2. 天津农学院 天津市水产生态及养殖重点实验室, 天津 300384;
3. 天津农学院 水产生态与养殖国家级实验教学示范中心, 天津 300384;
4. 上海海洋大学 海洋科学学院, 上海 201306;
5. 天津市水产研究所, 天津 300221
性成熟是渔业资源发育过程的重要阶段, 是渔业资源从幼体发育到成体并开始参与繁殖活动的重要转折[1]。初次性成熟体长(群体中处于该体长的个体已有50%性成熟, L50)作为普遍使用的性成熟指标[2], 被广泛应用于渔业资源的生长模式变动分析[3]、最大体长估算[4]、最小开捕规格确定[5]等方面, 是渔业资源评估和管理策略风险评价的关键参数之一[6-7], 为渔业资源的可持续利用提供了重要参考。
为获得初次性成熟体长的可靠信息, 学者们构建了各种形式的估算方法[8]。KORANTENG[9]基于性腺发育特征估算了欧洲鳀(Engraulis encrasicolus)的初次性成熟体长, 并提出该种类商业捕捞使用渔具的最小网目规格; FONTOURA等[10]认为在生长过程中, 性成熟会影响渔业资源的体长-体质量关系, 从而建立了包含初次性成熟体长的多项式体长-体质量关系, 并用于巴西宝莲灯鱼(Cheirodon ibicuhiensis)初次性成熟体长估算; MESQUITA等[11]指出雌性普通黄道蟹(Cancer pagurus)的性成熟会引起身体形态变化, 进而应用广义线性模型估算初次性成熟头胸甲宽; FROESE等[12]利用世界鱼类数据库(http://www.fishbase.org)中265个种类的467组成对的初次性成熟体长和von Bertalanffy生长方程参数渐近体长数据建立相关关系, 以用于在缺少恰当信息时的渔业资源初次性成熟体长估算。其中基于性腺发育的初次性成熟体长估算方法, 因其具有较好的精度[13], 在渔业资源研究中被广泛使用。
口虾蛄(Oratosquilla oratoria)隶属节肢动物门(Arthropoda)甲壳纲(Crustacea)口足目(Stomadistaloda)虾蛄科(Squillidae)口虾蛄属(Oratosquilla), 是多年生海产经济甲壳类[14], 为西北太平洋沿海各国重要的渔业捕捞对象之一[15]。口虾蛄1龄即可性成熟[16], 繁殖群体以2龄和3龄个体为主[17], 繁殖期从5月初到8月初[18]。口虾蛄渔业是我国近海渔业的重要组成部分, 其渔获量在我国近海渔获中占有重要比例[19]。有资料显示, 我国近海口虾蛄资源已呈明显的小型化趋势[20], 资源利用正面临逐渐增加的压力。国内学者对口虾蛄开展了广泛的研究[14, 16], 但尚未有基于性腺发育信息的初次性成熟体长的报道。本研究基于性腺发育估算雌性口虾蛄初次性成熟体长(L50), 以丰富口虾蛄繁殖生物学信息, 为开展渤海湾口虾蛄资源评估、确定最小可捕体长和渔业管理提供科学依据。
1 材料与方法 1.1 数据来源研究用口虾蛄来源于渤海湾(117°30′E~118°20′E, 38°25′N~39°20′N)渔业资源科学调查获得的渔获物样品, 调查以单船底层拖网的形式在2018年4—11月(除5月)逐月开展, 累计获得口虾蛄6 731尾。调查船总吨位64 t, 主机功率176 kW, 拖网网口宽度10 m, 囊网网目尺寸20 mm, 拖网调查作业平均拖速2.5 kn, 拖曳时间1 h。口虾蛄样品在低温状态下运回实验室, 经性别鉴定后进行头胸甲长、体长、全长和体质量等性状测量[21]; 对雌性口虾蛄个体解剖, 根据KODAMA等[22]的方法确定性腺发育分期, 称量性腺质量。指标测量时, 长度测量精确到0.1 cm, 质量测量精确到0.01 g。
1.2 研究方法 1.2.1 逻辑斯蒂模型基于雌性口虾蛄性腺发育分期, 确定性成熟的性腺发育分期判别标准[23-26]。个体性腺发育分期低于标准的为性未成熟, 性腺发育分期处于或者高于标准的为性成熟[27-28]。以普遍采用的长度间隔1 cm[14, 23]对口虾蛄体长进行分组, 统计各体长组中雌性性成熟个体数量占相应体长组雌性个体总量的比例。应用逻辑斯蒂方程拟合各体长组的性成熟比例与对应体长组的体长中值[2, 29], 以估算雌性口虾蛄初次性成熟体长。逻辑斯蒂模型如下:
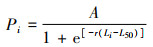 (1)
(1)
式中: Pi和Li分别为第i体长组对应的雌性口虾蛄性成熟个体的比例及该组的体长中值; A为性成熟比例渐近值; r为性成熟曲线系数; L50为50%雌性个体性成熟的体长(即初次性成熟体长)。
1.2.2 模型中性成熟比例渐近值以往的渔业资源初次性成熟体长研究, 逻辑斯蒂模型中性成熟比例渐近值多取为固定值1[30-31], 但有学者指出, 因种类而异, 该值并不总是为1[2, 8]。本研究为更加准确地揭示渤海湾雌性口虾蛄性成熟比例特征, 对于性成熟比例渐近值(A)的取值采用2种方法, 一种是取为固定值1, 另一种是将其作为待估参数, 与性成熟曲线系数(r)和初次性成熟体长(L50)同样处理, 在使用模型进行数据拟合的过程中一并进行估算[2, 24]。
1.2.3 性成熟判别标准普遍认为性成熟群体应包括在繁殖季节全部潜在的产卵个体[8, 32]。但是, 在已开展的性成熟研究中, 尚未有统一的性成熟判别标准[32-33]。以往基于性腺发育阶段判别个体是否处于性成熟时, 不同研究中采取的性成熟判别标准也不同[30, 33]。即使研究对象是同一种生物, 判别标准的差异也存在于各研究之间[23-26]。尽管这些差异与性腺发育被划分的阶段数有关[28, 34], 但标准的确定更多时候是因研究者的理解而异的[35-36]。大量文献[23, 25, 37]显示, 渔业资源性未成熟和性成熟的判别常以Ⅱ期、Ⅲ期或Ⅳ期为划分标准, 相对应的是性腺发育阶段为Ⅱ期及以上、Ⅲ期及以上或Ⅳ期及以上的个体为性成熟。本研究为更丰富地提供渤海湾雌性口虾蛄性成熟信息, 分别采取上述3种性成熟判别标准进行初次性成熟体长估算。
1.2.4 数据分析为计算逻辑斯蒂模型中参数的标准误, 对雌性口虾蛄样本采取无放回取样, 取样量为原始样本量的50%, 进行性成熟比例和对应体长组体长中值拟合, 重复30次, 30次估计值的标准差作为参数的标准误[10, 38]。
不同建模方法的待估计参数数量存在差异, 因此使用赤池信息准则(akaike's information criterion, AIC)[39]作为逻辑斯蒂曲线拟合效果的评价标准。所有的数据分析使用R 4.1.2软件[40]完成。
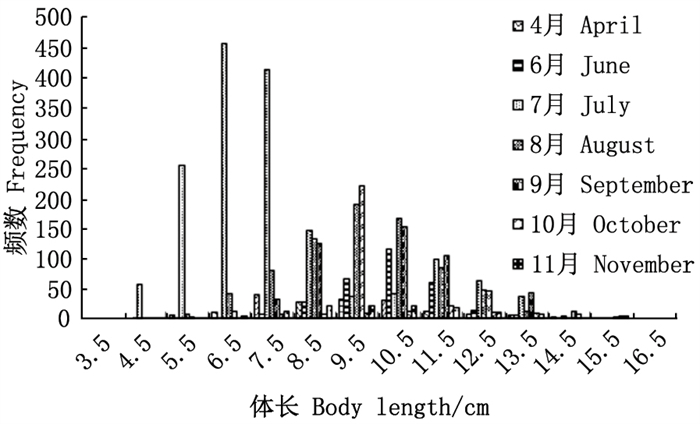
2 结果 2.1 样本结构及体长特征本研究累计收集雌性口虾蛄样本3 690尾, 约占样本总量的54.82%。各月雌性个体样本量中10月最少, 11月与10月的样本量相当, 7月份的样本最多。雌性口虾蛄头胸甲长为0.08~0.34 cm, 体长为4.02~15.83 cm, 全长为4.21~16.58 cm, 体质量为0.86~59.72 cm。其中, 雌性口虾蛄主要集中在体长中值为6.50~11.50 cm的体长组, 其中7月份的优势体长为中值5.50~8.50 cm组, 其他月份的优势体长组主要分布在中值7.50~11.50 cm组(图 1)。
|
图 1 雌性口虾蛄的体长分布 Fig. 1 Frequency distribution of length of female mantis shrimp |
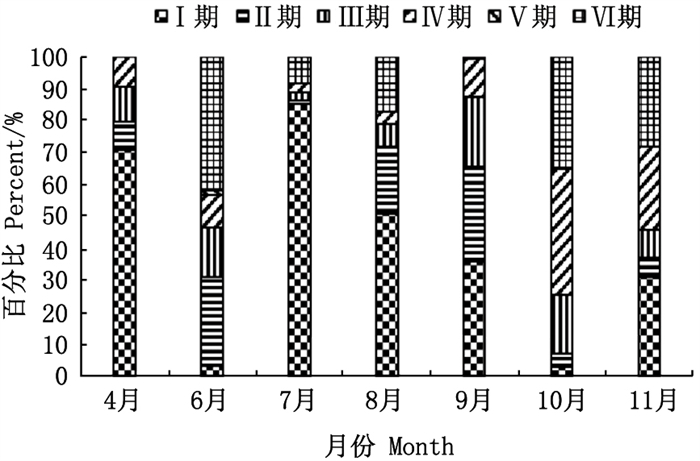
性腺发育处于Ⅰ~Ⅵ期的雌性口虾蛄个体在样本中均有发现。性腺发育处于完全成熟、临近产卵的Ⅴ期个体数量最少, 仅占0.16%, 处于Ⅰ期的个体数量最多, 约为58.26%(图 2)。统计各期的雌性口虾蛄来源, 性腺发育处于Ⅰ期的样本主要来源于7月, 贡献率达62.94%, 约占当月雌性口虾蛄个体数量的85.01%。性腺发育处于Ⅵ期的样本主要来源于6—8月, 贡献率为28.93%±1.72%, 其中7月的贡献率最大, 为30.53%, 6月和8月的Ⅵ期样本分别占各自月份雌性口虾蛄个体数量的41.90%和17.23%。4月未发现性腺发育处于Ⅴ期和Ⅵ期的雌性口虾蛄。
|
图 2 各月的性腺发育阶段状况 Fig. 2 Composition of gonadal development stages for female mantis shrimp in each month |
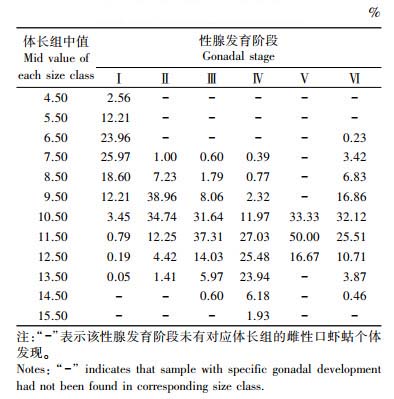
处于不同性腺发育期的雌性口虾蛄个体组成具有明显的体长特征(表 1)。性腺发育各期口虾蛄的体长范围相比较而言, 性腺发育Ⅰ期个体的体长范围最大, 覆盖了除两个最大体长组的其他全部10个, 以中值为5.50~9.50 cm的体长组为主, 约占该发育期个体总量的92.96%。性腺发育Ⅱ期、Ⅲ期和Ⅳ期个体体长最小值全部位于中值为7.50 cm体长组。Ⅱ期个体体长以9.50~11.50 cm为主, Ⅲ期以10.50~12.50 cm为主, Ⅳ期以11.50~13.50 cm为主, 分别占各自性腺发育期个体数量的85.94%、82.99%和76.45%。Ⅴ期的体长范围最小, 仅包含了中值为10.50~12.50 cm的体长组。Ⅵ期个体体长范围从中值6.50 cm到中值14.50 cm组, 以9.50~11.50 cm体长中值组为主。
|
表 1 各性腺发育阶段的体长组成 Tab.1 Distribution of length at each gonadal development stage for female mantis shrimp |
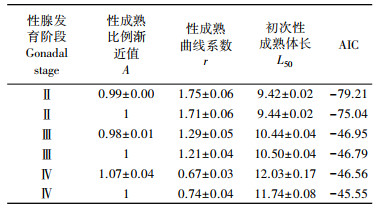
基于不同性成熟判定标准及性成熟比例渐近值处理方式的逻辑斯蒂模型拟合结果见表 2。当模型中性成熟比例渐近值为待估参数时, 以性腺发育Ⅱ期、Ⅲ期和Ⅳ期作为性成熟标志, 得到的雌性口虾蛄初次性成熟体长分别为(9.42±0.02)cm, (10.44±0.04)cm和(12.03±0.17)cm。当模型中性成熟比例渐近值取1时, 各性成熟标准下得到的初次性成熟体长分别为(9.44±0.02)cm, (10.50±0.04)cm和(11.74±0.08)cm。性成熟判定标准选取的性腺发育分期越大, 模型的拟合效果逐渐降低。对比两种性成熟比例渐近值处理方法, 采用可变性成熟比例渐近值建模, 得到的模型拟合效果均优于采取固定值1的处理方式。基于赤池信息准则, 将性腺发育Ⅱ期作为性成熟判定标准, 采用可变性成熟比例渐近值得到的模型拟合结果最优(AIC=-79.21), 雌性口虾蛄初次性成熟体长(L50)为(9.42±0.02)cm。
|
表 2 性成熟曲线模型的参数及比较 Tab.2 Estimates of the size at first maturation (L50) for female mantis shrimp using different maturation criteria and asymptote |
逻辑斯蒂模型被广泛用于渔业资源初次性成熟体长估算[41-42]。以往普遍认为随着体长的增加, 性成熟比例也增加并逐渐达到最大值100%[9, 22]。因此在研究过程中, 性成熟比例渐近值通常固定取值为1[22-23, 25]。但是, 有学者指出, 由于摄食不足、营养条件差或疾病等因素, 潜在性成熟个体而不能性成熟或跳过产卵阶段的现象在许多生物中存在[43]。这些种类的生物即使在繁殖盛期, 总是存在具有完全满足参与繁殖行为体长特征的个体仍有性未成熟的现象, 或是基于最严谨的性腺发育标准被判定为性成熟的个体仍不发生产卵行为[8]。因此应用逻辑斯蒂模型估算渔业资源初次性成熟体长时, 性成熟比例渐近值并不一定都是1。另外, 在各性腺发育期内, 随着体长增加, 渔业资源的数量普遍呈先增加后减小的倒扣钟特点[2, 11]。当采用逻辑斯蒂模型进行性成熟比例与体长关系拟合时, 即使最大体长的个体也存在不能被全部识别为100%性成熟的可能[2]。因此, 在估算初次性成熟体长时, 机械地将逻辑斯蒂模型中性成熟比例渐近值取为1, 必然会造成结果的偏差[2, 8]。本研究发现, 在繁殖盛期存在大个体的雌性口虾蛄性腺发育处于Ⅰ期的现象; 因此, 认为使用逻辑斯蒂模型拟合雌性口虾蛄性成熟比例与体长关系时, 模型中性成熟比例渐近值设定为待估参数是更恰当的。本研究建模的结果也证实了这点, 对比2种性成熟比例渐近值(A)赋值方法的拟合效果, 3种性成熟判定标准下, 均呈现出A为待估参数时的AIC值小于固定值1时的值。尽管增加待估参数的数量能够改善拟合结果[44], 但是赤池信息准则作为评价模型拟合效果的标准, 权衡了模型的复杂度和模型拟合数据的优良性[39]。本研究中, 当以性腺发育Ⅳ期为性成熟判别标准, 使用可变性成熟比例渐近值逻辑斯蒂模型拟合性成熟比例和体长关系时, 性成熟比例渐近值估计值为1.07±0.04, 拟合结果出现了过拟合的现象。因此, 为避免因性腺发育分期鉴定和性成熟判别偏差[45-46], 以及过拟合现象的发生[37], 在可变性成熟比例渐近值逻辑斯蒂模型参数估算过程中, 应引入性成熟比例渐进值不大于1的限制[2, 8], 以确保参数的估计值具有生物学意义。
性腺发育程度是渔业资源繁殖行为发生的基础[33, 47], 与体长关系密切[9], 因此体长结构特征可以间接地反映渔业资源的性成熟及繁殖行为的相关信息[26, 28]。本研究显示, 性腺发育处于Ⅱ期、Ⅲ期和Ⅳ期的雌性口虾蛄个体最小体长均处于7.00~7.99 cm组, 同时, 该体长组内也存在性腺发育Ⅵ期的个体; 说明无论是以性腺发育Ⅱ期, 还是以Ⅲ期或Ⅳ期作为雌性口虾蛄性未成熟和性成熟的界定标准, 渤海湾雌性口虾蛄最小性成熟体长[28]均介于7.00~7.99 cm并可完成产卵行为, 这与黄渤海海域其他相关研究的结果一致[16, 48]。雌性口虾蛄繁殖行为发生前, 性腺发育Ⅱ期个体体长以9.00~11.99 cm为主, Ⅲ期以10.00~ 12.99 cm为主, Ⅳ期以11.00~13.99 cm为主, 各期的优势体长均呈现随性腺发育逐渐增加的趋势, 说明在同一繁殖季节, 繁殖群体中大个体雌性口虾蛄的性腺发育较小个体早[22]。性腺发育Ⅵ期的个体中, 体长中值为10.50 cm、11.50 cm和12.50 cm组的个体量占各对应体长组样本总量的26.92%±1.87%, 其他体长组的个体量比例值最大仅为13.14%(出现在体长中值9.50 cm组)。说明体长10.00~12.99 cm的个体较其他体长组参与产卵活动的时间早[23], 在繁殖季节早期更适于被选作为人工繁育的亲体。而就性腺发育Ⅵ期的雌性口虾蛄, 体长以9.00~11.99 cm为主, 占该发育期个体总量的74.49%, 说明在自然环境条件下, 该体长范围的雌性口虾蛄对繁殖亲体起着主要贡献作用, 这与HAMANO等[15]的研究结果一致。通过描述性腺发育特征确定性成熟被认为是估算初次性成熟体长的最有效且普遍适用的方法[33, 37], 但在实际工作中, 采用的性腺发育分期标准存在较大差异[49-52]。就雌性口虾蛄而言, 存在5期[25, 34]和6期[22]两种性腺发育阶段划分方法, 因此选择恰当的性腺发育阶段作为性成熟判别标准就成为影响初次性成熟体长研究结果的关键因素之一。对比已知的两种性腺发育阶段划分方法, 尽管分期数量不同, 但对Ⅰ期的性腺发育特征描述基本一致[22, 25]。因此将Ⅰ期作为性未成熟, Ⅱ期及以上作为性成熟的判别标准估算雌性口虾蛄初次性成熟体长同时适用于两种不同的性腺发育阶段划分方法。
性成熟过程作为渔业资源从幼体发育到成体的重要阶段[38], 即包含了生长模式和繁殖器官等的外部形态学特征变化[10, 30], 也涉及性腺质量和颜色等的内部生理学特征变化[22-23], 这些可观察并可量化的改变为确定性成熟类型提供了依据[24, 38]。根据开展研究所依赖的生物学特征[45, 53], 性成熟分别被定义为生理学性成熟和形态学性成熟[1]。内部生理学特征变化标志着渔业资源繁殖行为的开始, 外部形态学特征变化则是渔业资源能够完成繁殖活动的基本保障[47]。从繁殖活动的开始到完成, 尽管渔业资源的生长通常会减缓, 但并未完全停止[26], 因此基于两类生物学特征得到的初次性成熟体长在多数情况下是不同的[1]。本研究得到的初次性成熟体长(9.42 cm±0.02 cm)小于基于生长模式变化得到的初次性成熟体长(11.01 cm)[38], 符合生理学初次性成熟体长小于形态学初次性成熟体长的基本规律[54]。渔业资源利用过程中, 大量的小规格或性未成熟个体被捕捞的现象在许多渔业中普遍存在[55-56], 一直是渔业管理重点解决的问题之一, 对渔业资源的结构稳定和可持续利用带来严重危害[57-58]。为保障有足够的亲体参与繁殖活动而使资源得以可持续利用, 初次性成熟体长被认为是渔业开发过程中可被容许的最小捕捞体长[55, 57, 59]。根据本研究结果, 建议渤海湾口虾蛄的开捕最小体长不小于9.42 cm。
| [1] |
WAIHO K, FAZHAN H, BAYLON J C, et al. On types of sexual maturity in brachyurans, with special reference to size at the onset of sexual maturity[J]. Journal of Shellfish Research, 2017, 36(3): 807-839. DOI:10.2983/035.036.0330 |
| [2] |
FONTOURA N F, BRAUN A S, MILANI P C C. Estimating size at first maturity (L50) from Gonadossomatic Index (GSI) data[J]. Neotropical Ichthyology, 2009, 7(2): 217-222. DOI:10.1590/S1679-62252009000200013 |
| [3] |
LORENZEN K. Toward a new paradigm for growth modeling in fisheries stock assessments: embracing plasticity and its consequences[J]. Fisheries Research, 2016, 180: 4-22. DOI:10.1016/j.fishres.2016.01.006 |
| [4] |
BINOHLAN C, FROESE R. Empirical equations for estimating maximum length from length at first maturity[J]. Journal of Applied Ichthyology, 2009, 25(5): 611-613. DOI:10.1111/j.1439-0426.2009.01317.x |
| [5] |
STARK J W. Contrasting maturation and growth of northern rock sole in the eastern Bering Sea and Gulf of Alaska for the purpose of stock management[J]. North American Journal of Fisheries Management, 2012, 32(1): 93-99. DOI:10.1080/02755947.2012.655845 |
| [6] |
TEMPLEMAN W, HODDER V M, WELLS R. Age, growth, year-class strength, and mortality of the haddock, Melanogrammus aeglefinus, on the southern Grand Bank and their relation to the haddock fishery of this area[J]. ICNAF Research Bulletin, 1978, 13: 31-52. |
| [7] |
TESFAHUN A. Overview of length-weight relationship, condition factor and size at first maturity of nile tilapia Oreochromis niloticus (L.) in different water bodies of ethiopia: a review[J]. Greener Journal of Biological Sciences, 2018, 8(3): 21-28. DOI:10.15580/GJBS.2018.3.060618077 |
| [8] |
TRIPPEL E A, HARVEY H H. Comparison of methods used to estimate age and length of fishes at sexual maturity using populations of white sucker (Catostomus commersoni)[J]. Canadian Journal of Fisheries and Aquatic Sciences, 1991, 48(8): 1446-1459. DOI:10.1139/f91-172 |
| [9] |
KORANTENG K A. Size at first maturity of the anchovy (Engraulis encrasicolus) in Ghanaian waters and suggestions for appropriate mesh size in its fishery[J]. Naga, 1993, 16(5): 29-30. |
| [10] |
FONTOURA N F, JESUS A S, LARRE G G, et al. Can weight/length relationship predict size at first maturity? A case study with two species of Characidae[J]. Neotropical Ichthyology, 2010, 8(4): 835-840. DOI:10.1590/S1679-62252010005000013 |
| [11] |
MESQUITA C, DOBBY H, SWEETING S, et al. Size-at-maturity of Brown Crab (Cancer pagurus) in Scottish waters based on gonadal and morphometric traits[J]. Fisheries Research, 2020, 229: 105610. DOI:10.1016/j.fishres.2020.105610 |
| [12] |
FROESE R, BINOHLAN C. Empirical relationships to estimate asymptotic length, length at first maturity and length at maximum yield per recruit in fishes, with a simple method to evaluate length frequency data[J]. Journal of Fish Biology, 2000, 56(4): 758-773. DOI:10.1111/j.1095-8649.2000.tb00870.x |
| [13] |
HASHIGUTI D T, SOARES B E, WILSON K L, et al. Comparing three methods to estimate the average size at first maturity: a case study on a Curimatid exhibiting polyphasic growth[J]. Ecology of Freshwater Fish, 2019, 28(2): 266-273. DOI:10.1111/eff.12451 |
| [14] |
徐海龙, 张桂芬, 乔秀亭, 等. 黄海北部口虾蛄体长及体质量关系研究[J]. 水产科学, 2010, 29(8): 451-454. XU H L, ZHANG G F, QIAO X T, et al. Relationship between body length and body weight in mantis shrimp Oratosquilla oratoria in Northern Yellow Sea[J]. Fisheries Science, 2010, 29(8): 451-454. DOI:10.3969/j.issn.1003-1111.2010.08.003 |
| [15] |
HAMANO T, MORRISSY N M. Growth of Oratosquilla oratoria (de Haan, 1844)(Stomatopoda) in the sea of Suo-Nada, Japan[J]. Crustaceana, 1992, 63(3): 263-276. DOI:10.1163/156854092X00415 |
| [16] |
刘海映, 谷德贤, 姜玉声, 等. 口虾蛄繁殖周期及生殖细胞发育的研究[J]. 大连海洋大学学报, 2013, 28(3): 269-272. LIU H Y, GU D X, JIANG Y S, et al. Annual reproductive cycle and germ cell development in mantis shrimp Oratosquilla oratoria[J]. Journal of Dalian Ocean University, 2013, 28(3): 269-272. DOI:10.3969/j.issn.2095-1388.2013.03.010 |
| [17] |
HAMANO T, MORRISSY N M, MATSUURA S. Ecological information on Oratosquilla oratoria (Stomatopoda, Crustacea) with an attempt to estimate the annual settlement date from growth parameters[J]. The Journal of Shimonoseki University of Fisheries, 1987, 36(1): 9-27. |
| [18] |
HAMANO T, MATSUURA S. Egg laying and egg mass nursing behaviour in the Japanese mantis shrimp[J]. Nippon Suisan Gakkaishi, 1984, 50(12): 1969-1973. DOI:10.2331/suisan.50.1969 |
| [19] |
王娜, 徐海龙, 武子山, 等. 塘沽上岸渔获物组成变化趋势分析[J]. 河北渔业, 2011(11): 32-34. WANG N, XU H L, WU Z S, et al. The change trend of the Catch Component in Tanggu Tianjin[J]. Hebei Fisheries, 2011(11): 32-34. DOI:10.3969/j.issn.1004-6755.2011.11.014 |
| [20] |
谷德贤, 刘茂利. 天津海域口虾蛄群体结构及资源量分析[J]. 河北渔业, 2011(8): 24-26. GU D X, LIU M L. Analysis on the Population structure and abundance of Oratosquilla oratoria in Tianjin Sea Area[J]. Hebei Fisheries, 2011(8): 24-26. DOI:10.3969/j.issn.1004-6755.2011.08.008 |
| [21] |
KUBO I, HORI S, KUMEMURA M, et al. A biological study on a Japanese edible mantis-shrimp Squilla oratoria de Haan[J]. Journal of Tokyo University of Fisheries, 1959, 45: 1-25. |
| [22] |
KODAMA K, SHIMIZU T, YAMAKAWA T, et al. Reproductive biology of the female Japanese mantis shrimp Oratosquilla oratoria (Stomatopoda) in relation to changes in the seasonal pattern of larval occurrence in Tokyo Bay, Japan[J]. Fisheries Science, 2004, 70(5): 734-745. DOI:10.1111/j.1444-2906.2004.00866.x |
| [23] |
KODAMA K, SHIRAISHI H, MORITA M, et al. Reproductive biology of the Japanese mantis shrimp Oratosquilla oratoria (Crustacea Stomatopoda): annual cycle of gonadal development and copulation[J]. Marine Biology Research, 2009, 5(5): 415-426. DOI:10.1080/17451000802644714 |
| [24] |
MILI S, BOURIGA N, MISSAOUI H, et al. Morphometric, reproductive parameters and seasonal variations in fatty acid composition of the mantis shrimp Squilla mantis (Crustacea: Stomatopoda) in the Gulf of Gabes (Tunisia)[J]. Journal of Life Sciences, 2011, 5(12): 1058-1071. |
| [25] |
KIM S, KIM H, BAE H, et al. Growth and reproduction of the Japanese mantis shrimp, Oratosquilla oratoria (De Haan 1844) in the coastal area of Tongyeong, Korea[J]. Ocean Science Journal, 2017, 52(2): 257-265. DOI:10.1007/s12601-017-0027-2 |
| [26] |
YAMADA R, KODAMA K, YAMAKAWA T, et al. Growth and reproductive biology of the small penaeid shrimp Trachysalambria curvirostris in Tokyo Bay[J]. Marine Biology, 2007, 151(3): 961-971. DOI:10.1007/s00227-006-0536-5 |
| [27] |
NANDIKESWARI R. Size at first maturity and maturity stages of Terapon jarbua (Forsskal, 1775) from Pondicherry Coast, India[J]. Journal of Fisheries, 2016, 4(2): 385-389. DOI:10.17017/j.fish.118 |
| [28] |
OVERTON J L, MACINTOSH D J. Estimated size at sexual maturity for female mud crabs (genus Scylla) from two sympatric species within Ban Don Bay, Thailand[J]. Journal of Crustacean Biology, 2002, 22(4): 790-797. DOI:10.1163/20021975-99990293 |
| [29] |
SOMERTON D A. A computer technique for estimating the size of sexual maturity in Crabs[J]. Canadian Journal of Fisheries and Aquatic Sciences, 1980, 37(10): 1488-1494. DOI:10.1139/f80-192 |
| [30] |
LOZANO-ÁLVAREZ E, BRIONES-FOURZÁN P, GRACIA A, et al. Relative Growth and Size at First Maturity of the Deep Water Shrimp, Heterocarpus ensifer (Decapoda, Pandalidae) from the Southern Gulf of Mexico[J]. Crustaceana, 2007, 80(5): 555-568. DOI:10.1163/156854007780765588 |
| [31] |
SALLANI W S. Reproductive biology of the mantis shrimp Erugosouilla massavensis from port said, egypt[J]. Egyptian Journal of Aquatic Biology and Fisheries, 2005, 9(1): 171-183. DOI:10.21608/ejabf.2005.1822 |
| [32] |
CARLUCCI R, D'ONGHIA G, SION L, et al. Selectivity parameters and size at first maturity in deep-water shrimps, Aristaeomorpha foliacea (Risso, 1827) and Aristeus antennatus (Risso, 1816), from the North-Western Ionian Sea (Mediterranean Sea)[J]. Hydrobiologia, 2006, 557(1): 145-154. DOI:10.1007/s10750-005-1317-8 |
| [33] |
PEIXOTO S, CALAZANS N, SILVA E F, et al. Reproductive cycle and size at first sexual maturity of the white shrimp Penaeus schmitti (Burkenroad, 1936) in northeastern Brazil[J]. Latin American Journal of Aquatic Research, 2018, 46(1): 1-9. DOI:10.3856/vol46-issue1-fulltext-1 |
| [34] |
徐善良, 王春琳, 梅文骧, 等. 口虾蛄Oratosquilla oratoria (De Haan)性腺特征及卵巢组织学观察[J]. 浙江水产学院学报, 1996, 15(1): 21-29. XU S L, WANG C L, MEI W X, et al. Observations on the characteristics of spermary ano ovarivm histology of Oratosquilla oratoria[J]. Journal of Zhejiang College of Fisheries, 1996, 15(1): 21-29. |
| [35] |
GREY K A. Estimates of the size of first maturity of the western rock lobster, Panulirus cygnus, using secondary sexual characteristics[J]. Australian Journal of Marine and Freshwater Research, 1979, 30(6): 785-791. DOI:10.1071/MF9790785 |
| [36] |
MELVILLE-SMITH R, DE LESTANG S. Spatial and temporal variation in the size at maturity of the western rock lobster Panulirus cygnus George[J]. Marine Biology, 2006, 150(2): 183-195. DOI:10.1007/s00227-006-0349-6 |
| [37] |
YANTI A, TRESNATI J, YASIR I, et al. Size at the maturity of sea cucumber Holothuria scabra. Is it an overfishing sign in Wallacea Region?[J]. IOP Conference Series: Earth and Environmental Science, 2020, 473: 012056. DOI:10.1088/1755-1315/473/1/012056 |
| [38] |
徐海龙, 薛薇, 谷德贤, 等. 基于两段式模型研究口虾蛄体长-体质量关系及估算初次性成熟体长[J]. 水产学报, 2022, 46(2): 207-214. XU H L, XUE W, GU D X, et al. Establishing length-weight relationship and predicting size at first maturity of Oratosquilla oratoria based on polyphasic model[J]. Journal of Fisheries of China, 2022, 46(2): 207-214. |
| [39] |
AKAIKE H. A new look at the statistical model identification[J]. IEEE Transactions on Automatic Control, 1974, 19(6): 716-723. DOI:10.1109/TAC.1974.1100705 |
| [40] |
R Core Team. R: a language and environment for statistical computing[EB/OL]. Vienna, Austria: R Foundation for Statistical Computing, 2021. https://www.R-project.org/.
|
| [41] |
CHEN Y, PALOHEIMO J E. Estimating fish length and age at 50% maturity using a logistic type model[J]. Aquatic Sciences, 1994, 56(3): 206-219. DOI:10.1007/BF00879965 |
| [42] |
BEACHAM T D. Variability in size and age at sexual maturity of American plaice and yellowtail flounder in the Canadian Maritimes region of the northwest Atlantic Ocean[R]. Department of Fisheries and Oceans, Fisheries Research Branch, Pacific, 1983.
|
| [43] |
RIDEOUT R M, TOMKIEWICZ J. Skipped spawning in fishes: more common than you might think[J]. Marine and Coastal Fisheries, 2011, 3(1): 176-189. DOI:10.1080/19425120.2011.556943 |
| [44] |
RICKER W E. Linear regressions in fishery research[J]. Journal of the Fisheries Board of Canada, 1973, 30(3): 409-434. DOI:10.1139/f73-072 |
| [45] |
LOWERRE-BARBIERI S K, GANIAS K, SABORIDO-REY F, et al. Reproductive timing in marine fishes: variability, temporal scales, and methods[J]. Marine and Coastal Fisheries, 2011, 3(1): 71-91. DOI:10.1080/19425120.2011.556932 |
| [46] |
SOSEBEE K A. Are density-dependent effects on elasmobranch maturity possible?[J]. Journal of Northwest Atlantic Fishery Science, 2005, 35: 115-124. DOI:10.2960/J.v35.m492 |
| [47] |
LóPEZ-MARTÍNEZ J, RÁBAGO-QUIROZ C, NEVÁREZ-MARTÍNEZ M O, et al. Growth, reproduction, and size at first maturity of blue shrimp, Litopenaeus stylirostris (Stimpson, 1874) along the east coast of the Gulf of California, Mexico[J]. Fisheries Research, 2005, 71(1): 93-102. DOI:10.1016/j.fishres.2004.06.004 |
| [48] |
盛福利, 曾晓起, 薛莹. 青岛近海口虾蛄的繁殖及摄食习性研究[J]. 中国海洋大学学报, 2009, 39(s1): 326-332. SHENG F L, ZENG X Q, XUE Y. Study on Propagation and Feeding Habits of Oratosquilla oratoria in the Inshore Waters of Qingdao[J]. Periodical of Ocean University of China, 2009, 39(s1): 326-332. |
| [49] |
SIVADASL M, NAIR P N R, BALASUBRAMANIAN K K, et al. Length weight relationship, relative condition, size at first maturity and sex ratio of Indian mackerel Rastrelliger kanagurta from Calicut[J]. Journal of the Marine Biological Association of India, 2006, 48(2): 274-277. |
| [50] |
FUJI A. STUDIES ON THE BIOLOGY OF THE SEA URCHIN: Ⅱ. Size at first maturity and sexuality of two Sea Urchins, Strongylocentrotus nudus and S. intermedius[J]. 北海道大學水産學部研究彙報, 1960, 11(2): 43-48. |
| [51] |
VASCONCELOS J, AFONSO-DIAS M, FARIA G. Atlantic chub mackerel (Scomber colias) spawning season, size and age at first maturity in Madeira waters[J]. Arquipel Life Mar Sci, 2012, 29: 43-51. |
| [52] |
TESFAYE G, TADESSE Z. Length-weight relationship, fulton's condition factor and size at first maturity of Tilapia, Oreochromis Niloticus L. in lakes Koka, Ziway and Langano (Ethiopian rift valley)[J]. Ethiopian Journal of Biological Sciences, 2008, 7(2): 139-157. |
| [53] |
PINHEIRO M A A, FRANSOZO A. Sexual maturity of the speckled swimming crab Arenaeus cribrarius (Lamarck, 1818) (Decapoda, Brachyura, Portunidae), in the Ubatuba littoral, São Paulo state, Brazil[J]. Crustaceana, 1998, 71(4): 434-452. DOI:10.1163/156854098X00536 |
| [54] |
BODIGUEL X, MAURY O, MELLON-DUVAL C, et al. A dynamic and mechanistic model of PCB bioaccumulation in the European hake (Merluccius merluccius)[J]. Journal of Sea Research, 2009, 62(2/3): 124-134. |
| [55] |
OGUTU-OHWAYO R, BALIRWA J S. Management challenges of freshwater fisheries in Africa[J]. Lakes & Reservoirs: Science, Policy and Management for Sustainable Use, 2006, 11(4): 215-226. |
| [56] |
OTIENO O N, KITAKA N, NJIRU J M. Length-weight relationship, condition factor, length at first maturity and sex ratio of Nile tilapia, Oreochromis niloticus in Lake Naivasha, Kenya[J]. International Journal of Fisheries and Aquatic Studies, 2014, 2(2): 67-72. |
| [57] |
GOUGH C L A, DEWAR K M, GODLEY B J, et al. Evidence of overfishing in small-scale fisheries in madagascar[J]. Frontiers in Marine Science, 2020, 7: 317. DOI:10.3389/fmars.2020.00317 |
| [58] |
MACINTOSH D J, THONGKUM C, SWAMY K, et al. Broodstock management and the potential to improve the exploitation of mangrove crabs, Scylla serrata (Forskål), through pond fattening in Ranong, Thailand[J]. Aquaculture Research, 1993, 24(2): 261-269. DOI:10.1111/j.1365-2109.1993.tb00549.x |
| [59] |
HILBORN R, WALTERS C J. Quantitative fisheries stock assessment: choice, dynamics and uncertainty[J]. Reviews in Fish Biology and Fisheries, 1992, 2(2): 177-178. DOI:10.1007/BF00042883 |
2. Tianjin Key Laboratory of Aqua-ecology and Aquaculture, Tianjin 300384, China;
3. National Demonstration Center for Experimental Aqua-ecology and Aquaculture Education, Tianjin Agriculture University, Tianjin 300384, China;
4. College of Marine Sciences, Shanghai Ocean University, Shanghai 201306, China;
5. Tianjin Fishery Institute, Tianjin 300221, China
 2022,
Vol. 31
2022,
Vol. 31

