2. 海洋动物系统分类与进化上海高校重点实验室, 上海 201306;
3. 水产科学国家级实验教学示范中心, 上海 201306;
4. 水产动物遗传育种中心上海市协同创新中心, 上海 201306
声信交流是广泛存在于鱼类中的一种快捷而有效的交流方式,已知约有100种左右的鱼类行为是与声音信号紧密关联的[1-2]。声音可以作为鱼类活动的一种信号,例如鱼类通过声音信号定位、捕食或逃避捕食者,或通过发声惊吓其他种群[2]。不少鱼类依靠特殊的声音信号来吸引配偶,达到成功交配[1-3]。同时,鱼类声音信号可作为鱼类个体辨别同物种与其他物种的依据[4-5],因此研究鱼类的发声具有重要意义。鱼类不具有声带,可靠身体不同部位的摩擦和振动产生声音,或者通过鱼鳔等器官发出各种不同的声音[6-7]。发声肌是鱼类主动发声的特殊组织结构,可通过发声肌快速收缩带动鱼鳔振动发声。鱼鳔联合附着鱼鳔上的发声肌构成了特殊的发声系统,发声肌快速收缩带动鱼鳔振动,进而发声[8-10]。繁殖季节,雄性发声鱼类通过发出求偶声吸引雌性到栖息地进行交配,故发声肌的发育对鱼类繁殖具有重大意义。发声肌可分为内在发声肌和外在发声肌:内在发声肌起源于鱼鳔壁并插入鱼鳔内;外在发声肌则只是附着于鱼鳔上,相对独立[11]。由于海洋鱼类声音录制难度较大,解剖发声系统的形态结构是研究其发声特征的常用手段[11-18]。
褐菖鲉(Sebastiscus marmoratus)为暖水性底层鱼类,典型的岛礁定居鱼类。褐菖鲉依靠发声肌-鱼鳔系统发声,其发声肌属于外在发声肌,一对发声肌左右对称分布于鱼鳔背侧两侧,前端从翼耳骨出发,后端与鱼鳔末端相连,见图 1[19]。在繁殖季节,发声鱼类雄性通常通过发声吸引雌性进入栖息地进行个体交配,发声肌越重的雄鱼越能得到雌性的青睐, 雄性的发声特性包括发声频率、振幅等参数与发声肌的收缩频率相关,发声肌收缩频率与发声肌质量、大小密切相关[11-21]。有研究[22]表明,一些发声鱼类的发声与性腺具有同步性。褐菖鲉属于卵胎生鱼类,繁殖季节约10月至翌年5月[23],且雄性褐菖鲉致力于求偶行为更甚于精子的产生,雄鱼求偶发声可能是雌鱼选择配偶的重要依据[20],由于雌鱼一般喜选择“体大强壮”的配偶,因此可以假设:褐菖鲉雄性发声肌的发育应该与其体长、质量等生长发育状态密切相关。为验证这个假设,运用解剖方法,对褐菖鲉不同性别的个体,进行发声肌发育及其个体发育的量化比较,探究二者间发育过程是否相关。

|
1.肝脏; 2.鱼鳔; 3.发声肌 1. Liver; 2. Swimming bladder; 3. Sonic muscle 图 1 褐菖鲉解剖侧面视图 Fig. 1 Dorsal view of anatomy map of S. marmoratus |
目前国内外关于褐菖鲉发声与发声机制的研究相对较少,研究褐菖鲉的发声机制的生物学机理,可以为声学技术在渔业的应用作出贡献,为鲉科鱼类的研究提供更多的依据。褐菖鲉肉质鲜美故具有很高的经济价值,胎卵生的褐菖鲉大规模人工养殖还很难实现,而依靠捕捞供应已导致褐菖鲉资源数量溃减。对褐菖鲉发声机制与发声行为的研究将对其人工繁殖和资源保护作出贡献。
1 材料与方法 1.1 实验材料实验用鱼在2016和2017年11—12月分2批次采样于浙江枸杞岛(122°45.787′E, 30°43.235′N),由渔民采用定置网具所捕获,处于繁殖期的雌雄个体性腺区别明显,可直接用肉眼观察辨别。其中:计数雌鱼62尾,全长(11.12~18.35) cm,平均(14.20±1.46) cm;雄鱼43尾,全长(10.91~18.35) cm,平均(14.79±1.78) cm。
1.2 实验方法取样和鉴定:(1)先测量鱼体全长、个体质量,再解剖采取生殖腺和发声肌样品。(2)用手术剪刀将实验鱼从肛门处沿上方剪开(图 1),除去鱼体一侧的肌肉和肋骨,暴露内脏器官,用解剖镊子将性腺区分出来,并利用解剖剪将其连接肛门处以及与鱼鳔两侧连接神经分别剪断,先完整地取出精巢或卵巢,置于培养皿中,并于解剖镜以及显微镜下观察,对照文献鉴定和记录性别以及性腺发育期。褐菖鲉在非繁殖期单从个体外在形态无法直接判定性别,但是繁殖期则性腺区别明显。(3)用镊子将发声肌周围腹侧部肌肉轻轻挑去,把该侧发声肌整个完全取出(图 2-3),进行测量、记录。

|
(a)腹侧视图; (b)局部侧面视图; (c)侧面视图;1.肝脏; 2.鱼鳔; 3.发声肌; 4.性腺; 5.第一背肋; 6.第二背肋; 7.第三背肋; 8.第四背肋; 9.第五腹肋; 10.第三腹肋; 11.第四腹肋; 12.翼耳骨 (a) Ventral view; (b) Partial side view; (c) Side view; 1. Liver; 2. Swimming bladder; 3. Sonic muscle; 4. Gonadal; 5. First dorsal rib; 6. Second dorsal rib; 7. Third dorsal rib; 8. Fourth dorsal rib; 9. Fifth abdominal rib; 10. Third abdominal rib; 11. Fourth abdominal rib; 12. Pterotic bone 图 2 褐菖鲉发声系统的形态结构 Fig. 2 Morphological structure of sound system |

|
(a)发声肌-鱼鳔腹面图; (b)发声肌-鱼鳔系统背面图 (a) The ventral view of sonic muscle-swimming bladder system; (b) The dorsal view of sonic muscle-swimming bladder 图 3 褐菖鲉发声肌-鱼鳔系统 Fig. 3 The sound production system of Sebastiscus marmoratus |
测量和记录:分别测量鱼体全长(total length, TL)、体质量(body mass,BM)、发声肌长度(sonic muscle length, SL),发声肌质量(sonic muscle mass, SM)、发声肌前端宽(sonicmuscle forefront wide,SWF)和最宽处宽度(一般位于发声肌末端前0.5 mm处,sonic muscle widest part, SWP)。发声肌长度与鱼体全长比值(SL/TL),定义为发声肌长度指数(length index);发声肌质量与体质量比值(SM/BM),定义为发声肌质量指数(mass index)。进行性腺发育前与发育期对比。
实验所得数据,应用SPSS 19.0、Minitab 17、Excel软件进行分析。
2 结果与分析 2.1 性腺发育期的鉴定实验解剖过程中发现,由于近海捕捞,取样的鱼性腺发育期多样,部分褐菖鲉个体性腺并未发育,全长小于9 cm的褐菖鲉。性腺尚未完全发育,呈白色细线状,即卵巢与精巢都处于Ⅰ期,无法辨别雌雄[24],因此在解剖后实验数据中不计入。样品中最小褐菖鲉全长为10.91 cm。
据文献[24]对褐菖鲉精巢与卵巢的时期划分可知,生殖群体中卵巢时相大多在Ⅲ、Ⅳ期,怀胎的卵巢中也有空滤泡与Ⅲ,Ⅳ期的卵子并存。采样时间为11—12月,为褐菖鲉非繁殖季节到繁殖季节的过渡期,因此性腺形态不一。发育前褐菖鲉性腺多为重复发育的Ⅱ期,且此时卵巢膜明显较厚。进入繁殖季节,大部分个体的卵巢快速从Ⅱ期发育到Ⅲ期,受精后进入怀胎期,可在显微镜下看到卵巢有明显的胚胎以及大量的空滤泡。实验发现,怀卵期的褐菖鲉发声肌相对怀胎期较小,故将卵巢生殖期分为发育前、发育中、怀胎期3个阶段分析。同样,也将精巢分为发育前与生殖期(发育中)两个阶段进行分析。
2.2 性腺发育前和生殖期发声肌形态的对比实验发现:性腺发育前雌雄鱼发声肌长度与全长无显著相关性(图 4,相关系数R2均小于0.6),雌鱼发声肌质量与体质量无相关性,雄鱼发声肌质量与体质量呈线性正相关(R2=0.842 2, 图 5);雌鱼性腺发育中发声肌长度与体长呈线性正相关(R2=0.814 1,P<0.01),雄鱼性腺发育中发声肌长度与体长呈线性正相关(R2=0.757 5,P<0.01, 图 6);雌雄鱼性腺发育中的发声肌质量与体质量都呈线性递增,雄鱼的相关性指数R2=0.850 7(|R|>0.8,P<0.01),有很高的相关性,雌鱼的相关性指数R2=0.642 8(|R|≈0.8,P<0.01),具有较好的相关性(图 7)。
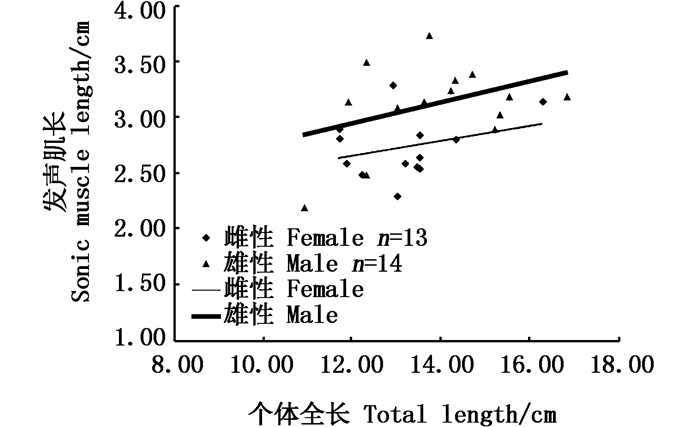
|
图 4 性腺发育前雌雄个体发声肌长与全长相关性分析图 Fig. 4 Relationship between sonic muscle length and total length of pre-gonadal development stage |

|
图 5 性腺发育前雌雄个体发声肌质量与体质量相关性分析图 Fig. 5 Relationship between sonic muscle mass and body mass of pre-gonadal development |
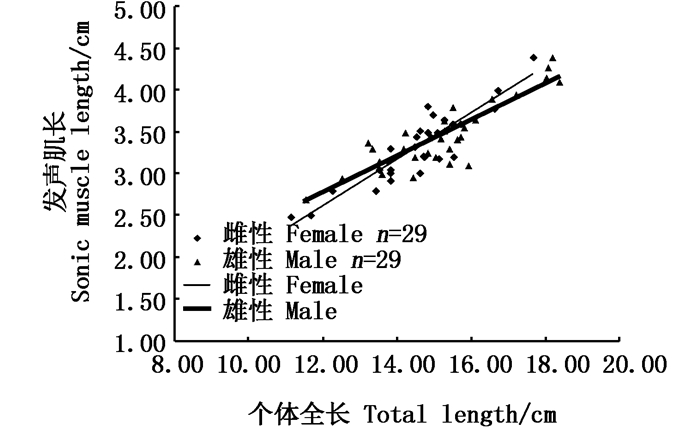
|
图 6 性腺发育中雌雄个体发声肌长与全长相关性分析图 Fig. 6 Relationship between sonic muscle length and total length of female (blue) and male (orange) in gonadal development stage |
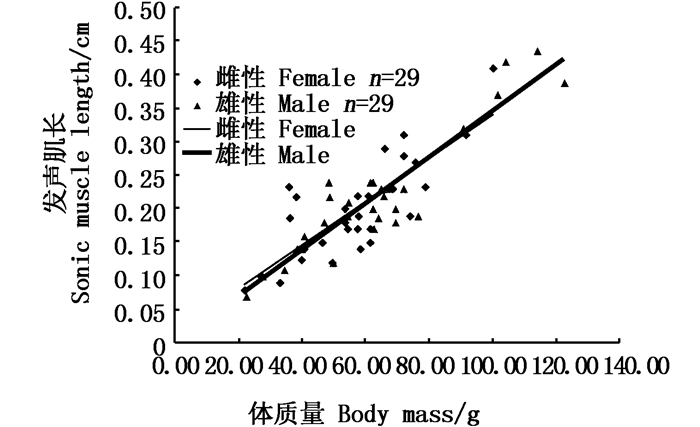
|
图 7 性腺发育中雌雄发声肌重量与体质量相关性分析图 Fig. 7 Relationship between sonic muscle weight and body weight of female (blue) and male (orange) in gonadal development stage |
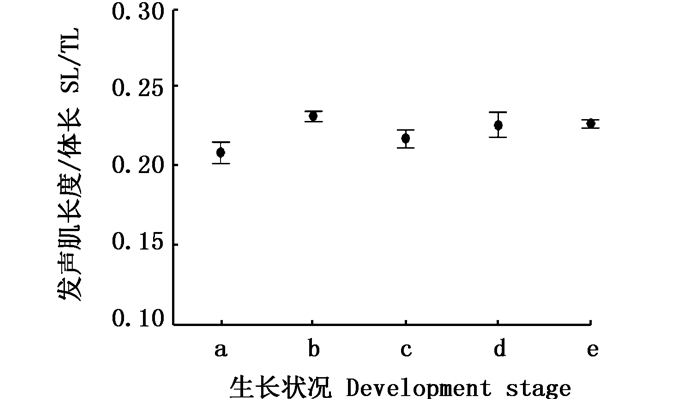
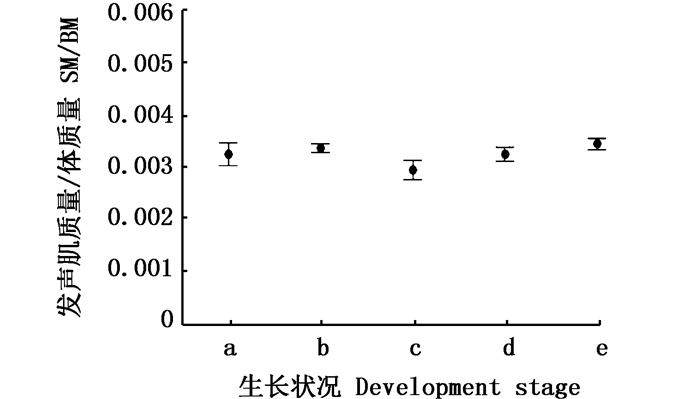
利用发声肌长度指数(SL/TL)和发声肌质量指数(SM/BM)进行量化对比。单因素方差检验(one-way ANOVA)显示,发声肌长度指数组别之间存在着显著性差异(P<0.05, 图 8),发声肌质量指数组别间不存明显差异(P>0.05,图 9)。再利用T双样本异方差假设检验进行两两对比差异分析,对比发现:发育中雌性个体发声肌长度指数(0.23±0.15)大于发育前(0.21± 0.03,P < 0.05),质量指数并没有显著性差异(0.003 4± 0.000 4, 0.003 3± 0.000 8, P>0.05);怀胎期的雌性发声肌长度指数小于发育中(0.22± 0.02,0.23± 0.15,P < 0.05),质量指数小于发育中个体(0.003 0± 0.000 7,0.003 4±0.000 4,P < 0.05),说明雌性个体发声肌在受精之后出现了萎缩;雄性发育前与生殖期的发声肌长度指数无显著差异(0.23±0.01, 0.23±0.03,P>0.05),发育前体质量与生殖期质量指数(0.003 3±0.000 5,0.003 5±0.000 6,P>0.05)无显著差异。
|
a.发育前雌性, n=13;b.发育中雌性, n=22;c.怀胎期雌性, n=13; d.发育前雄性, n=14; e.发育中雄性, n=29 a. Pre-gonadal developed Female, n=13; b. Female in the gonadal development stage, n=22; c. Fertilized female, n=13; d. Pre-gonadal developed Male, n=14; e. Male in the gonadal development stage, n=29 图 8 个体发声肌长度比较区间图 Fig. 8 Interval map for ratio of sonic muscle length to total length |
|
a.发育前雌性, n=13; b.发育中雌性, n=22; c.怀胎期雌性, n=13; d.发育前雄性, n=14; e.发育中雄性, n=29 a. Pre-gonadal developed Female, n=13; b. Female in the gonadal development stage, n=22; c. Fertilized female, n=13; d. Pre-gonadal developed Male, n=14; e. Male in the gonadal development stage, n=29 图 9 个体发声肌质量比较区间图 Fig. 9 Interval map for sonic ratio of muscle mass to total mass |
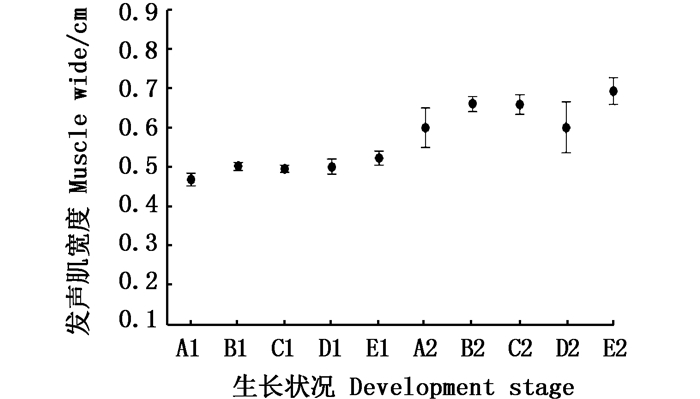
利用单因素方差分析法分别对发声肌的前端宽度(SWF)与最宽处宽度(SWP)(图 10)进行两两对比。SWF的对比:雌性性腺发育中[(0.50± 0.05)cm]大于发育前[(0.49± 0.03)cm,P < 0.05],怀胎期[(0.47± 0.03)cm]与发育中无显著差异(P>0.05);雄性性腺发育中与性腺发育前相近[(0.52± 0.04)cm,(0.50± 0.06)cm],无显著差异(P>0.05)。SWP的对比:雌性性腺发育中[(0.66± 0.09)cm]、怀胎期[(0.66±0.09)cm]和性腺发育前[(0.60± 0.09)cm]个体3者进行两两对比,无显著差异(P>0.05);雄性性腺发育中个体与发育前阶段[(0.69±0.11)cm, (0.63± 0.10)cm],也无显著差异(P>0.05)。宽度数值对比说明雄鱼褐菖鲉发育前后发声肌在宽度上没有明显的变化,雌鱼发育期宽度大于发育前说明了雌鱼发声肌在求偶发声中起作用。
|
A1.雌性发育前SWF;B1.雌性发育期SWF;C1.雌性怀胎期SWF;D1.雄性发育前SWF;E1.雄性发育期SWF;A2.雌性发育前SWP;B2.雌性发育期SWP;C2.雌性怀胎期SWP;D2.雄性发育前SWP; E2.雄性发育期SWP; 雌性发育前: n=4;雌性发育期: n=22;雌性怀胎期: n=13;雄性发育前: n=4;雄性发育期: n=12 A1. SWF of pre-gonadal developed Female; B1. SWF of Female in the gonadal development stage; C1. SWF of Fertilized individual; D1. SWF of pre-gonadal developed Male; E1. SWF of Male in the gonadal development stage; A2. SWP of pre-gonadal developed Female; B2. SWP of Female in the gonadal development stage; C2. SWP of Fertilized individual; D2. SWP of pre-gonadal developed Male; E2. SWP of Male in the gonadal development stage; Pre-gonadal developed Female, n=4; Female in the gonadal development stage, n=22; Fertilized individual, n=13;Pre-gonadal developed Male, n=4; Male in the gonadal development stage, n=12 图 10 发声肌的前端宽(SWF)与最宽处宽度(SWP)比较区间图 Fig. 10 Interval map for wide of forefront(SWF) and the widest part of sonic muscle(SWP) |
性腺发育中的个体,发声肌的形态数据都与个体发育呈线性正相关(图 6-7)。t检验:双样本异方差假设检验两两对比,发育期发声肌长度指数雄性大于雌性(0.23±0.03,0.21±0.03),但无显著性差异(P>0.05)。发育期雄性发声肌质量指数大于雌性(0.003 5±0.000 6,0.003 4±0.000 4),但无显著性差异(P>0.05)。雌性的发声肌长度相关性比雄性略高(雌:R2=0.814 1, 雄:R2=0.757 5,图 6),但雄性发声肌质量关联系数大于雌性(雌:R2 = 0.642 8,雄:R2=0.850 7,图 7)。
性腺发育前的褐菖鲉,雄性发声肌长度指数与雌性相近(0.23±0.03,0.21±0.03, P>0.05),雄性发声肌质量指数与雌性相近(0.003 3±0.000 5,0.003 3±0.000 8, P>0.05)。
利用t检验对发声肌宽度进行对比分析发现,雄性的发声肌宽度SWF与雌性(发育前:P>0.05,发育期:P>0.05)无显著性差异,非繁殖期发声肌的最宽处SWP无显著差异(发育前:P>0.05,发育期:P>0.05),说明在雌雄鱼发声肌在宽度上无明显差异。
3 讨论VANCE等[14]对3种石首鱼科鱼类斜纹犬牙石首鱼(Cynoscion regalis)、波纹绒须石首鱼(Micropogonias undulatus)和黄尾平口石首鱼(Leiostiom xanthurus)的发声肌发育进行对比,发现波纹绒须石首鱼的发声肌大小和体腔大小与性别并无显著相关性,3种石首科鱼类种间对比发现,依次从黄尾平口石首鱼到波纹绒须石首鱼,到斜纹犬牙石首鱼,发声肌大小和鱼个体大小比例成种间正相关性[14]。ALI等[13]对大陆架斜坡生存的3种深海鼬鱼科(Hoplobrotula armata, Neobythites longipe, Neobythites unimaculatus)的发声系统的发育和性别二态性进行研究发现,3种鼬鱼都有两对中央发声肌和两对侧发声肌,它们的长度和外观表型是相似的,然而3种鱼类的雄鱼中央发声肌质量都比雌鱼大,由相对较小的纤维构成,雌鱼侧发声肌普遍比较大,由大纤维构成。这3种鱼类不仅种类间的发声肌重量有差异,发声肌的发育与鱼体发育情况以及性别有关,且种内雄鱼发声的振幅和持续性各不同[13]。
在繁殖季节雄鱼通过发声吸引雌鱼,即雌鱼通过声音信号对雄鱼声音进行鉴别和选择,雄性求偶发声还可防止种间杂交的发生,以保证各自种群的唯一性和延续性[12-15, 25]。已有报道,发声振幅会随着发声肌的厚度发生改变[12],本实验数据表明繁殖期的褐菖鲉发声肌长度和质量与个体大小成正相关,即鱼体越大,发声肌越长、越重。因此,褐菖鲉发声特性随着体型变化而改变,发声可以传达体征与体质信息,利于吸引配偶。本实验研究发现,雄鱼在性腺发育前发声肌质量指数也呈正相关,褐菖鲉性情凶猛,通过发声进行御敌、通信、求偶等行为,在入侵领地的模拟实验中,表现其发声与领域入侵防御有关[19],也表明了雄性褐菖鲉的发声功能不仅在生殖行为中,而且与性腺发育前的有关领域入侵、防御等相关行为也有一定作用。
利用发声肌产生声音的鱼类,发声时通过高度收缩发声肌引起鱼鳔振动而发出声音[4, 12, 26]。在毒棘豹蟾鱼(Opsanus tau)、针潜鱼(Carapus acus)和斑光蟾鱼(Porichthys notatus)中大量存在的小清蛋白,可能在发声肌快速收缩中起着重要作用[27-28],但该蛋白已被证明在褐菖鲉发声肌中雌雄没有显著差异[29]。实验发现发声肌的宽度在性腺发育前与生殖期不具有显著差异,雌雄长度比和质量比均不具明显差异,所以雄鱼求偶发声并不是通过改变发声肌外在形态实现的。又根据文献[29]得知,褐菖鲉的发声肌具有超微结构中三联体发达,肌膜发达,肌质网发达,线粒体多且集中,以确保其发声肌快速收缩和放松,拥有持久的工作能力。进入繁殖期的褐菖鲉雄鱼有可能通过改变超微结构或者是使其运行状态不同,以达到改变声音振幅和频率的效果[29]。
褐菖鲉繁殖季节期间有两个高峰分别在11—12月、2—4月[23],本次褐菖鲉取材繁殖高峰期间11—12月,在枸杞岛近海捕捞得到雌性的个体比雄性多,与文献[20]报道一致,但是11月底所取样中性腺有不少还未发育,有可能是先进入繁殖期的褐菖鲉已向深海处转移。
| [1] |
TAVOLGA W M. Sound production and detection[J]. Fish Physiology, 1971, 5: 135-205. DOI:10.1016/S1546-5098(08)60047-3 |
| [2] |
FAY R R, POPPER A N. Comparative hearing:fish and amphibians[M]. New York: Springer, 1999: 363-411.
|
| [3] |
PRUZSINSZKY I, LADICH F. Sound production and reproductive behaviour of the armoured catfish Corydoras paleatus(Callichthyidae)[J]. Environmental Biology of Fishes, 1998, 53(2): 183-191. DOI:10.1023/A:1007413108550 |
| [4] |
MÉLOTTE G, VIGOUROUX R, MICHEL C, et al. Interspecific variation of warning calls in piranhas:a comparative analysis[J]. Scientific Reports, 2016, 6: 36127. DOI:10.1038/srep36127 |
| [5] |
PARMENTIER E, LAGARDōRE J P, VANDEWALLE P, et al. Geographical variation in sound production in the anemonefish Amphiprion akallopisos[J]. Proceedings of the Royal Society B:Biological Sciences, 2005, 272(1573): 1697-1703. DOI:10.1098/rspb.2005.3146 |
| [6] |
TOWER R W. The Production of sound in the drumfishes, the sea-robin and the toadfish[J]. Annals of the New York Academy of Sciences, 1908, 18(1): 149-180. DOI:10.1111/j.1749-6632.1908.tb55101.x |
| [7] |
PARMENTIER E, RAICK X, LECCHINI D, et al. Unusual sound production mechanism in the triggerfish Rhinecanthus aculeatus(Balistidae)[J]. Journal of Experimental Biology, 2017, 220: 186-193. DOI:10.1242/jeb.146514 |
| [8] |
BASS A H, LADICH F. Vocal-acoustic communication: from neurons to behavior[M]//WEBB J F, FAY R R, POPPER A N. Fish Bioacoustics. New York: Springer, 2008: 253-278.
|
| [9] |
FINE M L, MALLOY K L, KING C B, et al. Movement and sound generation by the toadfish swim bladder[J]. Journal of Comparative Physiology A, 2001, 187(5): 371-379. DOI:10.1007/s003590100209 |
| [10] |
KASUMYAN A O. Sounds and sound production in fishes[J]. Journal of Ichthyology, 2008, 48(11): 981-1030. DOI:10.1134/S0032945208110039 |
| [11] |
AMORIM M C P, VASCONCELOS R O, PARREIRA B. Variability in the sonic muscles of the Lusitanian toadfish (Halobatrachus didactylus):acoustic signals may reflect individual quality[J]. Canadian Journal of Zoology, 2009, 87(8): 718-725. DOI:10.1139/Z09-067 |
| [12] |
FINE M L, LIN H, NGUYEN B B, et al. Functional morphology of the sonic apparatus in the fawn cusk-eel Lepophidium profundorum(Gill, 1863)[J]. Journal of Morphology, 2007, 268(11): 953-966. DOI:10.1002/jmor.10551 |
| [13] |
ALI H A, MOK H K, FINE M L. Development and sexual dimorphism of the sonic system in deep sea neobythitine fishes:the upper continental slope[J]. Deep Sea Research Part Ⅰ:Oceanographic Research Papers, 2016, 115: 293-308. DOI:10.1016/j.dsr.2016.07.010 |
| [14] |
VANCE T L, HEWSON J M. Variability in sonic muscle size and innervation among three Sciaenids:Spot, Atlantic Croaker, and Weakfish[J]. Copeia, 2002, 2002(4): 1137-1143. DOI:10.1643/0045-8511(2002)002[1137:VISMSA]2.0.CO;2 |
| [15] |
PARMENTIER E, VANDEWALLE P, BRIÉ C, et al. Comparative study on sound production in different holocentridae species[J]. Frontiers in Zoology, 2011, 8(1): 12. |
| [16] |
PARMENTIER E, FONTENELLE N, FINE M L, et al. Functional morphology of the sonic apparatus in Ophidion barbatum(Teleostei, Ophidiidae)[J]. Journal of Morphology, 2006, 267(12): 1461-1468. DOI:10.1002/jmor.10496 |
| [17] |
LADICH F, FINE M L. Sound-generating mechanisms in fishes: a unique diversity in vertebrates[M]//LADICH F, COLLIN S P, MOLLER P, et al. Communication in Fishes. Enfield, NH: Science Publishers, 2006: 3-43.
|
| [18] |
PARMENTIER E, BOUILLAC G, DRAGICEVIC B, et al. Call properties and morphology of the sound-producing organ in Ophidion rochei(Ophidiidae)[J]. Journal of Experimental Biology, 2010, 213: 3230-3236. DOI:10.1242/jeb.044701 |
| [19] |
张旭光, 谢伟, 郭弘艺, 等. 褐菖鲉发声系统形态结构及其发声特征研究[J]. 上海海洋大学学报, 2013, 22(5): 672-677. ZHANG X G, XIE W, GUO H Y, et al. The morphology of sonic system and sound characteristics of the marbled rockfish Sebastiscus marmoratus[J]. Journal of Shanghai Ocean University, 2013, 22(5): 672-677. |
| [20] |
FUJITA H, KOHDA M. Male mating effort in the viviparous scorpionfish, Sebastiscus marmoratus[J]. Ichthyological Research, 1996, 43(3): 247-255. DOI:10.1007/BF02347597 |
| [21] |
CLARA M, AMORIM P. Diversity of sound production in fish[M]//LADICH F, COLLIN S P, MOLLER P, et al. Communication in Fishes. Enfield, NH: Science Publishers, 2006: 71-104.
|
| [22] |
任新敏, 高大治, 姚玉玲, 等. 大黄鱼的发声及信号特性研究[J]. 大连水产学院学报, 2007, 22(2): 123-128. REN X M, GAO D Z, YAO Y L, et al. Occurrence and characteristic of sound in large yellow croaker (Pseudosciaena crocea)[J]. Journal of Dalian Fisheries University, 2007, 22(2): 123-128. DOI:10.3969/j.issn.1000-9957.2007.02.009 |
| [23] |
吴常文. 浙江舟山近海褐菖鲉Sebastiscus marmoratus生物学研究[J]. 浙江海洋学院学报(自然科学版), 1999, 8(3): 185-190, 226. WU C W. Biological studies on Sebastiscus marmoratus off Zhoushan[J]. Journal of Zhejiang Ocean University (Natural Science), 1999, 8(3): 185-190, 226. DOI:10.3969/j.issn.1008-830X.1999.03.001 |
| [24] |
林丹军, 尤永隆. 卵胎生硬骨鱼褐菖卵巢的周期发育研究[J]. 动物学研究, 2000, 21(4): 269-274. LIN D J, YOU Y L. The ovarian cyclical development of ovoviviparous teleost, Sebastiscus marmoratus[J]. Zoological Research, 2000, 21(4): 269-274. DOI:10.3321/j.issn:0254-5853.2000.04.003 |
| [25] |
MOSHARO K K, LOBEL P S. Acoustic signals of two toadfishes from Belize:Sanopus astrifer and Batrachoides gilberti(Batrachoididae)[J]. Environmental Biology of Fishes, 2012, 94(4): 623-638. DOI:10.1007/s10641-011-9969-x |
| [26] |
ROME L C. Design and function of superfast muscles:new insights into the physiology of skeletal muscle[J]. Annual Review of Physiology, 2006, 68: 193-221. DOI:10.1146/annurev.physiol.68.040104.105418 |
| [27] |
TIKUNOV B A, ROME L C. Is high concentration of parvalbumin a requirement for superfast relaxation?[J]. Journal of Muscle Research and Cell Motility, 2009, 30(1/2): 57-65. |
| [28] |
PARMENTIER E, GENNOTTE V, FOCANT B, et al. Characterization of the primary sonic muscles in Carapus acus(Carapidae):a multidisciplinary approach[J]. Proceedings of the Royal Society of London. Series B:Biological Sciences, 2003, 270(1530): 2301-2308. DOI:10.1098/rspb.2003.2495 |
| [29] |
谢伟, 张旭光, 郭弘艺, 等. 褐菖鲉发声肌中小清蛋白的特性及肌肉纤维的超微结构特性[J]. 上海海洋大学学报, 2016, 25(3): 367-373. XIE W, ZHANG X G, GUO H Y, et al. Characteristics of the parvalbumin and the ultrastructure in the sonic muscle of the marbled rockfish, Sebastiscus marmoratus[J]. Journal of Shanghai Ocean University, 2016, 25(3): 367-373. |
2. Shanghai Universities Key Laboratory of Marine Animal Taxonomy and Evolution, Shanghai 201306, China;
3. National Demonstration Center for Experimental Fisheries Science Education, Shanghai 201306, China;
4. Shanghai Collaborative Innovation for Aquatic Animal Genetics and Breeding, Shanghai 201306, China
 2019,
Vol. 28
2019,
Vol. 28


