2. 上海海洋大学 水产科学国家级实验教学示范中心,上海 201306
电压门控钠通道(voltage-gated sodium channels,VGSC)由1个成孔α亚基和最多2个相关的β亚基组成,对于可兴奋组织包括脑神经元中动作电位的产生起着至关重要的作用[1-4]。电压门控钠离子通道主要由α亚基起作用,根据表达组织中细胞的不同,分为中枢和外周神经元型(神经型)、心肌型、骨骼肌/肌肉型电压门控钠离子通道[5-7]。其中Nav1.1( scn1a/SCN1A)属于在脑神经组织中表达的电压门控钠离子通道α亚基之一,对神经元功能起着至关重要的作用[1-2, 8]。
电压门控钠离子通道Nav1.1突变会导致脑神经疾病、脑放电异常等缺陷[2, 9-11] 。其中人类Nav1.1中的功能完全丧失,会导致严重的婴儿肌阵挛性癫痫(SMEI或Dravet综合征)[1, 8]。小鼠中Nav1.1功能丧失,会导致海马氨基丁酸(GABA)抑制神经元中的钠电流和动作电位放电严重受损[10]。
斑马鱼中Nav1.1有2个拷贝基因,即 scn1Laa (Nav1.1a)和 scn1Lab (Nav1.1b),两者具有67%的氨基酸序列相似性,在之前的研究中表明,斑马鱼 scn1Lab主要表达在中枢神经系统,与人类SCN1A表达更相似,而 scn1Laa同样在脑神经系统中表达,但主要在外周神经系统中表达,与 scn1Lab表达模式不重复[12-13]。已有研究[8, 13-14]利用斑马鱼 scn1Lab突变体构建Dravet综合征等癫痫疾病模型,用于研究和筛选抗癫痫类药物,但尚不清楚 scn1Laa在神经元的发育和功能中起到什么样的作用?
本研究利用CRISPR/Cas9基因编辑技术对斑马鱼基因 scn1Laa进行敲除,首次构建了可稳定遗传的 scn1Laa功能缺陷型(scn1Laa-/-)斑马鱼家系,并通过检测脑神经发育和相关基因表达以及行为等指标,对 scn1Laa在斑马鱼脑神经和运动行为中的作用进行了研究。
1 材料与方法 1.1 斑马鱼饲养所用斑马鱼为AB系,养殖于28 ℃恒温的循环水养殖系统内[15-16]。按照上海海洋大学动物伦理相关规定进行实验(1ACUC 2017009)。
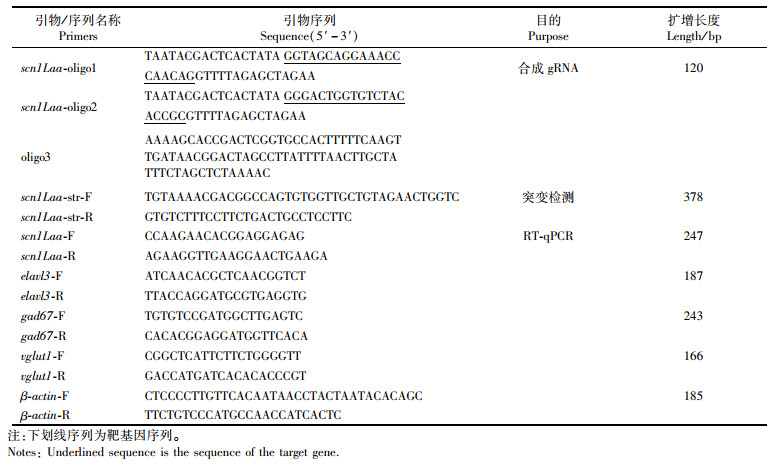
1.2 scn1Laa基因缺失型斑马鱼家系构建从加利福尼亚大学圣克鲁兹分校(UCSC)斑马鱼数据库(http://genome.ucsc.edu/index.html)中筛选出斑马鱼 scn1Laa基因(NM_200132.2)的2个相邻敲除靶点(序列见表 1),这2个靶点均位于其第一号外显子上。以“T7启动子序列+靶点序列+支架蛋白序列”的结构设计引物(表 1),对连接靶向序列与Cas9蛋白的支架蛋白序列进行PCR扩增,再经转录获得靶向 scn1Laa-guide RNA (gRNA),Cas9 mRNA的制备则经pT3ts-nCas9n质粒线性化后转录获得,以上具体操作参考廖永冠等[17]的步骤。显微注射中,将两种RNA(gRNA 100 pg,cas9 mRNA 500 pg)的混合液显微注射入Ⅰ细胞期的野生斑马鱼受精卵中,注射剂量参考实验室之前构建其他基因缺失家系每颗受精卵的注射量,约为1.4 nL[18]。一共进行了4批次注射,统计注射后胚胎发育到第3天的平均存活率约为56.8%(4次成活率分别为40.8%、67.2%、50.6%和68.6%)。经碱裂解法[18-20]提取受精卵或尾鳍DNA进行基因目的片段特异性引物对和荧光中心标记的引物运行“三引物”荧光PCR(表 1),即短小串联重复序列(short tandem repeat,STR)检测[21],在遗传分析仪ABI(Applied Biosystems,美国)3 130 xl或3 730 xl上使用毛细管电泳分离得到荧光标记的扩增子,通过GeneMapper或Genescan和Genotyper软件包(Applied Biosystems)处理*.fsa文件,比较分析进行敲除的样本峰与野生型对照峰,选出含非三倍数的突变峰的个体。进一步通过以上具有突变峰的扩增子进行亚克隆Sanger分子测序,挑选单克隆以检查 scn1Laa序列的实际突变情况。
|
表 1 本研究中的DNA合成序列 Tab.1 DNA sequences used in the research |
成活的注射个体中,scn1Laa的有义突变率约32.35%。筛选一对发生相同有义突变的个体与野生型个体进行交配,获得杂合突变个体(scn1Laa+/-),再从杂合突变个体的交配后代中筛选出3对 scn1Laa的纯合突变体和3对正常野生型斑马鱼(wild type siblings)。通过纯合个体的繁育,分别构建可稳定遗传的 scn1Laa纯合突变家系(scn1Laa-/-)以及具有相同遗传背景的对照组家系( scn1Laa+/+)。
1.3 斑马鱼生长检测将 scn1Laa+/+和scn1Laa-/-斑马鱼各设置3个平行组(30尾/组)于同一环境中进行饲养。待生长至90 dpf(days post fertilization),分别测定雌雄鱼个体标准体长(自吻端至椎骨末端的直线长度)和体质量(测定时间为进食后2 h),雌雄 scn1Laa+/+和scn1Laa-/-斑马鱼各组测定6尾。
1.4 cDNA制备及RT-qPCR每组取5 dpf的斑马鱼不少于6尾,参照RNAiso Plus(TaKaRa,日本)说明书提供的方法进行组织RNA抽提(5 dpf样本取头部组织)。用HiScriptⓇ Ⅲ 1st Strand cDNA Synthesis Kit(+gDNA wiper)(vazyme,中国)试剂,参照说明书,反应生成cDNA第一链,用于后续荧光实时定量PCR(RT-qPCR)。
对氨基丁酸能神经标记基因( gad67,AB183390.1)[22-24]、谷氨酸能神经标记基因( vglut1,NM_001098755.1)[25-27]和有丝分裂后(成熟)神经元标记基因( elavl3,XM_009299733.3)[28-30]的mRNA水平进行RT-qPCR检测,内参基因为 β-actin。相关半定量PCR引物见表 1。荧光实时定量PCR(RT-qPCR)步骤:按照Hieff UNICONⓇ Universal Blue RT-qPCR SYBR Green Master Mix(翊圣,中国)说明进行加样。用CFX96荧光定量PCR仪(Bio-rad,美国)进行qRT-PCR反应,反应条件如下:(1)预变性95 ℃,2 min;(2)变性和退火/延伸:40×(95 ℃,10 s;60 ℃,30 s);(3)溶解曲线:机器默认。
1.5 BrdU细胞增殖免疫组化取5 dpf野生型 scn1Laa+/+和突变型scn1Laa-/-仔鱼各10尾置于含有15% DMSO的E3中的10 mmol/L BrdU溶液(Sigma-Aldrich, 美国)中,在冰上孵育20 min, 28 ℃下放置5 min后于4% PFA中室温固定2 h;固定后的仔鱼经1×PBST洗涤后用150 mmol/L Tris-HCl(pH 9)于室温处理15 min,70 ℃下处理15 min;处理后仔鱼经1×PBST后-20 ℃丙酮渗透30 min;1×PBST洗涤后用2% H2O2室温光照下脱色素;2 mol/L HCl中室温处理1 h以暴露BrdU表位;然后用0.1 mol/L硼酸盐缓冲液(pH 8.5)室温中和20 min,再用1×PBST洗涤;在1×PBST/10%山羊血清/0.8% Triton-X/1% BSA中的4 ℃封闭缓冲液中过夜,用1∶ 200稀释的BrdU抗体(Mouse monoclonal anti-BrdU antibody,Sigma-Aldrich,美国)4 ℃下孵育24 h。接下来,仔鱼在1×PBST中洗涤3×5 min,然后在1×PBST/0.8% Triton-X中孵育5 min。应用二抗(Alexa Fluor 488标记山羊抗小鼠IgG(H+L),Beyotime)(1∶ 2 000稀释)在1×PBST,4 ℃下12 h。随后,仔鱼在1×PBST中洗涤3×5 min,然后在hochest33258(Abcam, 英国)(1∶ 1 000稀释)中1×PBST避光孵育20 min,1×PBST中洗涤3×5 min,在1×PBST中洗涤4 ℃过夜。使用共聚焦显微镜(Leica TCS SP8,美国)成像。
1.6 斑马鱼运动分析5 dpf斑马鱼仔鱼运动分析:参考GAO等[18]方法,将5 dpf的仔鱼(n = 6尾/组)放入含有500 μL培养基的12孔板(1只仔鱼/孔)中,并让其适应装置(ZebraBox,Viewpoint,法国)15 min。仔鱼在暗环境利用红外线跟踪200 min,记录间隔为10 min。每条斑马鱼所覆盖的移动距离以毫米(mm)记录,用ZebraLab(Viewpoint,法国)分析运动数据,去掉监控前后10 min,取中间180 min数据进行分析。
90 dpf斑马鱼成鱼运动分析:该阶段的运动行为检测使用90 dpf的雄性斑马鱼于ZebraTower(Viewpoint,法国)设备下进行监控分析检测。取直径为10 cm玻璃圆缸(外贴白纸,避免相互干扰)加普通饲养水400 mL,放入scn1Laa+/+和scn1Laa-/-组斑马鱼各2尾进行检测,共3组。每组监测时长30 min,取中间10 min进行分析。对检测的运动数据进行离线分析,运动分析设置参数:将运动速度设置为20~50 mm/s(运动轨迹为绿色),运动速度超过50 mm/s设置为爆发性运动(运动轨迹为红色),运动速度低于20 mm/s设置为“静止”(运动轨迹为黑色),每5秒记录1次,根据每5秒内的斑马鱼各组别运动类型记录次数(count),持续时间(dur)和运动距离(Dist),计算平均速度,分别作为斑马鱼运动量及冲动性的指标。
1.7 数据统计运动数据和体长、体质量以平均值±标准差(Mean ± SD)在折线图或柱状图上显示,距离用双尾独立t检验。相关数据的比较分析及制图通过GraphPad prism 8.0软件完成:scn1Laa+/+和scn1Laa-/-斑马鱼间的比较通过双尾独立t检验(方差不齐的经Welch校正)或曼-惠特尼(Mann-Whitney)独立检验进行。P < 0.05,差异显著;P < 0.01,P < 0.001和P < 0.000 1,差异极显著。
2 结果 2.1 scn1Laa-/-斑马鱼稳定遗传家系的建立通过敲除后的筛选及传代,构建了scn1Laa-/-斑马鱼的稳定遗传家系:通过对scn1Laa 基因进行测序发现,CRISPR/Cas9编辑导致scn1Laa 的序列发生有义突变,造成插入5个碱基的突变(图 1a);荧光毛细管电泳检测呈单峰,说明该突变为纯合突变(图 1d),并且scn1Laa 序列碱基的缺失,导致氨基酸编码提前终止于第二号外显子,而主要功能结构域的缺失造成蛋白功能丧失(图 1b)。观察5 dpf scn1Laa+/+和scn1Laa-/-斑马鱼仔鱼,外型并无明显差异(图 1c)。

|
(a) scn1Laa 敲除靶位点示意图和测序碱基突变, 粗体标注CRISPR/Cas9靶序列;(b)基因序列突变导致氨基酸编码提前终止,scn1Laa 插入5 bp,产生截短的氨基酸;(c)5 dpf scn1Laa-/-鱼表型观察,与野生型鱼表型无明显差异;(d)荧光毛细管电泳检测基因型突变。WT或者wild type表示野生型斑马鱼。 (a) Schematic diagram of CRISPR/Cas9 target site of scn1Laa and sequence changes after scn1Laa gene editing, nucleotides were labeled bold indicated the CRISPR/Cas9 target sites; (b)Truncated termination of amino acid encoding. the scn1Laa mutation of 5 bp insert produced the truncated amino acid sequence; (c) No noticeable morphologic difference was observed between the scn1Laa wild type sibling and scn1Laa mutant larvae at 5 days post fertilization (dpf); (d) Genotype mutation detection through capillary electrophoresis STR (short tandem repeats) analysis. WT or wild type, the scn1Laa wild type fish. 图 1 利用CRISPR/Cas9建立scn1Laa-/-斑马鱼家系 Fig. 1 CRISPR/Cas9-mediated scn1Laa mutant in zebrafish |
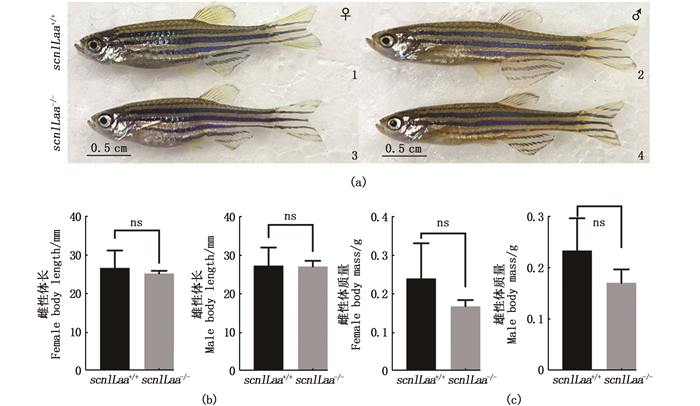
对90 dpf成年斑马鱼进行外观、体长、体质量等检测,结果表明对scn1Laa-/-突变型斑马鱼与野生型斑马鱼的外观进行比较,无论雌雄,均无明显差异(图 2a)。比较体质量,scn1Laa-/-雌鱼(P=0.062 3),雄鱼(P=0.07)均比野生型的轻,但是均无显著差异(图 2b)。比较scn1Laa-/-斑马鱼与野生型雌鱼(P=0.388 7)和雄鱼(P=0.814 1)的体长,无显著差异(图 2c)。
|
1.野生型雌性斑马鱼;2.野生型雄性斑马鱼;3. scn1Laa-/-雌鱼;4. scn1Laa-/-雄鱼。(b) scn1Laa-/-型与野生型90 d龄雌雄斑马鱼的身长统计比较;(c) scn1Laa-/-型与野生型90 d龄雌雄斑马鱼的体质量统计比较,ns表示无显著差异。 1. wild-type male zebrafish; 2. wild-type female zebrafish; 3. scn1Laa-/- male zebrafish; 4. scn1Laa-/- female zebrafish. (b) body length; (c) body mass. "ns" represent no significate difference. 图 2 scn1Laa-/-型与野生型90 d龄斑马鱼的外观和生长相关指数比较 Fig. 2 Comparison of phenotype and related growth index of scn1Laa-/- and wild-type zebrafish at 90 dpf |
为了检测scn1Laa 对斑马鱼脑神经元发育的影响,通过基因组织半定量和细胞增殖标记(BrdU)免疫组化的方法分别对5 dpf的斑马鱼进行检测。组织半定量结果显示,5 dpf scn1Laa-/-仔鱼与野生型比较,脑神经中氨基丁酸能神经标记基因(gad67 )mRNA表达相对升高(P=0.108 6),但是谷氨酸能神经标记基因(vglut1 )(P=0.000 3)和有丝分裂后神经元,即成熟神经元标记基因(elavl3 )(P=0.000 7)mRNA表达显著降低,即氨基丁酸能神经增多,但是谷氨酸能神经和成熟神经元减少(图 3a)。进一步通过BrdU标记增殖细胞,发现5 dpf scn1Laa-/-仔鱼与野生型比较,脑部细胞增殖明显比野生型的少(图 3b),同时通过统计脑部区域BrdU荧光信号也显示具有显著差异(P=0.000 3), 见图 3c。

|
(a)qRT-PCR检测神经标记基因表达,5 dpf scn1Laa-/-斑马鱼仔鱼与野生型比较,氨基丁酸能神经标记基因(gad67 )相对升高,谷氨酸能神经标记基因(vglut1 )和有丝分裂后神经元标记基因(elavl3 )显著减少;数据显示用平均值± SD表示(n=6,ns,无明显差异,***P < 0.001);(b)5 dpf scn1Laa-/-斑马鱼细胞增殖检测。BrdU荧光染色的5 dpf野生型斑马鱼(A)和scn1Laa-/-斑马鱼(B)头部z轴叠加荧光图片,C和D分别为分离出绿色荧光通道(BrdU)的野生斑马鱼和scn1Laa 缺失斑马鱼图片。白色三角箭头指示BrdU阳性信号,不规则框标注头部脑的位置;(c)5 dpf野生型斑马鱼和scn1Laa-/-斑马鱼BrdU阳性信号统计,数据显示为平均值±标准差,n=5,*表示P < 0.01。 (a) scn1Laa-/- larvae had an relative increased expression of GABAergic (gad67 ), an decreased expression of glutamatergic (vglut1 ) and postmitotic (elavl3 ) neuronal markers compared to WT siblings, as measured by RT-qPCR (real-time quantitative polymerase chain reaction) at 5 dpf, Data are expressed as mean± SD (n=6, ns, no significant difference, ***P < 0.001); (b) Proliferation of cells in the brain of scn1Laa-/- larvae. Representative z-slices of with BrdU staining of WT (A) and scn1Laa-/- (B) larvae head at 5 dpf. split-green channel images of WT (C) and scn1Laa-/- (D) larvae to the below, respectively. white triangular arrowheads indicated the BrdU positive signals, the irregular shape frame showed the location of the brain; (c) BrdU signals of brain in WT and scn1Laa-/- larvae head at 5 dpf. Data were shown as mean±SD. n=5, * indicated that P < 0.01. 图 3 scn1Laa 缺失导致斑马鱼脑神经发育异常 Fig. 3 Loss of scn1Laa influening the development of the cranial nerve in zebrafish |
通过对5 dpf scn1Laa-/-仔鱼和野生型仔鱼运动水平监测分析,发现缺失scn1Laa 后,导致5 dpf仔鱼整体运动比野生型更为激烈和频繁,同时进行单位时间内平均运动距离数据分析及统计学检验,结果表明差异极显著(P=0.007),见图 4。

|
(a)运动行为监测系统;(b)行为监测中实验样本scn1Laa-/-仔鱼和野生型仔鱼的分组监控位置;(c)监测软件记录的运动路线示意图,不同颜色代表在不同运动速度下的运动路线记录,其中红色代表爆发性(高速度,速度>50 mm/s)运动, 绿色代表正常(中等速度,20 mm/s < 速度 < 50 mm/s)运动,黑色代表小距离运动(低速度运动,速度 < 20 mm/s);(d)3 h仔鱼运动行为记录,每10 min计算1次运动距离,误差棒为标准差;(e)柱状图统计运动数据,计算每10 min运动平均距离,显示scn1Laa 缺失仔鱼运动距离较野生型斑马鱼远,***P < 0.001。 (a) Locomotion behavior monitoring system; (b) The locomotion monitor sample list of scn1Laa mutant and wild type sibling at 5dpf; (c) ZebraLab monitoring locomotion and tracking path display of scn1Laa mutant larvae and wild type siblings at 5 dpf, the red line showed the burst locomotion (velocity >50mm/s), the green line showed the locomotion velocity between 20mm/s and 50mm/s, the black line means locomotion velocity < 20mm/s; (d) 3 hours recording behavioral data of scn1Laa mutant and wild type sibling at 5 dpf. Time bins are 10 minutes, bars showed SD; (e) Boxplot showed the mean values (n=6 individuals per group) of distance per 10 min. scn1Laa mutant larvae move increase distance moved more than wild type larvae, ***P < 0.001. 图 4 scn1Laa-/-斑马鱼仔鱼行为学检测 Fig. 4 scn1Laa-/- Zebrafish larvae locomotion behavior detection |
进一步对scn1Laa-/-成鱼(90 dpf)进行运动水平分析,结果显示突变型比野生型斑马鱼运动更为频繁(图 5a和b),体现在小距离运动次数(速度 < 20 mm/s,P=0.002 2)和大距离运动次数(速度>50 mm/s,P=0.002 9)突变型均高于野生型斑马鱼,且具有显著差异。缺失scn1Laa 后,导致成年斑马鱼运动具有爆发性(图 5)。

|
(a)斑马鱼行为检测分组排布,每组各4尾鱼,包含2尾scn1Laa-/-鱼和2尾野生型鱼;(b)检测软件标记的运动路线示意图,其中最外侧红线为圈定检测范围,内侧红线条是运动速度>50 mm/s的爆发性运动轨迹,绿色线条是运动速度在20~50 mm/s的运动轨迹,黑色线条是速度 < 20 mm/s的运动轨迹;(c) scn1Laa-/-斑马鱼小距离运动行为统计(速度在20~50 mm/s);(d) scn1Laa-/-斑马鱼检测大距离运动统计(速度>50 mm/s);数据显示是平均值±标准差(n=6,**P < 0.01)。 (a) Schematic diagram of experiment group, 4 fish in a group, including 2 scn1Laa-/- zebrafish and 2 wild siblings. (b) ZebraLab monitoring locomotion and tracking path display, the red line showed the burst locomotion (velocity >50 mm/s), the green line showed the locomotion velocity between 20 mm/s and 50 mm/s, the black line means locomotion velocity < 20mm/s; (c) small locomotor activity count of scn1Laa-/- zebrafish in one minute (velocity between 20 mm/s and 50 mm/s); (d) large movement. count of scn1Laa-/- zebrafish in one minute (velocity >50 mm/s); Data are expressed as mean ± SD (n=6, **P < 0.01). 图 5 scn1Laa-/- 90 dpf斑马鱼运动行为检测 Fig. 5 scn1Laa-/- Zebrafish locomotion monitoring at 90 dpf |
目前关于斑马鱼电压门控离子通道Nav1.1(SCN1A)多集中在scn1Lab这个基因拷贝上,对另一个拷贝基因scn1Laa 研究较少。本研究通过CRISPR/Cas9技术成功构建的稳定遗传scn1Laa突变斑马鱼,研究scn1Laa缺失后对脑神经发育、运动行为和生长的影响。
已有研究[8, 13-14]表明scn1Lab 缺失仔鱼会出现由于代谢缺陷和癫痫发作而导致的早期死亡,但是在本文中构建的scn1Laa 缺陷斑马鱼仔鱼中并未发现这种情况,同时检测scn1Laa-/-成鱼生长等指数发现,缺失scn1Laa并未出现生长方面的影响,说明scn1Laa对于斑马鱼生长方面的影响较小。根据scn1Laa 突变型斑马鱼体质量相对低于野生型这一特征,我们推测是由于突变型的运动量显著高于野生型,从而导致能量消耗更多引起的,但不能排除突变型能否会摄食更多来补充所消耗的能量,导致体质量无显著差异,这部分内容还需要更进一步的研究。
同时,本研究结果表明scn1Laa 缺失后仔鱼脑部发育异常,主要体现在脑神经元发育变化。在氨基丁酸(GABA)类神经元内GABA能信号在大脑发育中具有重要作用[31-34]。氨基丁酸类神经元一般是起到抑制兴奋,与焦虑、自闭症等有密切关系[31-33, 35]。谷氨酰胺类神经元则一般起到兴奋作用,其中谷氨酰胺类神经主要指神经递质为谷氨酰胺的中枢神经,这些神经具有不同的谷氨酰胺受体,主要分为离子型(电压敏感,voltage sensitive)和代谢型(配体敏感,ligand sensitive),并且这些谷氨酰胺神经受体与神经元的发育和生长有关[8, 29, 31, 34]。与野生型仔鱼比较,scn1Laa-/-仔鱼氨基丁酸类神经元有相对增加,但是谷氨酰胺类神经大量减少,成熟神经元也较野生型减少,同时检测该阶段脑部细胞增殖情况也发现缺失scn1Laa后,脑部神经组织细胞增殖明显减少。说明scn1Laa参与斑马鱼脑神经发育和生长。
已有研究表明SCN1A(Nav1.1)参与的电压门控钠离子钠通道与一些脑疾病有关,例如原发性癫痫(Dravet综合征)、偏瘫性偏头痛和自闭症谱系障碍(ASD)[36-40]。已有研究所构建scn1Lab 缺失(与人类Nav1.1/ SCN1A具有更高的相似性)斑马鱼系已用于研究筛选治疗癫痫的药物,并且通过实验证明scn1Lab 功能缺陷斑马鱼具有焦虑、运动障碍、以及神经发育异常等Dravet综合征类似症状[8, 37, 41],同时scn1Lab缺失幼鱼由于Scn1Lab功能丧失导致神经元过度兴奋,出现“癫痫样”Dravet综合征,同时伴随发育早期身体色素沉淀增多以及早期鱼鳔缺损、增加斑马鱼早期死亡率[8, 41-44]。scn1Laa与scn1Lab结构功能相似,都在斑马鱼发育3 dpf开始高表达在脑神经系统中,但是scn1Laa 主要表达在外周神经组织中,scnLab 主要表达在中枢神经中[12]。通过本研究结果也证实scn1Laa功能与scn1Lab参与脑神经调节功能有一定相关性。首先,二者都在脑神经中大量表达,并且在发育早期(3 dpf)就大量表达,同时在敲除scn1Laa和scn1Lab的表型上有类似的地方,比如都出现运动行为异常,具有运动爆发性,而且敲除后都影响早期脑神经细胞增殖减少[8, 41-44]。另外,敲除这两个基因都出现谷氨酸能神经标记基因(vglut1)和有丝分裂后神经元标记基因(elavl3)显著减少,即兴奋类神经元和成熟神经元减少等类似表型,说明这两个基因在参与脑神经功能上具有相关性。但是具体调节和影响的方面具有一定差异性。氨基丁酸类神经元主要负责抑制神经兴奋,在scn1Lab缺失斑马鱼会导致氨基丁酸类神经类神经元显著减少[8],而scn1Laa 缺失斑马鱼与正常斑马鱼比较氨基丁酸类神经类神经元相对增加(P < 0.05),推测可能是因为scn1Laa 主要在外周神经而不是中枢神经中表达,对于抑制兴奋类的神经元影响更小一些。
Nav1.1 (Scn1a )基因与癫痫等脑放电异常的疾病相关联[2, 9, 39-40],而脑神经发育异常也会体现在运动行为上,缺失scn1Laa 后斑马鱼运动更为频繁,并且具有爆发性运动行为。推测可能是scn1Laa 功能缺失后,相关主导神经兴奋(如谷氨酰胺类神经元)、抑制的神经元(如氨基丁酸类神经元)表达发生变化,以及成熟神经元大量减少,影响了脑周围神经放电功能,从而导致运动神经出现调节障碍,表现为运动行为过于兴奋,出现频繁运动和爆发性运动等异常现象。
| [1] |
MUSTO E, GARDELLA E, MØLLER R S. Recent advances in treatment of epilepsy-related sodium channelopathies[J]. European Journal of Paediatric Neurology, 2020, 24: 123-128. DOI:10.1016/j.ejpn.2019.12.009 |
| [2] |
CATTERALL W A, KALUME F, OAKLEY J C. NaV1.1 channels and epilepsy[J]. The Journal of Physiology, 2010, 588(Pt 11): 1849-1859. |
| [3] |
KRUGER L C, ISOM L L. Voltage-gated Na+ channels: not just for conduction[J]. Cold Spring Harbor Perspectives in Biology, 2016, 8(6): a029264. DOI:10.1101/cshperspect.a029264 |
| [4] |
FRASER S P, OZERLAT-GUNDUZ I, BRACKENBURY W J, et al. Regulation of voltage-gated sodium channel expression in cancer: hormones, growth factors and auto-regulation[J]. Philosophical Transactions of the Royal Society B: Biological Sciences, 2014, 369(1638): 20130105. DOI:10.1098/rstb.2013.0105 |
| [5] |
CATTERALL W A. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels[J]. Neuron, 2000, 26(1): 13-25. DOI:10.1016/S0896-6273(00)81133-2 |
| [6] |
YU F H, CATTERALL W A. Overview of the voltage-gated sodium channel family[J]. Genome Biology, 2003, 4(3): 207. DOI:10.1186/gb-2003-4-3-207 |
| [7] |
PATINO G A, ISOM L L. Electrophysiology and beyond: multiple roles of Na+ channel β subunits in development and disease[J]. Neuroscience Letters, 2010, 486(2): 53-59. DOI:10.1016/j.neulet.2010.06.050 |
| [8] |
TIRABOSCHI E, MARTINA S, VAN DER ENT W, et al. New insights into the early mechanisms of epileptogenesis in a zebrafish model of Dravet syndrome[J]. Epilepsia, 2020, 61(3): 549-560. DOI:10.1111/epi.16456 |
| [9] |
WEISS L A, ESCAYG A, KEARNEY J A, et al. Sodium channels SCN1A, SCN2A and SCN3A in familial autism[J]. Molecular Psychiatry, 2003, 8(2): 186-194. DOI:10.1038/sj.mp.4001241 |
| [10] |
OGIWARA I, MIYAMOTO H, MORITA N, et al. Nav1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation[J]. Journal of Neuroscience, 2007, 27(22): 5903-5914. DOI:10.1523/JNEUROSCI.5270-06.2007 |
| [11] |
DE LERA RUIZ M, KRAUS R L. Voltage-gated sodium channels: structure, function, pharmacology, and clinical indications[J]. Journal of Medicinal Chemistry, 2015, 58(18): 7093-7118. DOI:10.1021/jm501981g |
| [12] |
NOVAK A E, TAYLOR A D, PINEDA R H, et al. Embryonic and larval expression of zebrafish voltage-gated sodium channel α-subunit genes[J]. Developmental Dynamics, 2006, 235(7): 1962-1973. DOI:10.1002/dvdy.20811 |
| [13] |
BARABAN S C, DINDAY M T, HORTOPAN G A. Drug screening in Scn1a zebrafish mutant identifies clemizole as a potential Dravet syndrome treatment[J]. Nature Communications, 2013, 4: 2410. DOI:10.1038/ncomms3410 |
| [14] |
SOURBRON J, SMOLDERS I, DE WITTE P, et al. Pharmacological analysis of the anti-epileptic mechanisms of fenfluramine in Scn1a mutant zebrafish[J]. Frontiers in Pharmacology, 2017, 8: 191. |
| [15] |
王燕飞, 徐义平, 鲍宝龙. 斑马鱼HCS基因全长cDNA序列及早期仔鱼阶段的空间表达[J]. 上海海洋大学学报, 2013, 22(3): 321-327. WANG Y F, XU Y P, BAO B L. Cloning and expression of HCS gene in zebrafish larvae[J]. Journal of Shanghai Ocean University, 2013, 22(3): 321-327. |
| [16] |
高磊, 黄亚娟, RACHIT P, 等. 斑马鱼 nos2a基因的生长调控作用[J]. 上海海洋大学学报, 2021, 30(4): 573-580. GAO L, HUANG Y J, RACHIT P, et al. Role of nos2a in regulating the growth of zebrafish (Danio rerio)[J]. Journal of Shanghai Ocean University, 2021, 30(4): 573-580. |
| [17] |
廖永冠, 李育媚, 鲍宝龙. opn1lw2基因在红光诱导斑马鱼皮肤色素细胞形成中的作用[J]. 动物学杂志, 2021, 56(4): 608-616. LIAO Y G, LI Y M, BAO B L. The role of opn1lw2 in the formation of skin pigment cells in zobrafish (Danio rerio) under red irradiation[J]. Chinese Journal of Zoology, 2021, 56(4): 608-616. |
| [18] |
GAO L, PENGLEE R, HUANG Y J, et al. CRISPR/Cas9-induced nos2b mutant zebrafish display behavioral abnormalities[J]. Genes, Brain and Behavior, 2021, 20(5): e12716. |
| [19] |
邱超达, 鲍宝龙. 紫光对斑马鱼皮肤黑色素细胞数量及相关基因表达的影响[J]. 上海海洋大学学报, 2022, 31(1): 1-10. QIU C D, BAO B L. Effect of uvillumination on number of melanocytes and experssion of related genes in sws1 mutant zebrafish[J]. Journal of Shanghai Ocean University, 2022, 31(1): 1-10. |
| [20] |
WILKINSON R N, ELWORTHY S, INGHAM P W, et al. A method for high-throughput PCR-based genotyping of larval zebrafish tail biopsies[J]. Biotechniques, 2013, 55(6): 314-316. DOI:10.2144/000114116 |
| [21] |
CARRINGTON B, VARSHNEY G K, BURGESS S M, et al. CRISPR-STAT: an easy and reliable PCR-based method to evaluate target-specific sgRNA activity[J]. Nucleic Acids Research, 2015, 43(22): e157. DOI:10.1093/nar/gkv802 |
| [22] |
MUELLER T, WULLIMANN M. Atlas of early zebrafish brain development: a tool for molecular neurogenetics[M]. London: Academic Press, 2016.
|
| [23] |
LIU H, LIU Z Z. Aggressive-like behavior and increased glycine transporters in a zebrafish model of CHARGE syndrome[J]. Behavioural Brain Research, 2020, 378: 112293. DOI:10.1016/j.bbr.2019.112293 |
| [24] |
CHEN S J, REICHERT S, SINGH C, et al. Light-dependent regulation of sleep and wake states by prokineticin 2 in zebrafish[J]. Neuron, 2017, 95(1): 153-168. e6. DOI:10.1016/j.neuron.2017.06.001 |
| [25] |
GOOD-AVILA S V, YEGOROV S, HARRON S, et al. Relaxin gene family in teleosts: phylogeny, syntenic mapping, selective constraint, and expression analysis[J]. BMC Evolutionary Biology, 2009, 9: 293. DOI:10.1186/1471-2148-9-293 |
| [26] |
VOLKMANN K, RIEGER S, BABARYKA A, et al. The zebrafish cerebellar rhombic lip is spatially patterned in producing granule cell populations of different functional compartments[J]. Developmental Biology, 2008, 313(1): 167-180. DOI:10.1016/j.ydbio.2007.10.024 |
| [27] |
NIMURA T, ITOH T, HAGIO H, et al. Role of reelin in cell positioning in the cerebellum and the cerebellum-like structure in zebrafish[J]. Developmental Biology, 2019, 455(2): 393-408. DOI:10.1016/j.ydbio.2019.07.010 |
| [28] |
KO C Y, TSAI M Y, TSENG W F, et al. Integration of CNS survival and differentiation by HIF2α[J]. Cell Death & Differentiation, 2011, 18(11): 1757-1770. |
| [29] |
CHUNG A Y, KIM M J, KIM D, et al. Neuron-specific expression of atp6v0c2 in zebrafish CNS[J]. Developmental Dynamics, 2010, 239(9): 2501-2508. DOI:10.1002/dvdy.22383 |
| [30] |
DEE C T, HIRST C S, SHIH Y H, et al. Sox3 regulates both neural fate and differentiation in the zebrafish ectoderm[J]. Developmental Biology, 2008, 320(1): 289-301. DOI:10.1016/j.ydbio.2008.05.542 |
| [31] |
HIGASHIJIMA S I, MANDEL G, FETCHO J R. Distribution of prospective glutamatergic, glycinergic, and GABAergic neurons in embryonic and larval zebrafish[J]. The Journal of Comparative Neurology, 2004, 480(1): 1-18. DOI:10.1002/cne.20278 |
| [32] |
CAPUTI A, MELZER S, MICHAEL M, et al. The long and short of GABAergic neurons[J]. Current Opinion in Neurobiology, 2013, 23(2): 179-186. DOI:10.1016/j.conb.2013.01.021 |
| [33] |
HUANG Z J, PAUL A. The diversity of GABAergic neurons and neural communication elements[J]. Nature Reviews Neuroscience, 2019, 20(9): 563-572. DOI:10.1038/s41583-019-0195-4 |
| [34] |
HABERMACHER C, ANGULO M C, BENAMER N. Glutamate versus GABA in neuron-oligodendroglia communication[J]. Glia, 2019, 67(11): 2092-2106. DOI:10.1002/glia.23618 |
| [35] |
SOUZA B R, ROMANO-SILVA M A, TROPEPE V. Dopamine D2 receptor activity modulates Akt signaling and alters GABAergic neuron development and motor behavior in zebrafish larvae[J]. Journal of Neuroscience, 2011, 31(14): 5512-5525. DOI:10.1523/JNEUROSCI.5548-10.2011 |
| [36] |
MANTEGAZZA M, BROCCOLI V. SCN1A /NaV1.1 channelopathies: mechanisms in expression systems, animal models, and human iPSC models[J]. Epilepsia, 2019, 60(s3): S25-S38. |
| [37] |
CHOW C Y, CHIN Y K Y, MA L L, et al. A selective NaV1.1 activator with potential for treatment of Dravet syndrome epilepsy[J]. Biochemical Pharmacology, 2020, 181: 113991. |
| [38] |
DEVINSKY O, CROSS J H, LAUX L, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome[J]. The New England Journal of Medicine, 2017, 376(21): 2011-2020. |
| [39] |
STEEL D, SYMONDS J D, ZUBERI S M, et al. Dravet syndrome and its mimics: beyond SCN1A[J]. Epilepsia, 2017, 58(11): 1807-1816. |
| [40] |
SCHEFFER I E, NABBOUT R. SCN1A-related phenotypes: epilepsy and beyond[J]. Epilepsia, 2019, 60(s3): 17-24. |
| [41] |
GRONE B P, QU T G, BARABAN S C. Behavioral comorbidities and drug treatments in a zebrafish scn1lab model of Dravet syndrome[J]. eNeuro, 2017, 4(4): ENEURO.0066-17. |
| [42] |
BRENET A, HASSAN-ABDI R, SOMKHIT J, et al. Defective excitatory/inhibitory synaptic balance and increased neuron apoptosis in a zebrafish model of Dravet syndrome[J]. Cells, 2019, 8(10): 1199. |
| [43] |
SOURBRON J, PARTOENS M, SCHELDEMAN C, et al. Drug repurposing for Dravet syndrome in scn1Lab-/- mutant zebrafish[J]. Epilepsia, 2019, 60(2): e8-e13. |
| [44] |
DINDAY M T, BARABAN S C. Large-scale phenotype-based antiepileptic drug screening in a zebrafish model of Dravet syndrome[J]. eNeuro, 2015, 2(4): ENEURO.0068-15. |
2. National Demonstration Center for Experimental Fisheries Science Education, Shanghai Ocean University, Shanghai 201306, China
 2022,
Vol. 31
2022,
Vol. 31


