘤的发展密切相关。本研究结果也表明,miR494促进结直肠癌EMT的发生。
综上所述, 本研究筛选出低表达的Lovo细胞株,通过慢病毒构建的miR494过表达细胞株迁移能力增强,且EMT的发生被促进,提示miR494对结直肠癌转移具有潜在的正向调控作用。其具体机制有待进一步研究。
| [1] |
Go S, Takehiro N, Hidetoshi E, et al. Surgical outcome of extended liver resections for colorectal liver metastasis compared with standard liver resections[J]. Mol Clin Oncol, 2018, 9(1): 104-111. |
| [2] |
万德森, 张苏展, 陈玉泽, 等. 贝伐珠单抗联合以5-FU为基础的双药化疗用于结直肠癌仅肝转移患者新辅助治疗:一项多中心单臂研究[J]. 实用肿瘤杂志, 2018, 33(1): 41-46. |
| [3] |
Dulundu E, Attaallah W, Tilki M, et al. Simultaneous resection for colorectal cancer with synchronous liver metastases is a safe procedure:Outcomes at a single center in Turkey[J]. Biosci Trends, 2017, 11(2): 235-242. DOI:10.5582/bst.2017.01019 |
| [4] |
Shiozawa K, Watanabe M, Ikehara T, et al. Comparison of contrast-enhanced ultrasonograpy with Gd-EOB-DTPA-enhanced MRI in the diagnosis of liver metastasis from colorectal cancer[J]. J Clin Ultrasound, 2017, 45(3): 138-144. DOI:10.1002/jcu.22421 |
| [5] |
Baltruskeviciene E, Schveigert D, Stankevicius V, et al. Down-regulation of miRNA-148a and miRNA-625-3p in colorectal cancer is associated with tumor budding[J]. Bmc Cancer, 2017, 17(1): 607. DOI:10.1186/s12885-017-3575-z |
| [6] |
程先硕, 刘萍, 杨之斌. miRNA-145在结直肠癌中的作用及临床应用价值研究进展[J]. 实用肿瘤杂志, 2019, 34(3): 273-276. |
| [7] |
Strubberg AM, Madison BB. MicroRNAs in the etiology of colorectal cancer:pathways and clinical implications[J]. Dis Model Mech, 2017, 10(3): 197-214. DOI:10.1242/dmm.027441 |
| [8] |
Chorti A, Bangeas P, Papavramidis TS, et al. Role of microRNA in the diagnosis and therapy of hepatic metastases from colorectal cancer[J]. MicroRNA (Shariqah, United Arab Emirates), 2018, 7(3): 167-177. |
| [9] |
Li W, Chang J, Tong D, et al. Differential microRNA expression profiling in primary tumors and matched liver metastasis of patients with colorectal cancer[J]. Oncotarget, 2017, 8(22): 35783-35791. |
| [10] |
Huang S, Tan X, Huang Z, et al. microRNA biomarkers in colorectal cancer liver metastasis[J]. J Cancer, 2018, 9(21): 3867-3873. DOI:10.7150/jca.28588 |
| [11] |
Zhao JJ, Yang J, Lin J, et al. Identification of miRNAs associated with tumorigenesis of retinoblastoma by miRNA microarray analysis[J]. ChNS, 2009, 25(1): 13-20. |
| [12] |
Kwak SY, Yang JS, Kim BY, et al. Ionizing radiation-inducible miR-494 promotes glioma cell invasion through EGFR stabilization by targeting p190B RhoGAP[J]. Biochimica et Biophysica Acta, 2013, 1843(3): 508-516. |
| [13] |
Zhang J, Zhu Y, Hu L, et al. miR-494 induces EndMT and promotes the development of HCC (Hepatocellular Carcinoma) by targeting SIRT3/TGF-β/SMAD signaling pathway[J]. Sci Rep, 2019, 9(1): 7213. DOI:10.1038/s41598-019-43731-4 |
| [14] |
Liu Y, Lai L, Chen Q, et al. MicroRNA-494 is required for the accumulation and functions of tumor-expanded myeloid-derived suppressor cells via targeting of PTEN[J]. J Immunol, 2012, 188(11): 5500-5510. DOI:10.4049/jimmunol.1103505 |
| [15] |
Cheng L, Kong B, Zhao Y, et al. miR-494 inhibits cervical cancer cell proliferation through upregulation of SOCS6 expression[J]. Oncol Lett, 2017, 15(3): 3075-3080. |
| [16] |
Yu Y, Yu X, Liu H, et al. miR-494 inhibits cancer initiating cell phenotypes and reverses resistance to lapatinib by downregulating FGFR2 in HER2 positive gastric cancer[J]. Int J Mol Med, 2018, 42(2): 998-1007. |
| [17] |
Li L, Li Z, Kong X, et al. Down-regulation of microRNA-494 via loss of SMAD4 increases FOXM1 and beta-catenin signaling in pancreatic ductal adenocarcinoma cells[J]. Gastroenterology, 2014, 147(2): 485-497. DOI:10.1053/j.gastro.2014.04.048 |
| [18] |
Yang Y, Tao X, Li CB, et al. MicroRNA-494 acts as a tumor suppressor in pancreatic cancer, inhibiting epithelial-mesenchymal transition, migration and invasion by binding to SDC1[J]. Int J Oncol, 2018, 53(3): 1204-1214. |
| [19] |
Liu L, Jiang Y, Zhang H, et al. Overexpressed miR-494 down-regulates PTEN gene expression in cells transformed by anti-benzo (a) pyrene-trans-7, 8-dihydrodiol-9, 10-epoxide[J]. Life Sci, 2010, 86(5/6): 192-198. |
| [20] |
Romano G, Acunzo M, Garofalo M, et al. MiR-494 is regulated by ERK1/2 and modulates TRAIL-induced apoptosis in non-small-cell lung cancer through BIM down-regulation[J]. Proc Nat Acad Sci U S A, 2012, 109(41): 16570-16575. DOI:10.1073/pnas.1207917109 |
| [21] |
Ohdaira H, Sekiguchi M, Miyata K, et al. MicroRNA-494 suppresses cell proliferation and induces senescence in A549 lung cancer cells[J]. Cell Prolif, 2012, 45(1): 32-38. DOI:10.1111/j.1365-2184.2011.00798.x |
| [22] |
Sun HB, Chen X, Ji H, et al. miR494 is an independent prognostic factor and promotes cell migration and invasion in colorectal cancer by directly targeting PTEN[J]. Int J Oncol, 2014, 45(6): 2486-2494. DOI:10.3892/ijo.2014.2665 |
| [23] |
王韵, 吉宁, 周敏, 等. microRNA-223过表达与抑制表达慢病毒载体的构建及鉴定[J]. 华西口腔医学杂志, 2015(5): 13-17. |
| [24] |
Shen PF, Chen XQ, Liao YC, et al. MicroRNA-494-3p targets CXCR4 to suppress the proliferation, invasion, and migration of prostate cancer[J]. Prostate, 2014, 74(7): 756-767. DOI:10.1002/pros.22795 |
| [25] |
Tang B, Liang W, Liao Y, et al. PEA15 promotes liver metastasis of colorectal cancer by upregulating the ERK/MAPK signaling pathway[J]. Oncol Rep, 2018, 41(1): 43-56. |
 2019, Vol. 34
2019, Vol. 34
