2. 上海冷链装备性能测试与节能评价公共服务平台, 上海 201306;
3. 上海市水产品加工与贮藏工程技术研究中心, 上海 201306;
4. 食品科学与工程国家级实验教学示范中心, 上海 201306
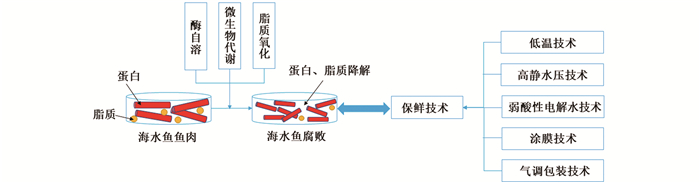
海水鱼含有高生物价值的蛋白质、维生素和矿物质,且饱和脂肪酸含量低,所富含的高质量多不饱和脂肪酸[如: 二十二碳六烯酸(Docosahexaenoic acid,DHA)和二十碳五烯酸(Eicosapentaenoic acid,EPA)]也已被证实与预防心血管疾病有关,并展示出良好的抗炎特性[1-2]。然而,由于海水鱼水分活度较高(0.98~0.99),鱼体内pH较适宜(6.2~6.5),并且存在众多小分子物质(如:游离氨基酸)。因此,海水鱼产品极易受到微生物、生物化学腐败的影响,必须在冷藏或冷冻的条件下进行贮藏。即便如此,冷藏和冷冻条件下的货架期一般也仅为5~7 d和6~12个月[3-6],尤其在冷藏的条件下海水鱼产品的货架期有限。据联合国粮食及农业组织(FAO)的数据统计,全球每年仍有超过20%的海水鱼产品因腐败变质而造成浪费,海水鱼的易腐性仍是在贮藏时所面临的最大挑战[7]。本研究重点介绍海水鱼捕捞死亡后在流通过程中的腐败因素,以及为控制其腐败所应用的保鲜技术的原理和应用效果(图 1)。
|
图 1 造成海水鱼腐败的因素和代表性保鲜技术 Fig. 1 Factors responsible for marine fish spoilage and representative preserving methods |
海水鱼捕捞上岸后,由于环境的不适应性常常会在短期内死亡。海水鱼死亡后,鱼体内迅速发生一系列的生物化学反应。各种成分的分解和新物质的生成是海水鱼腐败过程中风味和质构变化的主要原因。海水鱼的腐败和内源酶活性(自溶)、微生物代谢活动和脂质氧化息息相关[8]。
1.1 酶自溶腐败海水鱼在死亡后立即发生的变化主要是其内源酶作用的结果[9]。海水鱼中的内源酶活性较大,且因鱼体pH条件适宜,易导致其自溶。酶自溶对海水鱼产品的风味影响较小,但对质构影响较为显著[10]。在海水鱼贮藏的早期,微生物还处于较低水平,因此影响早期海水鱼腐败的因素主要为各种酶的作用[11]。表 1列出了几种内源酶对海水鱼造成的影响[8, 12]。

|
表 1 海水鱼冷藏中内源性酶的作用 Tab.1 The role of endogenous enzymes in refrigeration of marine fish |
酶自溶腐败主要是核苷酸分解酶参与的酶促降解,其次是某些内源蛋白酶和脂肪酶的作用。内源性的核苷酸分解酶在鱼体内所参与的降解反应:三磷酸腺苷(ATP) →二磷酸腺苷(ADP) →一磷酸腺苷(AMP) →次黄嘌呤核苷酸(IMP) →肌苷(HxR) →次黄嘌呤(Hx),这一反应步骤也与海水鱼的种类、肌肉类型和贮藏条件有关[13]。核苷酸分解酶的活性决定了K的大小,K与水产品的腐败程度有很好的相关性[14]。K的计算公式:
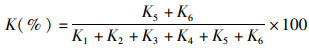 (1)
(1)
式中:K1为三磷酸腺苷;K2为二磷酸腺苷;K3为一磷酸腺苷;K4为次黄嘌呤核苷酸;K5为肌苷;K6为次黄嘌呤。
K值的大小会随着核苷酸分解酶活性的增加和贮藏期的延长逐步上升。新鲜海水鱼的K值较低,保持在10%左右;当K值为20%~40%时,海水鱼的品质仍可以接受;而当K值高于40%时,则表示海水鱼开始腐败变质,品质不可接受。此外,在海水鱼死后,体内的蛋白水解体系与内源性的细胞质钙蛋白酶、溶酶体组织蛋白酶(如组织蛋白酶B、L、H和D)和基质金属蛋白酶活性高度相关[15],内源性蛋白酶的高自溶活性会导致肌原纤维蛋白的软化降解,硬度下降,并产生一系列水解产物,如:小分子肽和游离氨基酸等。内源性脂肪酶也可降解海水鱼体内脂质,产生游离脂肪酸等水解产物,并易氧化生成羰基化合物,产生鱼腥味[1]。酶自溶腐败的产物有利于后续腐败微生物的生长,使得感官属性发生变化,造成变质。
1.2 微生物腐败微生物的生长是造成新鲜或弱冷保鲜(冷藏、冰藏等)海水鱼腐败变质的最重要因素[16]。起初鱼肉被认为是基本无菌的,然而在海水鱼死亡后,皮肤、鳃和肠道上的微生物会在表面释放一系列的水解酶,破坏表面结构,从而进入到肌肉组织中[17]。海水鱼特有的高水分活度、低酸度和高非蛋白氮含量可为微生物的生长提供良好的环境,导致微生物在其体内快速生长。同时,微生物生长所分泌的内源酶和胞外酶也会加快鱼肉蛋白质、脂质和其他营养物质的降解速率[18]。这会消耗鱼体内大量营养物质,并产生大量的腐败化合物,如:挥发性胺、生物胺、硫化物、醇、醛、酮等,从而对海水鱼的外观、质构和风味等造成恶劣的影响,使其品质不可逆地下降。微生物的生长还会伴随三甲胺(trimethylamine,TMA)的形成,基于此,TMA水平的高低是判断海水鱼腐败程度的另一个指标[8]。
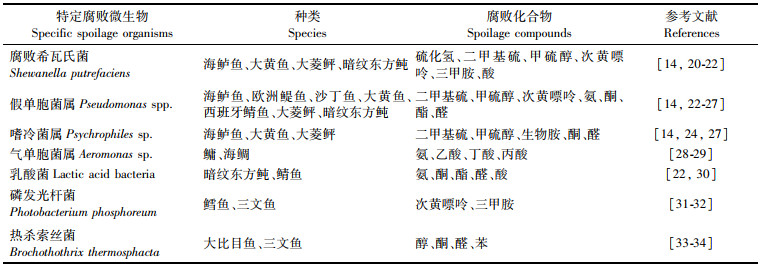
值得注意的是,虽然海水鱼死后会受到众多微生物种类的侵染,但往往只有小部分微生物会大量增殖并产生腐败代谢产物,最终导致产品变质,这部分微生物被称为特定腐败微生物(specific spoilage organisms,SSOs)。SSOs与鱼的种类、贮藏条件相关,表 2列出了部分海水鱼低温贮藏过程中的几种特定腐败微生物及其产生的腐败物质。此外,WICKRAMASINGHE等[19]研究发现,SSOs的生物膜可与肌原纤维蛋白质水解时产生的小分子物质结合形成黏液,这种黏液可保护SSOs的正常生长并加速肌肉组织的软化。因此,控制海水鱼贮藏过程中SSOs的数量及其生物膜的形成对保证海水鱼的品质至关重要。
|
表 2 部分海水鱼低温贮藏过程中的几种特定腐败微生物及其产生的腐败化合物 Tab.2 Several specific spoilage organisms and their spoilage compounds during low temperature storage of some marine fishes |
海水鱼鱼脂内长链脂肪酸(C14~C22)中的多不饱和脂肪酸含量高达40%,同时鱼体中又含有极易参与到氧化反应中的微量金属离子,例如铁、铜、钴等,这使脂质氧化造成的腐败在海水鱼贮藏过程中不可忽视[8]。脂质氧化可通过酶或非酶途径进行。酶促途径是内源性或嗜冷微生物代谢产生的脂肪酶对海水鱼脂质的作用,如三酰基甘油酰基水解酶可以水解甘油三酯生成游离脂肪酸,导致脂肪酸度增加,从而降低脂肪质量[12]。脂肪酶和游离脂肪酸的存在也会抑制鱼体内本身存在的抗氧化剂发生作用,因此认为脂质氧化反应在海水鱼死亡后即刻发生[17]。非酶途径一般可分为两步[35]:首先是海水鱼脂肪中的不饱和脂肪酸与氧气发生反应,生成无味的氢过氧化物(初级氧化产物),其次氢过氧化物分解生成羰基化合物(例如醛、酮等,二级氧化产物)。
脂质氧化对海水鱼品质的危害巨大,一是脂质氧化过程生成的二级氧化产物往往会伴随着不愉快的气味和风味,并严重危害消费者的健康;二是脂质氧化不仅造成脂肪变质,而且会加速鱼体内维生素的分解,从而导致海水鱼产品营养价值下降;三是脂质氧化产生的羰基化合物也会诱导肌原纤维蛋白变性,加快海水鱼产品的腐败[36]。此外,有研究[37]表明,活性氧(reactive oxygen species,ROS)会加快机体内各种氧化反应,因此,控制海水鱼贮藏过程中ROS的浓度可在一定程度上抑制海水鱼的脂质氧化。
2 保鲜技术控制海水鱼的腐败随着经济水平的提高,消费者越来越倾向购买不含防腐剂的食物,这对于常温下极易腐败食物——海水鱼的流通带来了严峻的挑战。人们通过各种保鲜技术来延缓海水鱼的腐败,并尽可能地延长其货架期。
2.1 低温技术在海水鱼捕捞后的运输、贮藏和销售期间,低温技术是非常必要的。低温技术大抵可分为冷藏、冰温、微冻和冻藏。低温技术的实施可以有效保证海水鱼的品质,延长海水鱼的货架期,并确保海水鱼加工厂的经济效益[38]。见表 3。

|
表 3 不同低温贮藏技术对海水鱼货架期的影响 Tab.3 Effects of different low temperature storage technologies on shelf life of marine fishes |
冷藏是将海水鱼贮藏在0~5 ℃(高于其初始冰点)的过程。冷藏可以通过降低内源酶的活性、抑制微生物的生长和减缓物理、化学反应来延长海水鱼的贮藏期[39]。此外,由于冷藏未在海水鱼体内形成冰晶,对海水鱼肌原纤维蛋白的破坏性较小,贮藏期内感官品质较高,因此受到许多消费者的青睐。但是,冷藏最大的缺点在于其所处的温度范围并不足以完全抑制各类腐败过程,尤其是某些嗜冷微生物的生长,因此,冷藏过程中微生物安全问题得不到有效解决[40]。NISAR等[41]研究发现,鲷鱼在4 ℃贮藏时初始总菌落数为3.70 log CFU/g,在第9天达到了7.9 log CFU/g,超过总菌落数限值(7.0 log CFU/g),嗜冷菌也表现出了相似趋势,冷藏期间微生物生长是影响海水鱼的货架期的主要因素。此外,在微生物丰度方面,ZHAO等[42]通过高通量测序技术研究了海鲈鱼在4 ℃下微生物种群的组成和动态演替,结果表明,微生物丰度随着冷藏贮藏期的延长而逐渐下降,且假单胞属数量最大,占所有微生物种群的25.41%,在海鲈鱼冷藏期间对假单胞属的控制尤为重要。因此,为了延长海水鱼的冷藏的货架期需要栅栏技术保鲜,如冷藏结合涂膜、气调包装等[43-44]。
2.1.2 冰温/超冰温贮藏冰温是指0 ℃以下初始冰点以上的温度范围,产品在此温度范围内进行贮藏时称为冰温贮藏。冰温贮藏技术不仅可以保持冷藏技术的优势,又能更进一步地降低鱼体内的生物、化学反应速率,延长海水鱼的货架期[45]。由于冰温贮藏的温度带较窄,冰温技术应用时的最大挑战是对环境温度的有效控制,精确和严格的温度控制是实现食品冰温贮藏的关键。CHAN等[46]将三文鱼分别贮藏于-1 ℃的冷却海水和0 ℃冰中,研究了这两种冰温贮藏条件下的品质差异。结果表明,冷却海水组与冰组相比可显著延缓微生物,尤其是产H2S菌的生长,并且呈现出良好品质特性。可见,将海水鱼置于冷却海水中进行贮藏是一种良好的冰温贮藏方法。
超冰温技术是指将产品的温度降低至其初始冰点以下,而不发生相变凝固——形成冰晶的一种贮藏技术,此时产品处于过冷态[47-48]。因此,产品温度虽低于初始冰点但不会受到冰晶的物理损伤[49]。然而,由于过冷态是一种临界状态,因此在超冰温贮藏中,一旦受到温度波动或外界振动的影响,产品的过冷态就会立即消失,鱼体内会生成晶核,晶核进一步形成冰晶,最终产品将转变为微冻状态。超冰温贮藏对环境要求严苛且操作困难[50-52],PARK等[53]通过每18 h降低0.5 ℃的方法(即逐步冷却法)实现了鲭鱼的过冷贮藏。研究结果表明,鲭鱼在-1.42 ℃的过冷态下进行贮藏时,与冷藏(1.01 ℃)相比脂质氧化和微生物腐败速率明显减缓,一级鲜度持续时间可接近9 d,是冷藏的2~3倍,且超冰温鲭鱼的持水力、质构等品质指标与冻藏相比均显著提升。
2.1.3 微冻贮藏微冻是指将产品温度降至低于其初始冰点1~2 ℃,食品部分冻结的贮藏过程。在微冻状态下,只有5%~30%的水会形成冰晶,且鲜鱼表面以下只会形成1~3 mm的冰晶层,所以有人称之为“部分冻结”或“壳式冻结”[54-55]。微冻海水鱼体内形成的少量冰可在运输产品时持续地创造低温环境,因此在流通海水鱼时无需在产品周围加入额外的冰,可极大地减少运输体积[56-57]。TRYGGVASON等[58]研究发现,通常海水鱼在低温物流中使用的包装容器为17 cm深度的聚苯乙烯泡沫箱,随着堆货深度的增加,产品间形成的挤压力会对产品品质造成影响,而微冻状态下流通的三文鱼,即使包装容器深度达到60 cm,也不易受挤压的影响,其汁液损失仍未明显增加,品质未发生明显变化。可见,微冻海水鱼在流通过程中的优势是巨大的。然而,尽管海水鱼在微冻贮藏时的环境参数与过冷贮藏相比更易控制,但微冻贮藏海水鱼也可能对质量产生负面影响。BAHUAUD等[59]报道三文鱼在微冻贮藏时会在细胞内和细胞外形成冰晶,造成溶酶体破裂,并释放出组织蛋白酶B和L,加速肌纤维之间的断裂。ZHAO等[60]还发现,在微冻贮藏过程中大黄鱼的肌球蛋白重链降解较严重,并伴随着Ca2+-ATPase活性和巯基官能团含量的下降。同时,微冻所在的温度范围位于最大冰晶生成带内,温度的波动会导致冰晶朝着多而大的方向发展,从而对海水鱼肌原纤维结构造成不可逆的损害,所以在微冻贮藏过程中应该避免过大的温度波动[61]。
2.1.4 冻藏冻藏海水鱼所采用的温度通常为-18~-30 ℃,在这个温度下,大约有80%的水结成冰,水分活度降低[62]。较低的水分活度与寒冷的温度使得微生物在冻藏期间几乎无法生长繁殖,因此微生物腐败机制对于冻藏海水鱼来说不太适用[63]。TAN等[64]研究发现,pH的变化是引起冻藏大黄鱼肌原纤维蛋白降解的关键原因。随着pH的降低,肌原纤维蛋白的展开程度加大,导致内部疏水基团暴露并因此造成肌原纤维蛋白的聚集降解。冰晶也是影响海水鱼冻藏品质的关键因素,超声辅助冻结技术、高压处理技术、添加抗冻剂等技术的实施可使冻藏鱼体内形成的冰晶更加细小,利于保证海水鱼的品质[65]。此外,由表 3可以看出,在所有的低温贮藏技术中,冻藏技术可以最大地延长海水鱼的货架期。尽管在货架期上冻藏相比其他低温贮藏技术有优势,但不可否认的是,冻藏对海水鱼产品的质量影响较大。由于冰晶的大量生成,会导致产品汁液损失增大,严重影响海水鱼的质构、风味和营养,造成品质下降[66]。
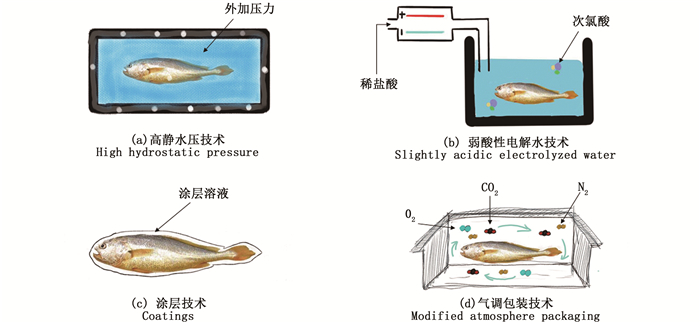
2.2 高静水压技术高静水压技术是一种海水鱼的非热贮藏技术,早于1990年开始在日本使用[72](图 2a)。高静水压技术的原理:海水鱼在高压下(通常为100~600 MPa)加工时,鱼体内的微生物和酶失活,从而有效地延长其货架期[73]。微生物对高静水压力的敏感性一般为真菌>革兰氏阴性菌>革兰氏阳性菌[72]。然而,该技术由于外加压力,实施的过程中可能对海水鱼的质构产生影响。但已有文献[35]表明,当外加压力在100~300 MPa时,这种影响是可逆的。ERKAN等[74]在研究高静水压技术对竹荚鱼的品质影响时得出,采用高静水压技术加工海水鱼时3种可能的最佳工艺:(1)220 MPa下,15~25 ℃加压5 min;(2)250 MPa下,7~15 ℃加压5 min;(3)330 MPa下,25 ℃、10 min,这些处理条件可显著降低竹
|
图 2 几种海水鱼保鲜技术示意图 Fig. 2 Schematic diagram of several marine fish preservation technologies |
弱酸性电解水技术由于其易操作性、良好的抗菌性和较低的成本,已在食品行业应用[77] (图 2b)。弱酸性电解水是通过电解中间无膜的电解室内较稀的氯化钠溶液或盐酸溶液(2%~6%)制得,生成主要成分为次氯酸的酸性溶液(有效氯浓度为10×10-6~30×10-6,pH为5.0~6.5)[78]。LIU等[79]报道称,弱酸性电解水对腐败希瓦氏菌和腐生葡萄球菌均有良好的杀菌特性,可通过破坏菌体的细胞膜,降低菌体细胞内抗氧化酶活性,最终导致它们的死亡。TANTRATIAN等[80]将其良好的杀菌特性归因于电解时产生的次氯酸和生物活性成分对细胞的杀伤作用。此外,在食品行业中,弱酸性电解水也已被证实是绿色、安全、无污染的[81-82]。在保鲜应用方面,弱酸性电解水常作为海水鱼低温贮藏前的预处理技术,减少鱼表面初始微生物数量。LAN等[83]研究发现暗纹东方鲀经弱酸性电解水(pH为6.35,有效氯浓度为30×10-6)处理10 min后,在4 ℃贮藏下,嗜冷菌、假单胞菌、希瓦氏菌等微生物均生长缓慢,货架期可达到8 d。但也有研究[84]认为,弱酸性电解水技术会破坏海水鱼肌原纤维蛋白的二级结构,从而影响其品质特性。
2.4 涂膜技术海水鱼的涂膜技术是将预制作好的涂膜溶液通过喷淋或浸渍的方式包裹在鱼体表面后进行贮藏的技术,通过抑制鱼体贮藏期内各类腐败过程,达到延长货架期的目的[85](图 2c)。该技术所采用的涂膜物质是由某些生物活性成分和作为基材的多糖类生物聚合物组成,其在鱼体表面厚度一般<0.3 mm,且是可食用的[86-88]。组成涂膜的生物活性成分,如酚类物质等,可有效抑制海水鱼贮藏期内细菌、真菌的生长以及脂质氧化腐败作用[89-91]。而多糖类生物聚合物,如海藻酸钠等,一可有效隔离食品外界的紫外线、氧气和微生物,二可有效防止物理损伤,三可充分包裹生物活性成分,使其在贮藏期内持续释放,进而发挥更好的效果[85, 92]。DEMIRCAN等[93]研究发现,鲭鱼经含有质量浓度1%柠檬精油的壳聚糖涂膜溶液处理2 min,并在2 ℃条件下贮藏9 d,其生物胺含量可比未经涂层处理组降低30%~40%。LAN等[94]研究了含有牛至精油的果胶涂膜溶液对冷藏大黄鱼的品质影响,结果表明经涂膜技术可有效减缓大黄鱼贮藏时的水分迁移,并可抑制内源酶活性以及延缓蛋白质的氧化进程。同时,近年来人们对涂膜中多种生物活性成分的复配效果研究较多。在机理方面,张家涛等[95]探索了含溶菌酶和茶多酚的壳聚糖涂膜对水产品特定腐败菌——腐败希瓦氏菌的抑制机理。研究发现,该涂膜表现出良好的抑菌性能,与只含有单一生物活性成分的涂膜相比,处理后菌体严重变形,细胞壁溶解加大,损伤更严重。在保鲜应用方面,杨华等[96]研究了含丁香酚和月桂精油的亚麻籽胶-壳聚糖涂膜对冷藏三文鱼鱼片的保鲜影响。结果表明,丁香酚和月桂精油的复配使用具有一定的协同增效作用,此外两种基材的复配使用也可有效地延长生物保鲜剂的作用时间,最终可使三文鱼的货架期延长6 d,具有良好的保鲜前景。
2.5 气调包装技术气调包装技术近几十年来发展迅速,已广泛应用于海水鱼的保鲜[97](图 2d)。该技术是通过改变产品周围气体条件,抑制微生物和脂质氧化腐败过程,从而延长海水鱼的货架期[9]。通常而言,气调包装技术主要采用不同比例的N2、CO2和O2的混合气体组成产品周围的气体环境微环境。其中,N2作为一种惰性气体可抑制海水鱼的氧化腐败,O2可减少海水鱼体内的渗出物并保持肉色,CO2可抑制好氧SSOs的生长并可在水介质中生成H2CO3,降低海水鱼在贮藏期的pH,达到延长货架期的作用[98]。其他气体,如He、Ar、Xe、Ne等在气调包装中有所研究,但由于成本过高,在实际生产中应用较少[99]。混合气体中各成分的比例取决于海水鱼产品对O2和CO2的敏感性以及SSOs生长习性[100]。例如,LI等[101]认为抑制假单胞菌的生长需较高比例的CO2浓度,而SAMPELS[102]认为抑制肉毒杆菌的生长则需较高比例的O2浓度。气调包装技术在海水鱼保鲜应用中,CHAN等[103]研究发现,三文鱼经气调包装(CO2与N2体积比为3∶2)处理后,腐败微生物的生长受到抑制,产品始终维持较低的pH,4 ℃条件下货架期可至18~20 d。LI等[101]还报道了高CO2浓度MAP(CO2、N2、O2体积比为12∶7∶1)可以显著降低暗纹东方鲀冷藏过程中(4 ℃)中的挥发性盐基总氮、K值和菌落总数,并可有效降低蛋白质的降解。
真空包装(vacuum package,VP)是将产品周围空气尽可能抽出并密封产品,维持高度减压的外界环境。由于降低了包装中97%~99%的氧气浓度,真空包装可抑制产品脂质氧化速率,并导致需氧腐败菌的不耐受[104]。此外,真空包装在海水鱼不同肌肉类型中的保鲜效果种有一定差异。LAHRECHE等[105]研究了真空包装对4 ℃贮藏下金枪鱼普通肌和暗色肌的品质影响,结果表明,真空包装对金枪鱼普通肌中的微生物抑制效果更好,而对于脂质氧化的抑制,普通肌则表现更为出色。
3 总结与展望海水鱼作为一种常温下高度易腐食品,其品质、感官特性在贮藏时极易发生变化。因此,通过采取有效的保鲜技术来延长海水鱼的货架期十分重要。低温技术是最常用的保鲜手段。其中,冻藏可极大地延长海水鱼货架期(6~12个月),但对肌原纤维结构的破坏较大,海水鱼在解冻后感官品质较低,且贮藏设备运转时所需的能耗较高,获得的经济效益总体较低。冰温、超冰温和微冻技术具有良好的发展潜力,在海水鱼感官品质得到保证的同时又可获得较理想的货架期,但在贮藏时对环境参数和操作的要求较高,实际应用中还存在着一定的局限性。相较而言,冷藏技术的成本低,且海水鱼在贮藏期内可保持较高的感官品质,是经济效益最高的一种低温保鲜技术,但其货架期往往受限(5~7 d),产品只能在短时间内进行流通,延长海水鱼在冷藏过程中的货架期已成为众多学者的研究目标。从海水鱼的腐败因素出发,为抑制酶自溶、微生物生长和脂质氧化的速率,新兴保鲜技术(如高静水压技术、弱酸性电解水技术、涂膜技术等)在食品行业中逐渐发展起来。冷藏与这些新兴技术的结合可有效地延长海水鱼的货架期,甚至可达到常规冷藏货架期的2~3倍。但新兴保鲜技术在发挥积极作用的同时,也需注意其带来的负面影响,如可能会降低质地、影响色差等。
未来可以将多种新兴技术与冷藏技术相结合,探索出不同技术之间最佳的结合条件,研究其对货架期的影响。而对于其他低温保鲜技术而言,探究其品质劣变的机理,并由此研发相应的保鲜工艺以及精准保鲜的相应设备也是十分必要的。此外,还可解析脂质氧化与蛋白氧化的关系,以及水产物流过程中色泽、风味、鲜度、质地等品质劣变和腐败损耗的生物学基础等,以更深入地了解海水鱼腐败过程,从而创新性地引入新技术,来满足人们对海水鱼品质保持和货架期延长的追求。
| [1] |
PRABHAKAR P K, VATSA S, SRIVASTAV P P, et al. A comprehensive review on freshness of fish and assessment: Analytical methods and recent innovations[J]. Food Research International, 2020, 133: 109157. DOI:10.1016/j.foodres.2020.109157 |
| [2] |
MEI J, MA X, XIE J. Review on natural preservatives for extending fish shelf life[J]. Foods, 2019, 8(10): 490. DOI:10.3390/foods8100490 |
| [3] |
CHU Y M, TAN M T, YI Z K, et al. Shelf-life prediction of glazed large yellow croaker (Pseudosciaena crocea) during frozen storage based on arrhenius model and long-short-term memory neural networks model[J]. Fishes, 2021, 6(3): 39. DOI:10.3390/fishes6030039 |
| [4] |
蓝蔚青, 张炳杰, 周大鹏, 等. 超声联合微酸性电解水处理对真空包装海鲈鱼冷藏期间品质变化的影响[J]. 食品科学, 2022, 43(5): 62-68. LAN W Q, ZHANG B J, ZHOU D P, et al. Effect of ultrasonic combined with slightly acidic electrolyzed water treatment on quality changes of vacuum-packaged sea bass (Lateolabrax japonicas) during refrigerated storage[J]. Food Science, 2022, 43(5): 62-68. |
| [5] |
DAI W L, GU S Q, XU M J, et al. The effect of tea polyphenols on biogenic amines and free amino acids in bighead carp (Aristichthys nobilis) fillets during frozen storage[J]. LWT, 2021, 150: 111933. DOI:10.1016/j.lwt.2021.111933 |
| [6] |
ZHENG H, TANG H B, YANG C X, et al. Evaluation of the slow-release polylactic acid/polyhydroxyalkanoates active film containing oregano essential oil on the quality and flavor of chilled pufferfish (Takifugu obscurus) fillets[J]. Food Chemistry, 2022, 385: 132693. DOI:10.1016/j.foodchem.2022.132693 |
| [7] |
FAO. The state of world fisheries and aquaculture 2020[R]. Rome: FAO, 2020: 47-65.
|
| [8] |
TAVARES J, MARTINS A, FIDALGO L G, et al. Fresh fish degradation and advances in preservation using physical emerging technologies[J]. Foods, 2021, 10(4): 780. DOI:10.3390/foods10040780 |
| [9] |
KONTOMINAS M G, BADEKA A V, KOSMA I S, et al. Recent developments in seafood packaging technologies[J]. Foods, 2021, 10(5): 940. DOI:10.3390/foods10050940 |
| [10] |
JIANG Q X, YANG F, JIA S N, et al. The role of endogenous proteases in degrading grass carp (Ctenopharyngodon idella) myofibrillar structural proteins during ice storage[J]. LWT, 2022, 154: 112743. DOI:10.1016/j.lwt.2021.112743 |
| [11] |
王建强, 陈景华, 郝发义, 等. 冷链物流对鲜肉新鲜度的影响及智能检测[J]. 包装工程, 2022, 43(1): 148-157. WANG J Q, CHEN J H, HAO F Y, et al. Effects of cold chain logistics on meat freshness and intelligent detection[J]. Packaging Engineering, 2022, 43(1): 148-157. DOI:10.19554/j.cnki.1001-3563.2022.01.019 |
| [12] |
王慧平, 张欢, 陈倩, 等. 鱼肉内源性蛋白酶对其贮藏期品质影响的研究进展[J]. 食品工业科技, 2021, 42(19): 429-435. WANG H P, ZHANG H, CHEN Q, et al. Research progress on effects of endogenous protease on quality of fish during storage[J]. Science and Technology of Food Industry, 2021, 42(19): 429-435. DOI:10.13386/j.issn1002-0306.2020080312 |
| [13] |
BOZIARIS I S. Introduction to seafood processing - assuring quality and safety of seafood[M]//Seafood Processing: Technology, Quality and Safety. Chichester: John Wiley & Sons, 2014: 1-8.
|
| [14] |
LIU W R, MEI J, XIE J. Effect of locust bean gum-sodium alginate coatings incorporated with daphnetin emulsions on the quality of Scophthalmus maximus at refrigerated condition[J]. International Journal of Biological Macromolecules, 2021, 170: 129-139. DOI:10.1016/j.ijbiomac.2020.12.089 |
| [15] |
LU H, LIANG Y H, ZHANG X M, et al. Effects of cathepsins on gel strength and water-holding capacity of myofibrillar protein gels from bighead carp (Aristichthys nobilis) under a hydroxyl radical-generation oxidizing system[J]. Foods, 2022, 11(3): 330. DOI:10.3390/foods11030330 |
| [16] |
谢晶. 海产品保鲜贮运技术与冷链装备[M]. 北京: 科学出版社, 2019: 1-8. XIE J. Seafood preservation and storage technology and cold chain facilities[M]. Beijing: Science Press, 2019: 1-8. |
| [17] |
RATHOD N B, RANVEER R C, BENJAKUL S, et al. Recent developments of natural antimicrobials and antioxidants on fish and fishery food products[J]. Comprehensive Reviews in Food Science and Food Safety, 2021, 20(4): 4182-4210. DOI:10.1111/1541-4337.12787 |
| [18] |
PELLISSERY A J, VINAYAMOHAN P G, AMALARADJOU M A R, et al. Spoilage bacteria and meat quality[M]//BISWAS A K, MANDAL P K. Meat Quality Analysis. New York: Academic Press, 2020: 307-334.
|
| [19] |
WICKRAMASINGHE N N, RAVENSDALE J, COOREY R, et al. Transcriptional profiling of biofilms formed on chilled beef by psychrotrophic meat spoilage bacterium, Pseudomonas fragi 1793[J]. Biofilm, 2021, 3: 100045. DOI:10.1016/j.bioflm.2021.100045 |
| [20] |
ZHOU Q Q, LI P Y, FANG S Y, et al. Preservative effects of gelatin active coating containing eugenol and higher CO2 concentration modified atmosphere packaging on Chinese sea bass (Lateolabrax maculatus) during superchilling (-0.9 ℃) storage[J]. Molecules, 2020, 25(4): 871. DOI:10.3390/molecules25040871 |
| [21] |
FU L L, WANG C, LIU N N, et al. Quorum sensing system-regulated genes affect the spoilage potential of Shewanella baltica[J]. Food Research International, 2018, 107: 1-9. DOI:10.1016/j.foodres.2018.01.067 |
| [22] |
LI P Y, PENG Y F, MEI J, et al. Effects of microencapsulated eugenol emulsions on microbiological, chemical and organoleptic qualities of farmed Japanese sea bass (Lateolabrax japonicus) during cold storage[J]. LWT, 2020, 118: 108831. DOI:10.1016/j.lwt.2019.108831 |
| [23] |
BONO G, OKPALA C O R, VITALE S, et al. Effects of different ozonized slurry-ice treatments and superchilling storage (-1 ℃) on microbial spoilage of two important pelagic fish species[J]. Food Science & Nutrition, 2017, 5(6): 1049-1056. |
| [24] |
BEKAERT K, DEVRIESE L, MAES S, et al. Characterization of the dominant bacterial communities during storage of Norway lobster and Norway lobster tails (Nephrops norvegicus) based on 16S rDNA analysis by PCR-DGGE[J]. Food Microbiology, 2015, 46: 132-138. DOI:10.1016/j.fm.2014.06.022 |
| [25] |
ZHENG R H, XU X R, XING J L, et al. Quality evaluation and characterization of specific spoilage organisms of spanish mackerel by high-throughput sequencing during 0 ℃ cold chain logistics[J]. Foods, 2020, 9(3): 312. DOI:10.3390/foods9030312 |
| [26] |
GE Y, ZHU J, YE X, et al. Spoilage potential characterization of Shewanella and Pseudomonas isolated from spoiled large yellow croaker (Pseudosciaena crocea)[J]. Letters in Applied Microbiology, 2017, 64(1): 86-93. DOI:10.1111/lam.12687 |
| [27] |
PEI J X, MEI J, YU H J, et al. Effect of gum tragacanth-sodium alginate active coatings incorporated with epigallocatechin gallate and lysozyme on the quality of large yellow croaker at superchilling condition[J]. Frontiers in Nutrition, 2022, 8: 812741. DOI:10.3389/fnut.2021.812741 |
| [28] |
LIU X C, ZHANG Y M, LI D P, et al. Characterization of the microbiota in lightly salted bighead carp (Aristichthys nobilis) fillets stored at 4 ℃[J]. Food Microbiology, 2017, 62: 106-111. DOI:10.1016/j.fm.2016.10.007 |
| [29] |
PARLAPANI F F, MEZITI A, KORMAS K A, et al. Indigenous and spoilage microbiota of farmed sea bream stored in ice identified by phenotypic and 16S rRNA gene analysis[J]. Food Microbiology, 2013, 33(1): 85-89. DOI:10.1016/j.fm.2012.09.001 |
| [30] |
STAMATIS N, ARKOUDELOS J. Quality assessment of Scomber colias japonicus under modified atmosphere and vacuum packaging[J]. Food Control, 2007, 18(4): 292-300. DOI:10.1016/j.foodcont.2005.10.009 |
| [31] |
DALGAARD P, GRAM L, HUSS H H. Spoilage and shelf-life of cod fillets packed in vacuum or modified atmospheres[J]. International Journal of Food Microbiology, 1993, 19(4): 283-294. DOI:10.1016/0168-1605(93)90020-H |
| [32] |
EMBORG J, LAURSEN B G, RATHJEN T, et al. Microbial spoilage and formation of biogenic amines in fresh and thawed modified atmosphere-packed salmon (Salmo salar) at 2 ℃[J]. Journal of Applied Microbiology, 2002, 92(4): 790-799. DOI:10.1046/j.1365-2672.2002.01588.x |
| [33] |
HOVDA M B, LUNESTAD B T, SIVERTSVIK M, et al. Characterisation of the bacterial flora of modified atmosphere packaged farmed Atlantic cod (Gadus morhua) by PCR-DGGE of conserved 16S rRNA gene regions[J]. International Journal of Food Microbiology, 2007, 117(1): 68-75. DOI:10.1016/j.ijfoodmicro.2007.02.022 |
| [34] |
RUDI K, MAUGESTEN T, HANNEVIK S E, et al. Explorative multivariate analyses of 16S rRNA gene data from microbial communities in modified-atmosphere-packed salmon and coalfish[J]. Applied and Environmental Microbiology, 2004, 70(8): 5010-5018. DOI:10.1128/AEM.70.8.5010-5018.2004 |
| [35] |
KONTOMINAS M G, BADEKA A V, KOSMA I S, et al. Innovative seafood preservation technologies: recent developments[J]. Animals, 2021, 11(1): 92. DOI:10.3390/ani11010092 |
| [36] |
NIE X B, ZHANG R C, CHENG L L, et al. Mechanisms underlying the deterioration of fish quality after harvest and methods of preservation[J]. Food Control, 2022, 135: 108805. DOI:10.1016/j.foodcont.2021.108805 |
| [37] |
NATHAN C, CUNNINGHAM-BUSSEL A. Beyond oxidative stress: an immunologist's guide to reactive oxygen species[J]. Nature Reviews Immunology, 2013, 13(5): 349-361. DOI:10.1038/nri3423 |
| [38] |
TSIRONI T, HOUHOULA D, TAOUKIS P. Hurdle technology for fish preservation[J]. Aquaculture and Fisheries, 2020, 5(2): 65-71. DOI:10.1016/j.aaf.2020.02.001 |
| [39] |
ZHOU G H, XU X L, LIU Y. Preservation technologies for fresh meat - a review[J]. Meat Science, 2010, 86(1): 119-128. DOI:10.1016/j.meatsci.2010.04.033 |
| [40] |
DONG Z, LUO C, GUO Y M, et al. Characterization of new active packaging based on PP/LDPE composite films containing attapulgite loaded with Allium sativum essence oil and its application for large yellow croaker (Pseudosciaena crocea) fillets[J]. Food Packaging and Shelf Life, 2019, 20: 100320. DOI:10.1016/j.fpsl.2019.100320 |
| [41] |
NISAR T, YANG X, ALIM A, et al. Physicochemical responses and microbiological changes of bream (Megalobrama ambycephala) to pectin based coatings enriched with clove essential oil during refrigeration[J]. International Journal of Biological Macromolecules, 2019, 124: 1156-1166. DOI:10.1016/j.ijbiomac.2018.12.005 |
| [42] |
ZHAO X, CHEN L, WONGMANEEPRATIP W, et al. Effect of vacuum impregnated fish gelatin and grape seed extract on moisture state, microbiota composition, and quality of chilled seabass fillets[J]. Food Chemistry, 2021, 354: 129581. DOI:10.1016/j.foodchem.2021.129581 |
| [43] |
刘文, 岳琪琪, 龚恒, 等. 包装调控方式对冷鲜鲟鱼肉微生物的抑制作用[J]. 包装工程, 2020, 41(9): 59-66. LIU W, YUE Q Q, GONG H, et al. Inhibition of microorganisms in chilled fresh sturgeon meat by packaging regulation[J]. Packaging Engineering, 2020, 41(9): 59-66. DOI:10.19554/j.cnki.1001-3563.2020.09.009 |
| [44] |
张冉, 杨丽丽, 李秋莹, 等. TP-Lips/LZM-CS复合缓释涂膜对美国红鱼鱼片贮藏品质的影响[J]. 包装工程, 2020, 41(1): 44-50. ZHANG R, YANG L L, LI Q Y, et al. Effects of TP-Lips/LZM-CS sustained-release composite coating on the storage quality of Sciaenops ocellatus fillets[J]. Packaging Engineering, 2020, 41(1): 44-50. DOI:10.19554/j.cnki.1001-3563.2020.01.007 |
| [45] |
张泽凌, 朱亚珠, 陈雁平. 不同贮藏条件对红娘鱼(Lepidotrigla microptera)品质变化规律的研究[J]. 肉类工业, 2019(10): 16-19. ZHANG Z L, ZHU Y Z, CHEN Y P. Study on the law of quality changes of the Lepidotrigla microptera under different storage conditions[J]. Meat Industry, 2019(10): 16-19. DOI:10.3969/j.issn.1008-5467.2019.10.006 |
| [46] |
CHAN S S, ROTABAKK B T, LØVDAL T, et al. Skin and vacuum packaging of portioned Atlantic salmon originating from refrigerated seawater or traditional ice storage[J]. Food Packaging and Shelf Life, 2021, 30: 100767. DOI:10.1016/j.fpsl.2021.100767 |
| [47] |
张博博, 孙钦, 许朝阳, 等. 超冰温技术研究进展[J]. 保鲜与加工, 2020, 20(6): 227-232. ZHANG B B, SUN Q, XU Z Y, et al. Research advance on controlled supercooling-point technology[J]. Storage and Process, 2020, 20(6): 227-232. |
| [48] |
KANG T, YOU Y, JUN S. Supercooling preservation technology in food and biological samples: a review focused on electric and magnetic field applications[J]. Food Science and Biotechnology, 2020, 29(3): 303-321. DOI:10.1007/s10068-020-00750-6 |
| [49] |
KANG T, HOPTOWIT R, JUN S. Effects of an oscillating magnetic field on ice nucleation in aqueous iron-oxide nanoparticle dispersions during supercooling and preservation of beef as a food application[J]. Journal of Food Process Engineering, 2020, 43(11): e13525. |
| [50] |
李剑, 高向阳, 马丽萍. 冰温技术在食品保藏中的应用与研究进展[J]. 江苏调味副食品, 2021(4): 10-13. LI J, GAO X Y, MA L P. Application and research progress of ice temperature technology in food storage[J]. Jiangsu Condiment and Subsidiary Food, 2021(4): 10-13. |
| [51] |
KANG T Y, SHAFEL T, LEE D Y, et al. Quality retention of fresh tuna stored using supercooling technology[J]. Foods, 2020, 9(10): 1356. DOI:10.3390/foods9101356 |
| [52] |
SULTANA T, LEE J I, PARK J H, et al. Supercooling storage for the transplantable sources from the rat and the rabbit: a preliminary report[J]. Transplantation Proceedings, 2018, 50(4): 1178-1182. DOI:10.1016/j.transproceed.2018.01.046 |
| [53] |
PARK D H, LEE S, LEE J, et al. Stepwise cooling mediated feasible supercooling preservation to extend freshness of mackerel fillets[J]. LWT, 2021, 152: 112389. DOI:10.1016/j.lwt.2021.112389 |
| [54] |
QIN L R, WU Y X, CHEN J W, et al. Effects of superchilling on quality of crayfish (Procambarus clarkii): water migration, biogenic amines accumulation, and nucleotides catabolism[J]. International Journal of Food Science and Technology, 2022, 57(1): 506-515. DOI:10.1111/ijfs.15438 |
| [55] |
ZHAO N N, YANG X Q, LI Y J, et al. Effects of protein oxidation, cathepsins, and various freezing temperatures on the quality of superchilled sturgeon fillets[J]. Marine Life Science & Technology, 2022, 4(1): 117-126. |
| [56] |
CLAUSSEN I C, INDERGÅRD E, GRINDE M. Comparative Life Cycle Assessment (LCA) of production and transport of chilled versus superchilled haddock (Melanogrammus aeglefinus) fillets from Norway to France[J]. Procedia Food Science, 2011, 1: 1091-1098. DOI:10.1016/j.profoo.2011.09.163 |
| [57] |
HOANG H M, BROWN T, INDERGARD E, et al. Life cycle assessment of salmon cold chains: comparison between chilling and superchilling technologies[J]. Journal of Cleaner Production, 2016, 126: 363-372. |
| [58] |
TRYGGVASON R I, MARGEIRSSON B, KARLSDÓTTIR M, et al. Effects of food container depth on the quality and yield of superchilled and iced Atlantic salmon[J]. Packaging Technology and Science, 2020, 33(8): 289-302. |
| [59] |
BAHUAUD D, MØRKØRE T, LANGSRUD Ø, et al. Effects of 1.5 ℃ Super-chilling on quality of Atlantic salmon (Salmo salar) pre-rigor Fillets: Cathepsin activity, muscle histology, texture and liquid leakage[J]. Food Chemistry, 2008, 111(2): 329-339. |
| [60] |
ZHAO J, LV W J, WANG J L, et al. Effects of tea polyphenols on the post-mortem integrity of large yellow croaker (Pseudosciaena crocea) fillet proteins[J]. Food Chemistry, 2013, 141(3): 2666-2674. |
| [61] |
EFSA Panel on Biological Hazards (BIOHAZ), KOUTSOUMANIS K, ALLENDE A, et al. The use of the so-called 'superchilling' technique for the transport of fresh fishery products[J]. EFSA Journal, 2021, 19(1): e06378. |
| [62] |
谢晶, 程浩. 冻藏水产品蛋白质变化与控制措施研究进展[J]. 上海海洋大学学报, 2021, 30(5): 905-912. XIE J, CHENG H. Effect of frozen storage on the myofibrillar protein of aquatic products[J]. Journal of Shanghai Ocean University, 2021, 30(5): 905-912. |
| [63] |
BROWN P, DAVE D. Current freezing and thawing scenarios employed by North Atlantic fisheries: their potential role in Newfoundland and Labrador's northern cod (Gadus morhua) fishery[J]. PeerJ, 2021, 9: e12526. |
| [64] |
TAN M T, YE J X, XIE J. Freezing-induced myofibrillar protein denaturation: Role of pH change and freezing rate[J]. LWT, 2021, 152: 112381. |
| [65] |
边楚涵, 谢晶. 冰晶对冻结水产品品质的影响及抑制措施[J]. 包装工程, 2022, 43(3): 105-112. BIAN C H, XIE J. Effects of ice crystal on frozen aquatic products and its inhibition measures[J]. Packaging Engineering, 2022, 43(3): 105-112. |
| [66] |
CROPOTOVA J, MOZURAITYTE R, STANDAL I B, et al. Superchilled, chilled and frozen storage of Atlantic mackerel (Scomber scombrus) fillets - changes in texture, drip loss, protein solubility and oxidation[J]. International Journal of Food Science & Technology, 2019, 54(6): 2228-2235. |
| [67] |
MOZURAITYTE R, STANDAL I B, CROPOTOVA J, et al. Superchilled, chilled and frozen storage of Atlantic mackerel (Scomber scombrus) - effect on lipids and low molecular weight metabolites[J]. International Journal of Food Science & Technology, 2021, 56(4): 1918-1928. |
| [68] |
DUUN A S, RUSTAD T. Quality of superchilled vacuum packed Atlantic salmon (Salmo salar) fillets stored at -1.4 and -3.6 ℃[J]. Food Chemistry, 2008, 106(1): 122-131. |
| [69] |
DUUN A, RUSTAD T. Quality changes during superchilled storage of cod (Gadus morhua) fillets[J]. Food Chemistry, 2007, 105(3): 1067-1075. |
| [70] |
CHANG K L B, CHANG J, SHIAU C Y, et al. Biochemical, microbiological, and sensory changes of sea bass (Lateolabrax japonicus) under partial freezing and refrigerated storage[J]. Journal of Agricultural & Food Chemistry, 1998, 46(2): 682-686. |
| [71] |
TOKAY F G, ALP A C, YERLIKAYA P. Production and shelf life of restructured fish meat binded by microbial transglutaminase[J]. LWT, 2021, 152: 112369. |
| [72] |
RASTOGI N K, RAGHAVARAO K S M S, BALASUBRAMANIAM V M, et al. Opportunities and challenges in high pressure processing of foods[J]. Critical Reviews in Food Science and Nutrition, 2007, 47(1): 69-112. |
| [73] |
HASSOUN A, SIDDIQUI S A, SMAOUI S, et al. Seafood processing, preservation, and analytical techniques in the age of industry 4.0[J]. Applied Sciences, 2022, 12(3): 1703. |
| [74] |
ERKAN N, VRETENER G, ALPAS H, et al. Effect of high hydrostatic pressure (HHP) treatment on physicochemical properties of horse mackerel (Trachurus trachurus)[J]. Food and Bioprocess Technology, 2011, 4(7): 1322-1329. |
| [75] |
KUNG H F, LIN C S, LIU S S, et al. High pressure processing extend the shelf life of milkfish flesh during refrigerated storage[J]. Food Control, 2022, 134: 108768. |
| [76] |
FERNANDES P A R, MOREIRA S A, FIDALGO L G, et al. Food preservation under pressure (Hyperbaric storage) as a possible improvement/alternative to refrigeration[J]. Food Engineering Reviews, 2015, 7(1): 1-10. |
| [77] |
LIU L, LAN W Q, PU T T, et al. Combining slightly acidic electrolyzed water and slurry ice to prolong the shelf‐life of mackerel (Pneumatophorus japonicus)[J]. Journal of Food Processing and Preservation, 2021, 45(9): e15762. |
| [78] |
WANG H H, DUAN D B, WU Z Y, et al. Primary concerns regarding the application of electrolyzed water in the meat industry[J]. Food Control, 2019, 95: 50-56. |
| [79] |
LIU L, LAN W Q, WANG Y B, et al. Antibacterial activity and mechanism of slightly acidic electrolyzed water against Shewanella putrefaciens and Staphylococcus saprophytic[J]. Biochemical and Biophysical Research Communications, 2022, 592: 44-50. |
| [80] |
TANTRATIAN S, KAEPHEN K. Shelf-life of shucked oyster in epigallocatechin-3-gallate with slightly acidic electrolyzed water washing under refrigeration temperature[J]. LWT, 2020, 118: 108733. |
| [81] |
马江林, 木泰华, 张苗. 超声波与微酸性电解水在食品杀菌保鲜中的应用研究进展[J]. 食品安全质量检测学报, 2021, 12(13): 5244-5250. MA J L, MU T H, ZHANG M. Research progress on application of ultrasonic and slightly acidic electrolyzed water in food sterilization and preservation[J]. Journal of Food Safety and Quality, 2021, 12(13): 5244-5250. |
| [82] |
ZHANG C L, ZHANG Y Y, ZHAO Z Y, et al. The application of slightly acidic electrolyzed water in pea sprout production to ensure food safety, biological and nutritional quality of the sprout[J]. Food Control, 2019, 104: 83-90. |
| [83] |
LAN W Q, SUN Y Q, FENG H J, et al. Effects of slightly acidic electrolyzed water pretreatment combined with compound bio-preservatives on quality and microbiota changes of refrigerated obscure pufferfish (Takifugu obscurus)[J]. Journal of Food Processing and Preservation, 2022, 46(2): e16287. |
| [84] |
IRAM A, WANG X M, DEMIRCI A. Electrolyzed oxidizing water and its applications as sanitation and cleaning agent[J]. Food Engineering Reviews, 2021, 13(2): 411-427. |
| [85] |
CHAKRABORTY P, NATH D, HOQUE M, et al. Biopolymer-based antimicrobial coatings for aquatic food products: A review[J]. Journal of Food Processing and Preservation, 2022, 46(4): e16465. |
| [86] |
DÍAZ-MONTES E, CASTRO-MUÑOZ R. Edible films and coatings as food-quality preservers: An overview[J]. Foods, 2021, 10(2): 249. |
| [87] |
CASTRO-MUÑOZ R, GONZÁLEZ-VALDEZ J. New trends in biopolymer-based membranes for pervaporation[J]. Molecules, 2019, 24(19): 3584. |
| [88] |
MORALES-JIMÉNEZ M, GOUVEIA L, YÁÑEZ-FERNÁNDEZ J, et al. Production, preparation and characterization of microalgae-based biopolymer as a potential bioactive film[J]. Coatings, 2020, 10(2): 120. |
| [89] |
SHAHIDI F, HOSSAIN A. Preservation of aquatic food using edible films and coatings containing essential oils: a review[J]. Critical Reviews in Food Science and Nutrition, 2022, 62(1): 66-105. |
| [90] |
YONG H M, LIU J. Active packaging films and edible coatings based on polyphenol-rich propolis extract: A review[J]. Comprehensive Reviews in Food Science and Food Safety, 2021, 20(2): 2106-2145. |
| [91] |
王倩, 蓝蔚青, 侯旻, 等. 银杏叶提取液对腐生葡萄球菌的作用机理[J]. 食品与生物技术学报, 2018, 37(9): 971-976. WANG Q, LAN W Q, HOU M, et al. Antimicrobial mechanism of Ginkgo biloba leaf extracts (GBLE) against Saprophytic staphylococcus[J]. Journal of Food Science and Biotechnology, 2018, 37(9): 971-976. |
| [92] |
SAHRAEE S, MILANI J M, REGENSTEIN J M, et al. Protection of foods against oxidative deterioration using edible films and coatings: A review[J]. Food Bioscience, 2019, 32: 100451. |
| [93] |
DEMIRCAN B, ÁZDESTAN OCAK Á. The effects of ethyl lauroyl arginate and lemon essential oil added edible chitosan film coating on biogenic amines formation during storage in mackerel fillets[J]. Journal of Food Processing and Preservation, 2021, 45(5): e15454. |
| [94] |
LAN W Q, SUN Y Q, CHEN M L, et al. Effects of pectin combined with plant essential oils on water migration, myofibrillar proteins and muscle tissue enzyme activity of vacuum packaged large yellow croaker (Pseudosciaena crocea) during ice storage[J]. Food Packaging and Shelf Life, 2021, 30: 100699. |
| [95] |
张家涛, 武娇, 杨华, 等. 原位合成纳米SiOx/LZM/TP-CS涂膜对腐败希瓦氏菌的抑菌机理[J]. 包装工程, 2021, 42(23): 76-83. ZHANG J T, WU J, YANG H, et al. Antibacterial mechanism of the in-situ synthesis of nano-SiOx/LZM/TP-CS composite coatings against Shewanella putrefaciens[J]. Packaging Engineering, 2021, 42(23): 76-83. |
| [96] |
杨华, 王雅妮, 孙晓冬, 等. FG/CS逐层复合保鲜涂膜对三文鱼鱼片品质的影响[J]. 包装工程, 2022, 43(1): 98-105. YANG H, WANG Y N, SUN X D, et al. Effects of FG/CS layer-by-layer composite coatings on storage quality of Salmon fillets[J]. Packaging Engineering, 2022, 43(1): 98-105. |
| [97] |
AMARAL R A, PINTO C A, LIMA V, et al. Chemical-based methodologies to extend the shelf life of fresh fish—a review[J]. Foods, 2021, 10(10): 2300. |
| [98] |
KONTOMINAS M G. Packaging | modified atmosphere packaging of foods[M]//Encyclopedia of Food Microbiology. 2nd ed. New York: Academic Press, 2014: 1012-1016.
|
| [99] |
DJENANE D, RONCALÉS P. Carbon monoxide in meat and fish packaging: advantages and limits[J]. Foods, 2018, 7(2): 12. |
| [100] |
HUSSAIN M A, SUMON T A, MAZUMDER S K, et al. Essential oils and chitosan as alternatives to chemical preservatives for fish and fisheries products: A review[J]. Food Control, 2021, 129: 108244. |
| [101] |
LI P Y, TAN M T, WANG J B, et al. Influence of high CO2 modified atmosphere packaging on some quality characteristics of fresh farmed pufferfish (Takifugu obscurus) during refrigerated storage[J]. Czech Journal of Food Sciences, 2020, 38(2): 123-130. |
| [102] |
SAMPELS S. The effects of storage and preservation technologies on the quality of fish products: a review[J]. Journal of Food Processing and Preservation, 2015, 39(6): 1206-1215. |
| [103] |
CHAN S S, SKARE M, ROTABAKK B T, et al. Evaluation of physical and instrumentally determined sensory attributes of Atlantic salmon portions packaged in modified atmosphere and vacuum skin[J]. LWT, 2021, 146: 111404. |
| [104] |
姚倩儒, 陈历水, 李慧, 等. 冷鲜肉保鲜包装技术现状和发展趋势[J]. 包装工程, 2021, 42(9): 194-200. YAO Q R, CHEN L S, LI H, et al. Current situation and development trend of packaging technology for chilled fresh meat[J]. Packaging Engineering, 2021, 42(9): 194-200. |
| [105] |
LAHRECHE T, DURMUŞ M, KOSKER A R, et al. Effects of different plant (Marjoram and Olive leaf) extracts on quality characteristics of red and ordinary muscles of vacuum—packaged tuna—like fillets[J]. Applied Food Research, 2022, 2(1): 100034. |
2. Shanghai Professional Technology Service Platform on Cold Chain Equipment Performance and Energy Saving Evaluation, Shanghai 201306, China;
3. Shanghai Engineering Research Center of Aquatic Product Processing and Preservation, Shanghai 201306, China;
4. National Experimental Teaching Demonstration Center for Food Science and Engineering, Shanghai Ocean University, Shanghai 201306, China
 2022,
Vol. 31
2022,
Vol. 31

