2. 上海海洋大学 水产科学国家级实验教学示范中心, 上海 201306
无论是自然捕捞还是人工养殖的鱼类,都不同程度地存在土腥味。特别是淡水鱼类,如莫桑比克罗非鱼(Oreochromis mossambicus)、鲤(Cyprinus carpio)、鲢(Hypophthalmichthys molitrix)、草鱼(Ctenopharyngodon idella)等土腥味尤为严重[1]。土腥味成分复杂,指的是多种化合物质的一个总称[2],主要的化合物是土味素Geosmin (Trans-1, 10-dimethyl-trans-9-decalol, 反-1, 10-二甲基-反-9-萘烷醇)和2-甲基异茨醇(2-methylisoborneol, 2-MIB),由放线菌(Actinomycetes)和浮游藻类产生。近年来,我国的东钱湖、滇池和熊河水库等水体中的鱼类都发现存在2-MIB异味问题[3-5]。水产品养殖、加工、销售等整个产业链也会因异味问题而造成巨大的经济损失[6-7]。
2-MIB是由水体中放线菌和蓝细菌(Cyanobacteria)等产生的单萜类化合物[8],具有强烈的霉味。2-MIB是水中土腥味的主要来源,饮用水中的土腥味已成为消费者投诉中最常见的问题[9]。研究表明,9~42 ng/L的2-MIB就能使水体及水产品产生土腥味[10]。鱼腥藻(Anabaena)、颤藻(Oscillatoria)和念珠藻(Nostoc commune Vauch)等淡水藻类可以产生2-MIB[11-12],该代谢产物通过鱼鳃进入鱼体累积,导致鱼体产生特有的腥味,降低鱼肉的品质,进而影响养殖业的经济效益[13-15]。因此,了解2-MIB在鱼体内的分子机制,对水产养殖业具有重要意义。
同为土腥味的萜类化合物土味素Geosmin,已发现在斑马鱼胚胎发育过程中可同时诱导多种反应,包括氧化应激、细胞凋亡和内分泌紊乱[16],而2-MIB对鱼类的影响尚没有引起重视。本研究通过2-MIB暴露斑马鱼,调查其对斑马鱼基因表达的影响,进一步了解2-MIB对斑马鱼的免疫机能产生哪些影响。
1 材料与方法 1.1 样本来源及实验设计本实验斑马鱼采购于上海市岚灵花鸟市场。饲养3 d[(27.0 ± 1)℃],每天早晚各饲喂1次,适应环境[17]。将3月龄的斑马鱼暴露于质量浓度为42 ng/L的2-MIB(Sigma-Aldrich,USA)24 h(每隔4 h换1次药物,保证药物质量浓度不变),对照组为未经2-MIB处理的斑马鱼。2-MIB处理组和未经2-MIB处理的对照组各设3个平行(每组15尾)。
1.2 RNA提取、文库构建和高通量测序使用Trizol法分别提取用2-MIB处理24 h和未经2-MIB处理的斑马鱼的鳃组织RNA,采用NEBNextR UltraTM RNA Library Prep Kit for IlluminaTM (NEB, USA)完成cDNA文库的构建,基于illumina Novaseq 6000TM高通量平台,进行转录组测序。
1.3 生物信息学分析对测序得到的fastq格式的原始读长进行过滤,去除低质量读长,获得净读长。选取HISAT软件将过滤后的测序序列进行基因组定位分析,比对到RNA Seq测序数据中的spliced read。使用DESeq进行差异基因DEGs(Differentially expressed genes)分析,筛选标准为P < 0.05。采用差异基因表达量FPKM层次聚类分析,分别用H-cluster、K-means和SOM等3种方法对差异基因的相对表达水平值log2r进行聚类。采用GOseq进行GO富集分析。使用KOBAS(2.0)进行Pathway富集分析,确定差异表达基因参与的最主要生化代谢途径和信号转导途径。
1.4 荧光定量PCR(RT-qPCR)Trizol法提取鳃、肝脏及头肾总RNA,使用HiScript Ⅲ RT SuperMix for qPCR (+gDNA wiper)(Vazyme,南京)反转录成cDNA,以cDNA为模板,选择elongation factor 1-alpha(elfa)作为内参基因,使用ABI 7500实时荧光定量PCR仪(Applied Biosystems,USA)进行RT-qPCR[18]。使用qPCR SYBR Geen Master Mix(YENSEN,上海),体系为总体积20 μL(其中包含2 μL特异性引物和1 μL的cDNA),按照以下步骤进行PCR反应:在95 ℃初始变性2 min;随后进行40个循环(95 ℃ 10 s,60 ℃ 30 s)。设置3个重复组,相对表达量计算采用2-ΔΔCt法,使用GraphPad Prism 6.0软件统计分析,实验结果用平均值±标准误(Mean±SE)表示,采用Multiple t-tests比较各基因的表达差异。所涉及到的基因引物序列在表 1中列出。

|
表 1 用于荧光定量PCR的引物序列 Tab.1 Primer sequences for real-time PCR |
取2-MIB处理组和对照组斑马鱼的鳃、肝脏组织,并用预冷0.9%生理盐水进行冲洗,用滤纸吸干称重后置于1.5 mL离心管中,依照总SOD活性检测试剂盒说明书(Beyotime,上海)加入样品制备液,冰上研磨取上清液作为待测样品,按照SOD说明书采用黄嘌呤氧化酶法,使用酶标仪进行测定,所有测定均重复3次,使用GraphPad Prism 6.0软件对数据进行t-test(unpaired)分析。
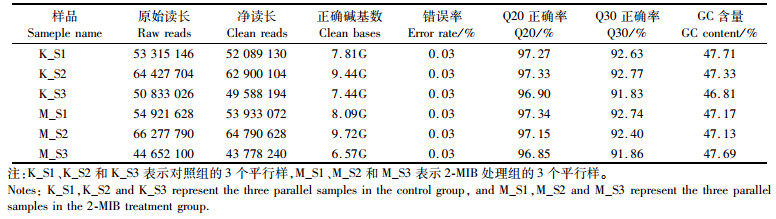
2 结果 2.1 2-MIB暴露对鳃组织转录组表达水平的影响通过对2-MIB处理组和对照组各3个平行的斑马鱼鳃组织进行转录组测序,共获得327 079 368条净读长,各样品Q20正确率均在96.85%以上,Q30正确率均在91.83%以上,GC含量分布在46%到48%之间,可见测序数据质量较高,见表 2转录组测序数据质量。
|
表 2 转录组测序数据质量 Tab.2 Transcriptome sequencing data quality |
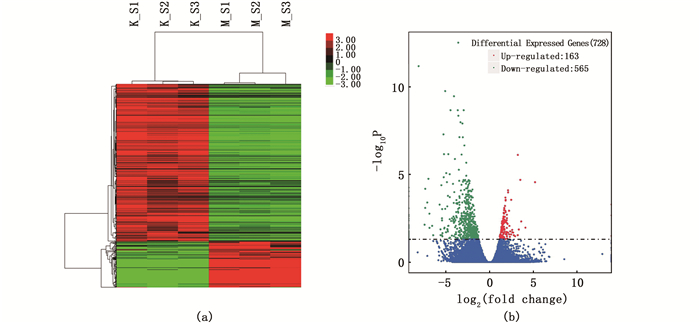
对比2-MIB处理组和对照组,暴露2-MIB的斑马鱼展现出明显的差异基因表达谱[图 1(a)]。与对照组相比,2-MIB处理组共发现728个差异表达基因(P < 0.05),其中163个基因上调,565个基因下调[图 1(b)]。
|
(a)聚类分析图,红色表示基因表达水平高,绿色表示基因表达水平低;(b)差异基因火山图,有显著性差异表达的基因用红色点(上调)和绿色点(下调)表示,无显著性差异表达的基因用蓝色点表示;横坐标代表基因在不同样本中表达倍数变化;纵坐标代表基因表达量变化差异的统计学显著性。 (a)Cluster analysis diagram, red indicates high gene expression level, green indicates low gene expression level; (b)Volcano Plot of differential genes, with red dots (up-regulated) and green dots (down-regulated) representing genes with significant differential expression, and blue dots representing genes with no significant differential expression; The abscissa represents the variation of gene expression multiple in different samples; The ordinate represents the statistically significant difference in gene expression. 图 1 聚类分析图与差异基因火山图 Fig. 1 Cluster analysis diagram and volcano plot of differential genes |
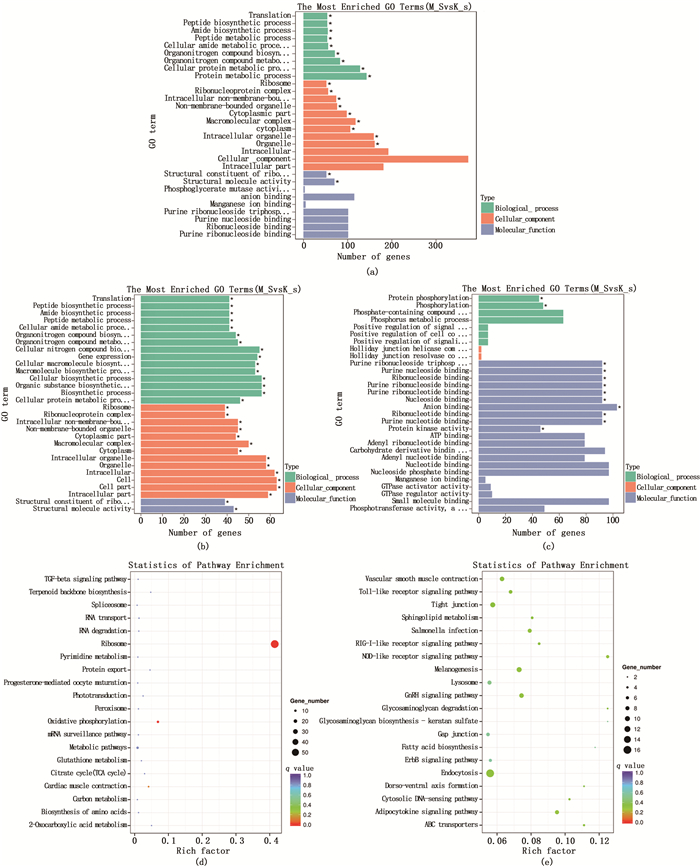
根据GO分析,共筛选出30条重要的富集记录,其中包括9个生物学过程,12个细胞成分和9个分子功能[图 2(a)]。如GO注释所示,生物过程中的“protein metabolic process(蛋白质代谢过程)”,细胞成分中的“organelle(细胞器)”和分子功能中的“structural molecule activity(结构分子活性)”的类别术语最具有代表性。分别对上调差异基因、下调差异基因进行GO富集分析[图 2(b)和(c)]。其中:上调差异基因富集到的GO生物过程包括细胞酰胺代谢过程(cellular amide metabolic process)、肽代谢过程(peptide metabolic process)、细胞蛋白质代谢过程(cellular protein metabolic process)、细胞大分子生物合成(cellular macromolecule biosynthetic process)等;下调差异基因富集到的GO生物过程包括蛋白磷酸化过程(protein phosphorylation)、磷代谢过程(phosphorus metabolic process)、信号转导的正调控(positive regulation of signal transduction)、细胞通信(positive regulation of cell communication)等。
|
(a) 总差异基因的GO富集柱状图,纵坐标为富集的GO term,横坐标为该term中差异基因个数;(b) 显著上调基因的GO归类;(c) 下调差异基因的GO归类;(d) 上调差异基因KEGG富集散点图;(e) 下调差异基因KEGG富集散点图,纵轴表示pathway名称,横轴表示Rich factor,点的大小表示此pathway中差异表达基因个数多少,而点的颜色对应于不同的qvalue范围。 (a) GO enrichment of total differential genes, The ordinate is the enriched GO term, and the abscissa is the number of differential genes in the term; (b) GO classification of up-regulated differential genes; (c) GO classification of down-regulated differential genes; (d) Distribution plot of up-regulated differential genes KEGG pathway enrichment; (e) Distribution plot of down-regulated differential genes KEGG pathway enrichment, The vertical axis represents Pathway name, the horizontal axis Rich factor, the dot size represents the number of differentially expressed genes in this Pathway, and the dot color corresponds to different q value ranges. 图 2 GO富集与KEGG代谢通路分析 Fig. 2 GO enrichment and KEGG metabolic pathway analysis |
对差异基因进行KEGG分析,其中上调差异基因富集到的KEGG通路显著性较高的分别是Ribosome(线粒体)、Oxidative phosphorylation(氧化磷酸化)、Cardiac muscle contraction(心肌收缩)[图 2(d)]。在下调差异基因KEGG通路中与免疫相关的通路主要有Toll-like receptor signaling pathway(Toll样受体信号通路)、NOD-like receptor signaling pathway(NOD样受体信号通路)、Salmonella infection(沙门氏菌感染)、RIG-I-like receptor signaling pathway(RIG-I样受体信号通路)及Adipocytokine signaling pathway(脂肪细胞因子信号通路)[图 2(e)]。
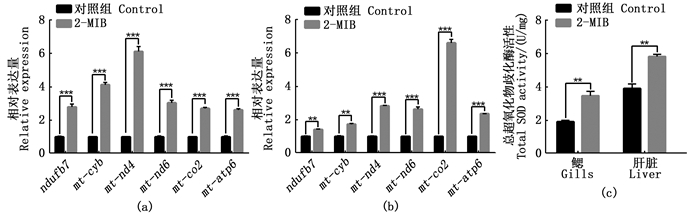
2.3 2-MIB对氧化磷酸化相关基因表达和SOD活性的影响基于转录组结果KEGG通路中氧化磷酸化通路表达上调,利用RT-qPCR检测氧化磷酸化相关的基因在对照组和2-MIB处理组中的表达情况。与对照组相比,2-MIB处理组斑马鱼鳃和肝脏中,氧化磷酸化通路中的ndufb7、mt-cyb、mt-nd4、mt-nd6、mt-co2、mt-atp6基因mRNA表达水平上调,差异极显著(P < 0.01)[图 3(a)和(b)]。
|
(a) 氧化磷酸化相关基因在鳃的表达;(b) 氧化磷酸相关基因在肝脏的表达;(c) 对照组和2-MIB处理组斑马鱼鳃、肝脏SOD活性; * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001具有显著统计学差异,无显著性差异未显示。 (a) Expression of oxidative phosphorylation related genes in gills; (b) Expression of Oxidative Phosphoric Acid Related Genes in Liver; (c) SOD activity in gill and liver of zebrafish in control group and 2-MIB treatment group; * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001 represents statistical significance, no significant difference is not shown. 图 3 氧化磷酸化相关基因表达差异分析和SOD活性变化 Fig. 3 Differential analysis of oxidative phosphorylation related genes expression and change of SOD activity |
进一步检测超氧化物歧化酶SOD活性变化,与对照组相比,2-MIB处理组斑马鱼鳃和肝脏的SOD活性均升高,且差异极显著(P < 0.01)[图 3(c)]。
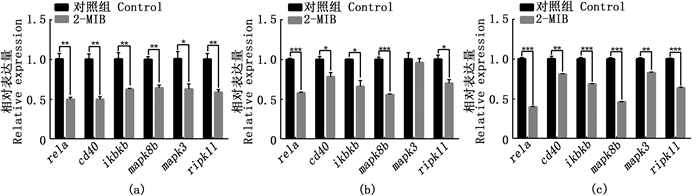
2.4 2-MIB对Toll样受体信号通路基因表达的影响基于转录组结果KEGG通路中免疫通路Toll样受体信号通路表达下调,利用RT-qPCR进一步检测该通路相关基因在斑马鱼鳃、肝脏和头肾的表达情况。与对照组相比,2-MIB处理组斑马鱼鳃、肝脏和头肾中,rela、cd40、ikbkb、mapk8b、ripk1l基因表达水平显著下调(P < 0.05)[图 4(a)、(b)和(c)] mapk3基因表达除了在肝脏中表达无显著性差异外,在鳃和头肾组织中均显著下调[图 4(b)]。
|
(a) 免疫相关基因在鳃的表达;(b) 免疫相关基因在肝脏的表达;(c) 免疫相关基因在头肾的表达; * P < 0.05, ** P < 0.01, ***P < 0.001, ****P < 0.0001具有显著统计学差异,无显著性差异未显示。 (a) Expression of immune-related genes in gills; (b) Expression of immune-related genes in liver; (c) Expression of immunorelated genes in head kidney; * P < 0.05, ** P < 0.01, *** P < 0.001, ****P < 0.0001 represents statistical significance, no significant difference is not shown. 图 4 Toll样受体信号通路相关基因表达差异分析 Fig. 4 Analysis of differential expression of genes related to Toll-like receptor signaling pathway |
通过KEGG通路分析发现,在2-MIB暴露处理斑马鱼鳃转录组中,氧化磷酸化(oxidative phosphorylation)信号通路的显著富集。氧化磷酸化参与细胞三磷酸腺苷(ATP)的产生,此过程发生于线粒体中,参与氧化磷酸化的体系是由5种复合物组成,分布于线粒体的内膜上,构成电子传递链。ndufb7基因和mt-nd4基因都编码复合物Ⅰ(NADH氧化酶)的亚基,且ndufb7基因编码的亚基具有结合NADH和氧化功能,复合物Ⅰ负责将电子从NADH转移到呼吸链中[19-20]。mt-cyb、mt-nd6、mt-atp6基因则是线粒体代谢是否正常的重要指标[21]。my-cyb基因编码复合物Ⅲ(细胞色素c氧化还原酶)中的细胞色素c,其负责催化电子从泛醇转移到细胞色素c,在能量产生中具有重要作用[22]。复合物Ⅳ(细胞色素c氧化酶)在催化氧还原为水的过程中起着核心作用,而mt-co2基因突变会影响复合物Ⅳ中亚基Ⅱ的活性[22]。mt-atp6基因编码复合物V(ATP合成酶)的亚基,负责协助质子参与ATP合成[23]。2-MIB处理斑马鱼,诱导ndufb7、mt-cyb、mt-nd4、mt-nd6、mt-co2、mt-atp6等氧化磷酸化相关基因在鳃和肝脏中表达,表明水体中的2-MIB会刺激斑马鱼提高氧化磷酸化水平。氧化磷酸化产生的活性氧,如过氧化氢、超氧化物等产生的自由基会造成细胞损伤甚至病变[24-25]。超氧化物歧化酶(SOD)是一种抗氧化酶,催化超氧阴离子自由基(O2-·)歧化为H2O2和O2[26]。当产生的活性氧超过生物体的抗氧化防御系统能力时,过多的超氧阴离子自由基会引起膜脂的过氧化,干扰细胞代谢甚至造成细胞死亡,因此超氧阴离子自由基被认为是污染物致毒的重要途径和机制[27-28]。研究[29]发现,经锑处理的斑马鱼鳃和肝脏SOD活性均比对照组高,由于暴露在锑中,氧化胁迫加剧,使得SOD产生代偿性表达。2-MIB处理组鳃和肝脏SOD活性均比对照组高,这表明暴露在2-MIB中,斑马鱼体内自由基大量产生。研究[30]表明,自由基的累积会引发氧化应激反应,而氧化应激会导致机体各种生理功能紊乱。
3.2 2-MIB降低斑马鱼Toll样受体信号通路相关基因的表达2-MIB暴露处理斑马鱼,转录组分析显示Toll样受体信号道路、NOD样受体信号道路、沙门氏菌感染、RIG-Ⅰ样受体信号道路、脂肪细胞因子信号道路等免疫相关通路发生下调,提示水体中的2-MIB可能降低鱼类的免疫力。Toll样受体能识别特定病原体,可诱导肿瘤坏死因子(TNF)、白细胞介素(IL)及干扰素(IFN)等刺激细胞产生清除病原体的效应分子[31-33]。在草鱼感染多子小瓜虫(Ichthyophthirius multifiliis)早期,Toll样受体信号通路表达上调[34]。在Toll样受体信号通路中,ripk1l基因编码的RIP1蛋白, 可激活MAPK信号通路和NF-κB信号通路[35]。mapk3基因和mapk8b基因属于MAPK家族成员,MAPK在细胞内对各种细胞外刺激的反应中起着关键作用[36]。MAPK可作用于激活蛋白-1(activator protein-1, AP-1),从而参与调节细胞增殖、分化、凋亡以及炎症等多种过程[37]。有研究[38]发现,mapk3基因参与斑马鱼抵抗鲤春病毒血症病毒(Spring viremia of carp virus,SVCV)感染反应。在Toll样受体信号通路中,RIP1蛋白促进了ikbkb基因编码的IKKβ蛋白的活化,激活NF-κB信号通路从而参与免疫反应[39]。rela基因属于NF-κB家族成员,调节性T细胞(Tregs)在免疫稳态和预防自身免疫性疾病中起重要作用,研究[40]发现,调节性T细胞缺失rela基因后引发炎症,rela基因对于维持调节性T细胞生物学功能具有重要作用。在半滑舌鳎(Cynoglossus semilaevis)中发现cd40基因参与抵抗哈维氏弧菌(Vibrio harveyi)的免疫应答反应[41]。2-MIB暴露,抑制了斑马鱼鳃、肝脏和头肾等主要免疫组织的Toll样受体信号通路相关基因的表达,rela、cd40、ikbkb、mapk8b、mapk3、ripk1l在经2-MIB处理后均显著下调。这提示我们:广泛存在于水体中的2-MIB,除了会导致鱼类产生土腥味外,还会降低Toll样受体信号通路相关基因的表达;而2-MIB会不会降低鱼类对病原体的识别能力,有待进一步的探究。
本研究首次发现暴露2-MIB提高斑马鱼氧化磷酸化水平,同时降低Toll样受体信号通路相关基因的表达,这为我们在土腥味研究方面提供重要启示,除了考虑导致水产品异味外,2-MIB等土腥味物质还会影响水生动物的生理功能。
| [1] |
JÓNSDÓTTIR R, ÓLAFSDÓTTIR G, CHANIE E, et al. Volatile compounds suitable for rapid detection as quality indicators of cold smoked salmon (Salmo salar)[J]. Food Chemistry, 2008, 109(1): 184-195. DOI:10.1016/j.foodchem.2007.12.006 |
| [2] |
杨玉平, 熊光权, 程薇, 等. 水产品异味物质形成机理、检测及去除技术研究进展[J]. 食品科学, 2009, 30(23): 533-538. YANG Y P, XIONG G Q, CHENG W, et al. Review on formation mechanism, analysis and removal technologies of fishy off-odor[J]. Food Science, 2009, 30(23): 533-538. DOI:10.3321/j.issn:1002-6630.2009.23.119 |
| [3] |
HUANG X, HUANG Z F, CHEN X P, et al. The predominant phytoplankton of Pseudoanabaena holding specific biosynthesis gene-derived occurrence of 2-MIB in a drinking water reservoir[J]. Environmental Science and Pollution Research, 2018, 25(19): 19134-19142. DOI:10.1007/s11356-018-2086-z |
| [4] |
WANG Z J, LI R H. Effects of light and temperature on the odor production of 2-methylisoborneol-producing Pseudoanabaena sp. and geosmin-producing Anabaena ucrainica (Cyanobacteria)[J]. Biochemical Systematics and Ecology, 2015, 58: 219-226. DOI:10.1016/j.bse.2014.12.013 |
| [5] |
ZHANG T, ZHENG L L, LI L, et al. 2-methylisoborneol production characteristics of Pseudoanabaena sp. FACHB 1277 isolated from Xionghe Reservoir, China[J]. Journal of Applied Phycology, 2016, 28(6): 3353-3362. DOI:10.1007/s10811-016-0864-x |
| [6] |
HANSON T R. Economic impact of off-flavor to the U.S. catfish industry[M]//RIMANDO A M, SCHRADER K K. Off-Flavors in Aquaculture. American Chemical Society, 2003: 13-29.
|
| [7] |
PERSSON P E. The source of muddy odor in bream (Abramis brama) from the Porvoo Sea Area (Gulf of Finland)[J]. Journal of the Fisheries Board of Canada, 1979, 36(8): 883-890. DOI:10.1139/f79-126 |
| [8] |
BENTLEY R, MEGANATHAN R. Geosmin and methylisoborneol biosynthesis in streptomycetes: evidence for an isoprenoid pathway and its absence in non-differentiating isolates[J]. FEBS Letters, 1981, 125(2): 220-222. DOI:10.1016/0014-5793(81)80723-5 |
| [9] |
LI L, ZHU C W, XIE C Q, et al. Kinetics and mechanism of Pseudoanabaena cell inactivation, 2-MIB release and degradation under exposure of ozone, chlorine and permanganate[J]. Water Research, 2018, 147: 422-428. DOI:10.1016/j.watres.2018.10.023 |
| [10] |
徐立蒲, 潘勇, 曹欢, 等. 渔业水体中土腥异味物质的研究进展[J]. 大连海洋大学学报, 2010, 25(6): 528-534. XU L P, PAN Y, CAO H, et al. The current research status of odorous compounds in fishery waters[J]. Journal of Dalian Ocean University, 2010, 25(6): 528-534. DOI:10.3969/j.issn.1000-9957.2010.06.010 |
| [11] |
殷守仁, 徐立蒲. 淡水浮游藻类与鱼体异味关系的初步研究[J]. 大连水产学院学报, 2003, 18(2): 156-157. YIN S R, XU L P. A preliminary study on the relationship between freshwater algae and the off-flavor in fishes[J]. Journal of Dalian Fisheries University, 2003, 18(2): 156-157. |
| [12] |
乔庆林. 水产品特有的食源性危害与控制研究的进展[J]. 现代渔业信息, 2009, 24(6): 9-15. QIAO Q L. Research progress in special food-born hazards and controls in fishery products[J]. Modern Fisheries Information, 2009, 24(6): 9-15. |
| [13] |
HOWGATE P. Tainting of farmed fish by geosmin and 2-methylisoborneol: a review of sensory aspects and of uptake/depuration[J]. Aquaculture, 2004, 234(1/4): 155-181. |
| [14] |
殷守仁, 徐立蒲, 赵文, 等. 水产动物异味的研究[J]. 大连水产学院学报, 2003, 18(2): 135-140. YIN S R, XU L P, ZHAO W, et al. A review on the studies of off-flavour in aquatic animals[J]. Journal of Dalian Fisheries University, 2003, 18(2): 135-140. |
| [15] |
PERSSON P E. Uptake and release of environmentally occurring odorous compounds by fish: A review[J]. Water Research, 1984, 18(10): 1263-1271. DOI:10.1016/0043-1354(84)90031-9 |
| [16] |
ZHOU W C, WANG J L, ZHANG J L, et al. Environmentally relevant concentrations of geosmin affect the development, oxidative stress, apoptosis and endocrine disruption of embryo-larval zebrafish[J]. Science of the Total Environment, 2020, 735: 139373. DOI:10.1016/j.scitotenv.2020.139373 |
| [17] |
高磊, 黄亚娟, PENGLEE Rachit, 等. 斑马鱼nos2a基因的生长调控作用[J]. 上海海洋大学学报, 2021, 30(4): 573-580. GAO L, HUANG Y J, PENGLEE R, et al. Role of nos2a in regulating the growth of zebrafish (Danio rerio)[J]. Journal of Shanghai Ocean University, 2021, 30(4): 573-580. |
| [18] |
邱超达, 鲍宝龙. 紫光对斑马鱼皮肤黑色素细胞数量及相关基因表达的影响[J]. 上海海洋大学学报, 2022, 31(1): 1-10. QIU C D, BAO B L. Effects of UV illumination on number of melanocytes and expression of related genes in sws1 mutant zebrafish[J]. Journal of Shanghai Ocean University, 2022, 31(1): 1-10. |
| [19] |
SU F, ZHOU F F, ZHANG T, et al. Quantitative proteomics identified 3 oxidative phosphorylation genes with clinical prognostic significance in gastric cancer[J]. Journal of Cellular and Molecular Medicine, 2020, 24(18): 10842-10854. DOI:10.1111/jcmm.15712 |
| [20] |
VALDIVIESO Á G, MARCUCCI F, TAMINELLI G, et al. The expression of the mitochondrial gene MT-ND4 is downregulated in cystic fibrosis[J]. Biochemical and Biophysical Research Communications, 2007, 356(3): 805-809. DOI:10.1016/j.bbrc.2007.03.057 |
| [21] |
WALLACE L, MEHRABI S, BACANAMWO M, et al. Expression of mitochondrial genes MT-ND1, MT-ND6, MT-CYB, MT-COI, MT-ATP6, and 12S/MT-RNR1 in colorectal adenopolyps[J]. Tumor Biology, 2016, 37(9): 12465-12475. DOI:10.1007/s13277-016-5101-3 |
| [22] |
HEIDARI M M, MIRFAKHRADINI F S, TAYEFI F, et al. Novel point mutations in mitochondrial MT-CO2 gene may be risk factors for coronary artery disease[J]. Applied Biochemistry and Biotechnology, 2020, 191(3): 1326-1339. |
| [23] |
KUCHARCZYK R, DAUTANT A, GOMBEAU K, et al. The pathogenic MT-ATP6 m.8851T>C mutation prevents proton movements within the n-side hydrophilic cleft of the membrane domain of ATP synthase[J]. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 2019, 1860(7): 562-572. |
| [24] |
RATTAN S I S. Theories of biological aging: genes, proteins, and free radicals[J]. Free Radical Research, 2006, 40(12): 1230-1238. |
| [25] |
VALKO M, LEIBFRITZ D, MONCOL J, et al. Free radicals and antioxidants in normal physiological functions and human disease[J]. The International Journal of Biochemistry & Cell Biology, 2007, 39(1): 44-84. |
| [26] |
陈鸿鹏, 谭晓风. 超氧化物歧化酶(SOD)研究综述[J]. 经济林研究, 2007, 25(1): 59-65. CHEN H P, TAN X F. Literature review of researches on superoxide dismutase[J]. Nonwood Forest Research, 2007, 25(1): 59-65. |
| [27] |
WILLIAMS J S, KUNKEL T A. Ribonucleotides in DNA: origins, repair and consequences[J]. DNA Repair, 2014, 19: 27-37. |
| [28] |
LUO H Y, WANG B, LI Z R, et al. Preparation and evaluation of antioxidant peptide from papain hydrolysate of Sphyrna lewini muscle protein[J]. LWT-Food Science and Technology, 2013, 51(1): 281-288. |
| [29] |
徐琨, 杨爱江, 胡霞, 等. 锑在斑马鱼不同组织中的积累及其对抗氧化系统的影响[J]. 生物技术通报, 2021, 37(4): 145-154. XU K, YANG A J, HU X, et al. Antimony accumulation and its effect on antioxidation system in different tissues of Danio rerio[J]. Biotechnology Bulletin, 2021, 37(4): 145-154. |
| [30] |
刘淑兰, 翟少伟. 氧化应激对鱼类的影响及其模型的研究进展[J]. 饲料博览, 2012(2): 48-51. LIU S L, ZHAI S W. Research progress of effects of oxidative stress on fish and oxidative stress model[J]. Feed Review, 2012(2): 48-51. |
| [31] |
PALTI Y. Toll-like receptors in bony fish: from genomics to function[J]. Developmental & Comparative Immunology, 2011, 35(12): 1263-1272. |
| [32] |
LIU G, ZHANG L, ZHAO Y. Modulation of immune responses through direct activation of Toll-like receptors to T cells[J]. Clinical & Experimental Immunology, 2010, 160(2): 168-175. |
| [33] |
何玉洁, 潘建平. 病原菌对NOD样受体及Toll样受体信号通路介导的固有免疫逃逸机制研究进展[J]. 浙江大学学报(医学版), 2017, 46(2): 218-224. HE Y J, PAN J P. Progress on mechanisms for pathogens to evade NOD-like receptor and Toll-like receptor signaling pathways[J]. Journal of Zhejiang University (Medical Sciences), 2017, 46(2): 218-224. |
| [34] |
赵飞, 谭爱萍, 孔璐璐, 等. 多子小瓜虫感染后草鱼TLR信号通路基因的表达动态[J]. 南方农业学报, 2019, 50(9): 1885-1892. ZHAO F, TAN A P, KONG L L, et al. The dynamics of gene expression profiles in toll-like receptor (TLR) signaling pathway of grass carp (Ctenopharyngodon idella) after infection with Ichthyophthirius multifiliis[J]. Journal of Southern Agriculture, 2019, 50(9): 1885-1892. |
| [35] |
李莉, 闫言. 受体相互作用蛋白激酶RIP1在细胞信号传导途径中作用的研究进展[J]. 基础医学与临床, 2011, 31(10): 1165-1167. LI L, YAN Y. Progress of study on functions of RIP1 in cell signal transduction[J]. Basic & Clinical Medicine, 2011, 31(10): 1165-1167. |
| [36] |
CHIOU M J, WANG Y D, KUO C M, et al. Functional analysis of mitogen-activated protein kinase-3 (MAPK3) and its regulation of the promoter region in zebrafish[J]. DNA and Cell Biology, 2007, 26(11): 781-790. |
| [37] |
韩畅畅, 万福生. AP-1的研究进展[J]. 中国细胞生物学学报, 2017, 39(10): 1357-1362. HAN C C, WAN F S. Research progress on AP-1[J]. Chinese Journal of Cell Biology, 2017, 39(10): 1357-1362. |
| [38] |
ENCINAS P, GARCIA-VALTANEN P, CHINCHILLA B, et al. Identification of multipath genes differentially expressed in pathway-targeted microarrays in zebrafish infected and surviving spring viremia carp virus (SVCV) suggest preventive drug candidates[J]. PLoS One, 2013, 8(9): e73553. |
| [39] |
HAYDEN M S, GHOSH S. Signaling to NF-κB[J]. Genes & Development, 2004, 18(18): 2195-2224. |
| [40] |
RONIN E, DI RICCO M L, VALLION R, et al. The NF-κB RelA transcription factor is critical for regulatory T cell activation and stability[J]. Frontiers in Immunology, 2019, 10: 2487. |
| [41] |
位战飞, 郭华, 李海龙, 等. 半滑舌鳎CD40基因克隆及其免疫功能分析[J]. 中国水产科学, 2016, 23(5): 1063-1072. WEI Z F, GUO H, LI H L, et al. Cloning and immunologic function analysis of CD40 from half-smooth tongue sole (Cynoglossus semilaevis)[J]. Journal of Fishery Sciences of China, 2016, 23(5): 1063-1072. |
2. National Demonstration Center for Experimental Fisheries Science Education, Shanghai Ocean University, Shanghai 201306, China
 2022,
Vol. 31
2022,
Vol. 31


