2. 上海海洋大学 水产科学国家级实验教学示范中心, 上海 201306;
3. 上海海洋大学 上海水产养殖工程技术研究中心, 上海 201306;
4. 象山县水产技术推广站, 浙江 象山 315700
皱紫菜[1]是我国南方重要的紫菜野生品种之一,多分布于福建和广东沿岸,尤其是闽南东山岛以南[2]。海洋环境的不断变化,如温室效应引起的海水回温现象,使紫菜出现大规模的烂苗和掉苗,造成了巨大的经济损失[3]。有研究[4]表明坛紫菜、长紫菜和皱紫菜的叶状体在高温胁迫条件下,皱紫菜具有较强的抗性。叶绿素a(Chl.a)、藻蓝蛋白(PC)和藻红蛋白(PE)是紫菜中含有的3种主要光合色素蛋白,它们的含量和相互之间的比例决定了紫菜的质量和叶状体的颜色[5-6]。颜色可以作为遗传育种中的遗传标记。因此,皱紫菜色素突变体的研究,不仅对它的遗传学和生理学具有重要意义,对养殖品种改良也具有重要意义。
目前有关紫菜色素突变体的研究,主要集中在坛紫菜和条斑紫菜,研究者利用它们的色素突变体开展了一系列的遗传学和育种学研究。OHME等[7]利用色素突变体的杂交实验培育出新品系;严兴洪等[8-9]利用坛紫菜色素突变体与野生型品系的杂交实验进一步证明了坛紫菜减数分裂位置;WANG等[10]利用色素突变体研究4种紫菜的早期发育模式和形态发生;也有研究者[11-13]利用色素突变体种间和种内杂交培育出大量的紫菜新品种(品系)。目前,色素突变体主要通过诱变获得,紫菜中应用最广泛的诱变剂为化学诱变剂MNNG和物理诱变剂γ射线[14]。有研究者利用紫外线辐照条斑紫菜和坛紫菜的原生质体,分别获得了体形变异体[15]和红色变异体[16],但未对变异体的稳定性进行验证。紫外线辐照可引起碱基转换、颠换、移码突变或缺失,从而达到变异的效果[17]。相比化学诱变剂MNNG,紫外线具有无需添加任何化学药品、无二次污染、高效率、清洁、低成本等优点[18]。
本文首次采用紫外线辐照皱紫菜叶状体,以期分离出皱紫菜的色素突变体,为皱紫菜的遗传育种研究奠定基础。
1 材料与方法 1.1 实验材料与培养实验材料为皱紫菜野生型品系(PC-WT),采自广东省汕头市南澳岛,经酶解后长出的再生叶状体雌雄分别单独培养,成熟后利用其单性生殖获取纯合的丝状体[19],后者被保存供使用。取适量的自由丝状体,经打碎后制成悬浮液,后者被均匀地喷洒在灭菌过的文蛤(Meretrix meretrix)壳上,黑暗处理4 d后,将其置于温度23 ℃、光照密度10 μmol photons/(m2·s)、光照周期10L:14D下培养,10 d后,清洗贝壳,并将光照密度调整为50 μmol photons/(m2·s)。待丝状体长满整个壳面后,将其转移到温度28 ℃、光照密度20 μmol photons/(m2·s)、光照周期8L:16D下培养,每7天更换1次培养液。待贝壳丝状体成熟后,取1个贝壳置于含5根尼龙单丝的塑料杯中,在温度23 ℃下充气,次日将附有壳孢子的尼龙单丝置于充气瓶中,在温度23 ℃、光照密度50 μmol photons/(m2·s)、光照周期10L:14D下充气培养,每5天更换一半培养液。所用培养液为添加MES的灭菌海水[20]。
1.2 紫外线辐照和色素突变体的分离待PC-WT品系的叶状体长度约1 cm时,随机取30株,分成3组,每组10株平铺在培养皿底部,并吸去藻体表面水分,进行紫外线辐照处理,辐照剂量分别为40、80和120 μW/cm2,5 min后,将叶状体分别放入含有培养液的充气瓶中进行黑暗处理,24 h后,将其移至温度23 ℃、光照密度50 μmol photons/(m2·s)、光照周期10L:14D下充气培养,4周后,显微观察并记录叶状体上出现的色素变异细胞块的种类和数量,每个剂量组统计5株叶状体。采用国际标准色卡-德国RAL色卡,对色素突变体颜色进行判断。继续培养一段时间后,将叶状体分别进行酶解处理,获得它们的单离细胞,后者被培养成再生叶状体[19]。45 d后,从再生叶状体中,挑选出颜色变异的进行单独培养,培养条件同上。当叶状体长到一定大小时,利用单性生殖,分别获取它们的纯合丝状体(品系)[19]。
1.3 色素突变品系的叶状体活体吸收光谱和色素蛋白含量的测定PC-WT品系和色素突变品系的F1叶状体被培养至45 d时,用分光光度计(UV-1750,日本岛津公司)分别测定他们的活体吸收光谱和主要光合色素蛋白含量。活体吸收光谱和叶绿素a(chlorophyll a, Chl.a)含量的测定方法参照ARUGA等[6],藻红蛋白(phycoerythrin, PE)和藻蓝蛋白(phycocyanin, PC)含量的测定方法参照KATO[21]等。
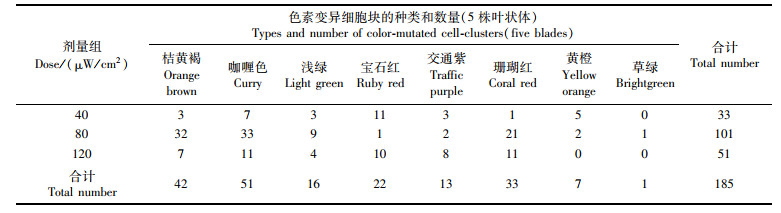
2 结果 2.1 诱变效果经不同剂量的紫外线辐照处理3 d后,PC-WT品系叶状体上出现不同颜色的变异细胞,4周后,变异细胞长成不同大小的细胞块,且野生型细胞和变异细胞块之间的界限清晰,如图版-1~9中所示,变异细胞块有草绿、黄绿、浅绿、宝石红、珊瑚红、交通紫、黄橙、桔黄褐、咖喱色等颜色,其中,宝石红和珊瑚红色块细胞分裂速度较快,黄绿和浅绿色块较慢。如表 1所示,对照组的叶状体上未观察到色素变异细胞块,在辐照组中,剂量为80 μW/cm2时色素变异细胞块的数量最多,在色块的种类上,咖喱色块最多,桔黄褐和珊瑚红色块次之,黄绿色块最少。不同剂量组中,各颜色色块出现的比率差异较大:剂量为40 μW/cm2时,宝石红色出现的比率最大;剂量为80 μW/cm2时,色块主要以桔黄褐色和咖喱色为主;剂量为120 μW/cm2时,咖喱色、宝石红色和珊瑚红色出现的比率较高。

|
1-9.分别为草绿、黄绿、浅绿、宝石红、珊瑚红、交通紫、黄橙、桔黄褐和咖喱色的色素变异细胞块(标尺为50 μm); 10-14.分别是PC-WT(野生型),PC-HL(黄绿色),PC-QL(浅绿色),PC-JH(桔黄褐色)和PC-ZH(宝石红色)的F1叶状体(标尺为2 cm) 1-9. Bright green, yellow green, light green, ruby red, coral red, traffic purple, yellow orange, orange brown and curry color-mutated cell-clusters, respectively(Bar=50 μm); 10-14. F1 gametophytic blade of PC-HL, PC-JH, PC-QL and PC-ZH strain in Pyropia crispata after being cultured for 45 days, respectively (Bar=2 cm) 图版 经紫外线辐照后在皱紫菜野生型品系(PC-WT)叶状体上形成的不同颜色变异细胞块的显微照片,以及PC-WT品系和色素突变品系(PC-HL,PC-JH,PC-QL,PC-ZH)的F1叶状体 Plate Plate Micrographs of color-mutated cell-clusters appeared in the gametophytic blade of the wild-type strain (PC-WT) in Pyropia crispata after being irradiated with ultraviolet ray, and F1 gametophytic blade of PC-WT and mutant strains (PC-HL, PC-JH, PC-QL, PC-ZH) in Pyropia crispata |
将含有色素变异细胞块的叶状体进行酶解,获得大量的单离体细胞,后者被培养约60 d后,从再生叶状体中挑选到了桔黄褐、宝石红、黄绿和浅绿等颜色的单色突变体,分别命名为PC-JH、PC-ZH、PC-HL和PC-QL。利用单性生殖分别获得各色素突变体的纯合丝状体(品系),后者成熟后释放的壳孢子长成叶状体(图版-10~14),其颜色与它们的母体叶状体颜色一致,表明颜色能稳定遗传。
|
表 1 皱紫菜野生型品系(PC-WT)的叶状体经不同剂量的紫外线辐照后再培养28 d时出现的色素变异细胞块的种类和数量 Tab.1 Types and numbers of color-mutated cell-clusters appeared in the blades of the wild-type strain in Pyropia crispata(PC-WT)after being irradiated by ultraviolet ray in different doses and cultured for 28 days |
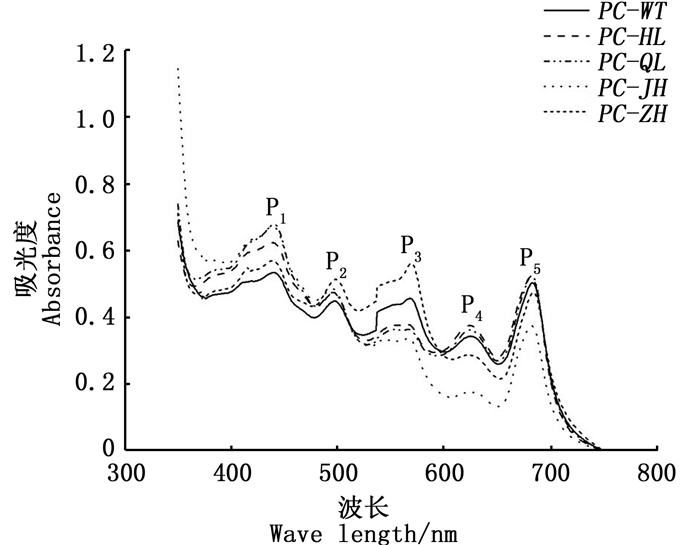
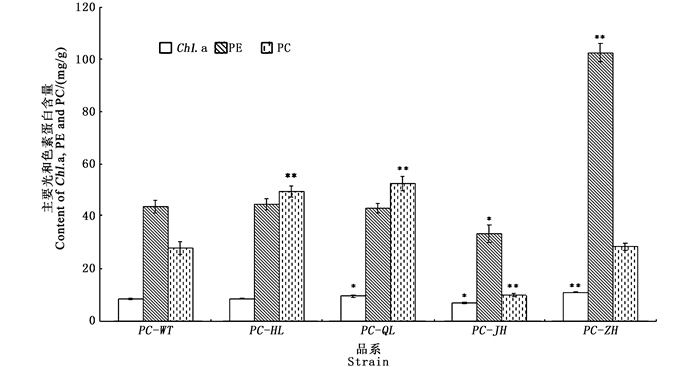
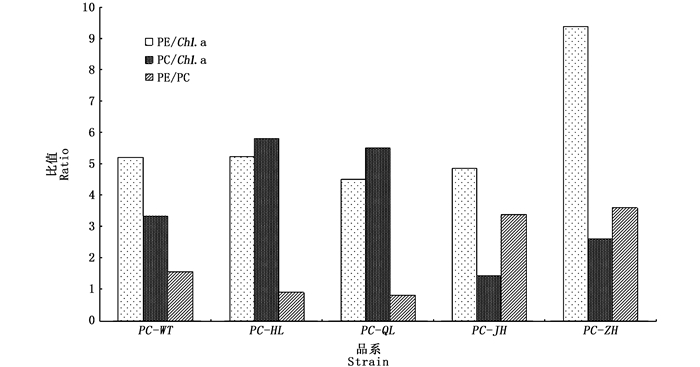
图 1为日龄45 d的F1叶状体在波长350~750 nm的活体吸收光谱,各品系均出现了5个明显的吸收峰,分别标记为P1、P2、P3、P4、P5,但各品系在5个吸收峰处的特性(吸收峰的形状、高度和各峰顶所在的波长位置)存在差异。相比PC-WT品系:PC-HL和PC-QL的5个峰均向短波长移动了1~3 nm;PC-JH的P1、P2和P3峰均向短波长移动2 nm;PC-ZH的P1峰向短波长移动了4 nm,P4峰向短波长移动了3 nm。各色素突变品系的Chl.a、PE和PC含量如图 2所示:2个绿色型突变品系(PC-HL和PC-QL)之间的主要光合色素蛋白含量的差异不大,且它们的PE含量与PC-WT也没有显著差异,但它们的PC含量均显著高于PC-WT;PC-JH的PE和PC含量均明显低于PC-WT;PC-ZH的PE含量显著高于PC-WT,但它的PC含量与PC-WT无显著差异。各色素突变品系和PC-WT品系的F1叶状体的3种色素蛋白之间的比值如图 3所示,与PC-WT品系相比,4种色素突变品系的PE/Chl.a、PC/Chl.a和PE/PC的值表现出明显的差异,除了2个绿色型突变品系之间的差异不大,另外2个色素突变品系之间也表现出较大的差异。
|
图 1 皱紫菜野生型品系(PC-WT)和各色素突变品系(PC-HL, PC-QL, PC-JH, PC-ZH)的F1叶状体(45 d)的活体吸收光谱 Fig. 1 In vivo absorption spectra of F1 gametophytic blades of the wild-type strain (PC-WT) and pigment-mutant strains (PC-HL, PC-QL, PC-JH, PC-ZH) of Pyropia crispata after being cultured for 45 days |
|
*表示与PC-WT品系差异显著(P<0.05); **表示与PC-WT品系差异极显著(P<0.01) * meant significant difference with PC-WT strain (P<0.05), ** meant highly significant difference with PC-WT strain (P < 0.01) 图 2 皱紫菜野生型品系(PC-WT)和各色素突变品系(PC-HL, PC-QL, PC-JH, PC-ZH)的F1叶状体(45 d)的主要光合色素蛋白含量 Fig. 2 Contents of chlorophyll a (Chl. a), phycoerythrin (PE), phycocyanin (PC) in F1 gametophytic blades of the wild-type strain (PC-WT) and pigment-mutant strains (PC-HL, PC-QL, PC-JH, PC-ZH) of Pyropia crispata after being cultured for 45 days |
|
图 3 皱紫菜野生型品系(PC-WT)和各色素突变品系(PC-HL, PC-QL, PC-JH, PC-ZH)的F1叶状体(45 d)中3种色素蛋白之间的比值 Fig. 3 PE/Chl. a, PC/Chl. a and PE/PC ratio in F1 gametophytic blades of the wild-type strain(PC-WT) and pigment-mutant strains (PC-HL, PC-QL, PC-JH, PC-ZH) of Pyropia crispata after being cultured for 45 days |
目前,紫外线已广泛应用于高等植物的生长[22-24]和育种[25-26]研究。20世纪90年代,我国研究者开始将紫外线作为诱变剂应用于紫菜[15-16, 27]。但上述研究的材料主要是紫菜的原生质体,本实验尝试采用紫外线辐照皱紫菜的叶状体,并获得了良好的诱变效果。在一定范围内,剂量越高变异细胞块越多,但剂量达到120 μW/cm2时反而变少,其中,剂量为80 μW/cm2获得的变异细胞块数最多。在变异细胞块的种类上,以黄色型变异细胞块居多。另外,紫外线剂量不同,细胞块的颜色偏好性也不相同,这与γ射线诱变条斑紫菜[28]和坛紫菜[33]叶状体后以形成红色型为主有所不同,有待进一步研究。
近30年来,研究者分别用MNNG和γ射线分离出大量的条斑紫菜[29-31]和坛紫菜[32-34]的色素突变体,后者的应用极大地推动了这2种紫菜的遗传学和育种学研究。利用紫外线辐照皱紫菜的叶状体,分离出了4个纯色的色素突变体,分别为PC-JH(桔黄褐色)、PC-ZH(宝石红色)、PC-HL(黄绿色)、PC-QL(浅绿色),通过单性生殖分别获得纯合品系,各突变品系的F1叶状体的颜色和形态均一,且与其母体叶状体相同,表明各突变品系的颜色等突变性状均能稳定遗传。各色素突变品系颜色的变异不仅导致了3种色素蛋白的含量及其相互间比值的变化,而且活体吸收光谱的性质也发生了不同程度的变化。与野生型品系(PC-WT)相比,2个绿色型突变品系(PC-HL和PC-QL)的PC含量和PC/Chl.a值明显增加,PE/PC值明显降低,比值接近于1,这与条斑紫菜人工绿色突变体的表现较一致[35]。桔黄褐色突变品系(PC-JH)的3种色素蛋白含量均降低,且PC/Chl.a值也较低,但PE/Chl.a和PE/PC值较高,这与条斑紫菜桔黄色型突变体[29]的变化差异较大。宝石红色突变品系(PC-ZH)的PE含量明显增加,相应地PE/Chl.a和PE/PC值也明显增加,这与条斑紫菜红色型自然色素突变体(C-22)的变化一致[6]。藻类是通过光合色素吸收转化光能进行碳固定而生长的,理论上光合色素含量越高,吸收利用的光能越多,生长速率越快[4]。在坛紫菜色素突变体研究中,叶状体的生长速率均是光合色素含量高的品系高于含量低的品系。在本实验中,皱紫菜宝石红色突变品系(PC-ZH)的光合色素含量(Chl.a+PE+PC)最高,理论上具有生长方面的优势,可优先被选择为良种培育的材料。
综上所述,紫外线对皱紫菜叶状体具有良好的诱变效果,本实验分离到4个皱紫菜的色素突变品系,它们将成为皱紫菜遗传育种研究的重要材料。
| [1] |
SUTHERLAND J E, LINDSTROM S C, NELSON W A, et al. A new look at an ancient order:genericrevision of the Bangiales (Rhodophyta)[J]. Journal of Phycology, 2011, 47(5): 1131-1151. DOI:10.1111/j.1529-8817.2011.01052.x |
| [2] |
曾呈奎, 张德瑞, 张峻甫, 等. 中国经济海藻志[M]. 北京: 科学出版社, 1962: 103-104. ZENG C K, ZHANG D R, ZHANG J F, et al. China's economic seaweed[M]. Beijing: Science Press, 1962: 103-104. |
| [3] |
王华芝, 严兴洪, 李琳. 条斑紫菜(Porphyra yezoensis)耐高温品系的筛选及特性分析[J]. 海洋与湖沼, 2012, 43(2): 363-369. WANG H Z, YAN X H, LI L. Selection and characterization of a high-temperature resistant strain of Porphyra yezoensis Ueda (Bangiales, Rhodophyta)[J]. Oceanologia et Limnologia Sinica, 2012, 43(2): 363-369. |
| [4] |
陈伟洲, 许俊宾, 吴文婷, 等. 三种紫菜叶状体对高温胁迫的生理响应[J]. 热带海洋学报, 2015, 34(1): 49-55. CHEN W Z, XU J B, WU W T, et al. Physiological responses of three species of Pyropia thallus to high temperature stress[J]. Journal of Tropical Oceanography, 2015, 34(1): 49-55. DOI:10.3969/j.issn.1009-5470.2015.01.007 |
| [5] |
ARUGA Y. Color and the pigments of Porphyra yezoensis[J]. Iden, 1980, 34(9): 8-13. |
| [6] |
ARUGA Y, MIURA A. In vivo absorption spectra and pigment contents of the two types of color mutants of Porphyra[J]. Japanese Journal of Phycology, 1984, 32(3): 243-250. |
| [7] |
OHME M, KUNIFUJI Y, MIURA A. Cross experiments of the color mutants in Porphyra yezoensis Ueda[J]. Japanese Journal of Phycology, 1986, 34: 101-106. |
| [8] |
YAN X H, LI L, ARUGA Y. Genetic analysis of the position of meiosis in Porphyra haitanensis Chang et Zheng (Bangiales, Rhodophyta)[J]. Journal of Applied Phycology, 2005, 17(6): 467-473. DOI:10.1007/s10811-005-2752-7 |
| [9] |
严兴洪, 李琳, 有贺佑胜. 坛紫菜减数分裂位置的杂交试验分析[J]. 水产学报, 2006, 30(1): 1-8. YAN X H, LI L, ARUGA Y. Cross experiments and analysis of the position of meiosis in Porphyra haitanensis(Rhodophyta)[J]. Journal of Fisheries of China, 2006, 30(1): 1-8. |
| [10] |
WANG J F, ZHU J Y, ZHOU W, et al. Early development patterns and morphogenesis of blades in four species of Porphyra (Bangiales, Rhodophyta)[J]. Journal of Applied Phycology, 2010, 22(3): 297-303. DOI:10.1007/s10811-009-9459-0 |
| [11] |
吴宏肖, 严兴洪, 宋武林, 等. 坛紫菜与Pyropia radi种间杂交重组优良品系的选育与特性分析[J]. 水产学报, 2014, 38(8): 1079-1088. WU H X, YAN X H, SONG W L, et al. Selection and characterization of an improved strain produced by genetic recombinant of interspecific hybridization between Pyropia haitanensis and Pyropia radi[J]. Journal of Fisheries of China, 2014, 38(8): 1079-1088. |
| [12] |
刘美君, 黄林彬, 严兴洪. 条斑紫菜种内杂交优良品系的筛选与特性分析[J]. 中国水产科学, 2015, 22(1): 33-43. LIU M J, HUANG L B, YAN X H. Isolation and characterization of the improved strain HW-4 by in-traspecific hybridization in Pyropia yezoensis[J]. Journal of Fishery Sciences of China, 2015, 22(1): 33-43. |
| [13] |
DING H C, LV F, WU H X, et al. Selection and characterization of an improved strain produced by inter-species hybridization between Pyropia sp.from India and Pyropia haitanensis from China[J]. Aquaculture, 2018, 491: 177-183. DOI:10.1016/j.aquaculture.2018.03.015 |
| [14] |
李淑平, 严兴洪. 60Co-γ射线辐照对长紫菜的诱变效果及优良品系分离与特性分析[J]. 海洋学报, 2015, 37(10): 69-79. LI S P, YAN X H. Isolation and characterization of an improved strain of Pyropia dentata (Bangiales, Rhodophyta) after being irradiated by 60Co-γ ray[J]. Acta Oceanologica Sinica, 2015, 37(10): 69-79. DOI:10.3969/j.issn.0253-4193.2015.10.007 |
| [15] |
戴继勋, 张全启, 包振民, 等. 紫菜原生质体的纯系培育、诱变处理和种间细胞融合的研究[J]. 海洋与湖沼, 1990, 21(3): 293-296. DAI J X, ZHANG Q Q, BAO Z M, et al. Studies on the pure line culture, mutagenization and interspecific fusion of Porphyra protoplasts[J]. Oceanologia et Limnologia Sinica, 1990, 21(3): 293-296. |
| [16] |
严兴洪. 紫外线辐射与条斑紫菜原生质体后代发育和变异[J]. 上海水产大学学报, 1992, 1(1/2): 71-78. YAN X H. Effects of UV irradiation on the development and variations of protoplast progenies in Porphyra yezoensis[J]. Journal of Shanghai Fisheries University, 1992, 1(1/2): 71-78. |
| [17] |
刘宝.角蛋白降解菌的紫外线和硫酸二乙酯复合诱变[D].南京: 南京农业大学, 2012: 14. LIU B.Complex mutagenesis of keratinose degrading strain with ultraviolet ray and diethyl sufate[D].Nanjing: Nanjing Agricultural University, 2012: 14. |
| [18] |
何培民, 秦松, 严小军, 等. 海藻生物技术及其应用[M]. 北京: 化学工业出版社, 2007: 59-61. HE P M, QIN S, YAN X J, et al. Seaweed biological technology and application[M]. Beijing: Chemical Industry Press, 2007: 59-61. |
| [19] |
严兴洪, 李琳, 陈俊华, 等. 坛紫菜的单性生殖与遗传纯系分离[J]. 高技术通讯, 2007, 17(2): 205-210. YAN X H, LI L, CHEN J H, et al. Parthenogenesis and isolation of genetic pure strains in Porphyra haitanensis (Bangiales, Rhodophyta)[J]. High Technology Letters, 2007, 17(2): 205-210. DOI:10.3321/j.issn:1002-0470.2007.02.019 |
| [20] |
王素娟, 张小平, 徐志东, 等. 坛紫菜营养细胞和原生质体培养的研究Ⅰ[J]. 海洋与湖沼, 1986, 17(3): 217-221. WANG S J, ZHANG X P, XU Z D, et al. A study on the cultivation of the vegetative cells and protoplasts of P.yezoensis[J]. Oceanologia et Limnologia Sinica, 1986, 17(3): 217-221. |
| [21] |
KATO M, ARUGA Y. Comparative studies on the growth and photosynthesis of the pigmentation mutants of Porphyra yezoensis in laboratory culture[J]. Japanese Journal of Phycology, 1984, 32: 333-347. |
| [22] |
赵东旭, 杨新芳, 刘亚丽, 等. 紫外线辐射对小麦幼苗生长的影响[J]. 河南科学, 1996, 14(s1): 32-35. ZHAO D X, YANG X F, LIU Y L, et al. Effects of ultraviolet radiation on growth of wheat seedlings[J]. Henan Sciences, 1996, 14(s1): 32-35. |
| [23] |
张敏.紫外线对霞多丽葡萄品质及相关生化物质的影响[D].晋中: 山西农业大学, 2016: 16-30. ZHANG M.The affect of ultraviolet radiation to the quality and relevant substances of Chardonnay[D].Jinzhong: Shanxi Agricultural University, 2016: 16-30. |
| [24] |
岳明, 王勋陵. 紫外线辐射对小麦和燕麦竞争性平衡的影响--小麦和燕麦生物量结构与冠层结构[J]. 环境科学学报, 1999, 19(5): 526-531. YUE M, WANG Y L. Effect of enhanced ultraviolet radiation on competitive balance of wheat and oat-the structure of biomass and canopy[J]. Acta Scientiae Circumstantiae, 1999, 19(5): 526-531. DOI:10.3321/j.issn:0253-2468.1999.05.012 |
| [25] |
陈爱芹.紫外线诱变处理对马铃薯茎段再生植株生理生化变异的影响[D].长沙: 湖南农业大学, 2008: 26-34. CHEN A Q.Physiological and biochemical variation of the potato stem section regeneration plants by ultraviolet radiation[D].Changsha: Hunan Agricultural University, 2008: 26-34. |
| [26] |
李波, 贾秀峰, 焦德志, 等. 紫外线诱变苜蓿愈伤组织抗旱性的研究[J]. 干旱地区农业研究, 2005, 26(6): 103-105. LI B, JIA X F, JIAO D Z, et al. Influence of ultraviolet ray to drought resistance of alfalfa calluses[J]. Agricultural Research in the Arid Areas, 2005, 26(6): 103-105. DOI:10.3321/j.issn:1000-7601.2005.06.021 |
| [27] |
黄世玉, 王志勇. 紫外线辐射坛紫菜离体细胞的初步试验[J]. 集美大学学报, 1997, 2(1): 25-30. HUANG S Y, WANG Z Y. Preliminary experiment on isolated cells from Porphyra haitanensis (Rhodophyta) radiated by ultraviolet ray[J]. Journal of Jimei University, 1997, 2(1): 25-30. |
| [28] |
严兴洪, 张淑娟, 黄林彬. 60Co-γ射线对条斑紫菜(Porphyra yezoensis)的诱变效果与色素突变体分离[J]. 海洋与湖沼, 2009, 40(1): 56-61. YAN X H, ZHANG S J, HUANG L B. Induction and isolation of pigmentation mutants of Porphyra yezoensis Ueda (Bangiales, Rhodophyta) by 60Co-γ ray irradiation[J]. Oceanologia et Limnologia Sinica, 2009, 40(1): 56-61. DOI:10.3321/j.issn:0029-814X.2009.01.009 |
| [29] |
YAN X H, ARUGA Y. Induction of pigmentation mutants by treatment of monospore germlings with NNG in Porphyra yezoensis Ueda (Bangiales, Rhodophyta)[J]. Algae, 1997, 12(1): 39-52. |
| [30] |
YAN X H, FUJITA Y, ARUGA Y. Induction and characterization of pigmentation mutants in Porphyra yezoensis(Bangiales, Rhodophyta)[J]. Journal of Applied Phycology, 2000, 12(1): 69-81. DOI:10.1023/A:1008129119065 |
| [31] |
王素娟, 马凌波, 许璞, 等. 60Co-γ射线诱变条斑紫菜丝状体的研究[J]. 海洋科学, 1999(4): 43-47. WANG S J, MA L B, XU P, et al. Study on using gamma-ray to induce mutation in conchocelis of Porphyra yezoensis[J]. Marine Sciences, 1999(4): 43-47. DOI:10.3969/j.issn.1000-3096.1999.04.017 |
| [32] |
李琳, 严兴洪. 坛紫菜绿色突变体的分离与特性分析[J]. 上海水产大学学报, 2006, 15(1): 30-35. LI L, YAN X H. Isolation and characterization of a green type mutant in Porphyra haitanensis Chang et Zheng (Bangiales, Rhodophyta)[J]. Journal of Shanghai Fisheries University, 2006, 15(1): 30-35. |
| [33] |
匡梅, 许璞, 王素娟. γ-射线对条斑紫菜和坛紫菜诱变作用的初步研究[J]. 上海水产大学学报, 1997, 6(4): 241-245. KUANG M, XU P, WANG S J. A preliminary study of the mutagensis of γ-rays on Porphyra yezoensis and P.haitanensis[J]. Journal of Shanghai Fisheries University, 1997, 6(4): 241-245. |
| [34] |
严兴洪, 梁志强, 宋武林, 等. 坛紫菜人工色素突变体的诱变与分离[J]. 水产学报, 2005, 29(2): 166-172. YAN X H, LIANG Z Q, SONG W L, et al. Induction and isolation of artificial pigmentation mutants in Porphyra haitanensis Chang et Zheng (Bangiales, Rhodophyta)[J]. Journal of Fisheries of China, 2005, 29(2): 166-172. |
| [35] |
严兴洪, 田中次郎, 有贺佑胜. 条斑紫菜色彩突变体的诱导、分离和特性分析[J]. 水产学报, 2000, 24(3): 221-228. YAN X H, TANAKA J, ARUGA Y. Isolation and characterization of pigmentation mutants in Porphyra yezoensis Ueda (Bangiales, Rhodophyta)[J]. Journal of Fisheries of China, 2000, 24(3): 221-228. |
2. National Demonstration Center for Experimental Fisheries Science Education, Shanghai Ocean University, Shanghai 201306, China;
3. Shanghai Engineering Research Center of Aquaculture, Shanghai Ocean University, Shanghai 201306, China;
4. Xiangshan Fisheries Technical Extension Center, Xiangshan 315700, Zhejiang, China
 2019,
Vol. 28
2019,
Vol. 28


