2. 鲁东大学 生命科学学院, 山东 烟台 264025;
3. 上海海洋大学 水产与生命学院, 上海 201306
我国海藻资源极其丰富,栽培面积及产量均居世界首位。海藻不但含有丰富的碳水化合物(如海藻胶、甘露醇、淀粉等),还含有蛋白质和碘、钾、钙、镁、钠、锰、硒等矿物质及多种维生素,是一种优良的饲料添加剂,有益于养殖动物的健康[1]。
许多报道均表明在饲料中添加海藻粉有利于改善养殖动物的生长表现。例如:在饲料中添加0~15%水平的石莼粉可显著提高红罗非鱼(Oreochromis sp.)的生长表现[2];在饲料中添加5%的泡叶藻(Ascophyllum nodosum)、紫菜(Porphyra yezoensis)和石莼(Ulva pertusa)促进了真鲷(Pagrus major)的生长[3];在饲料中添加紫菜(最高达10%水平)也促进了虹鳟(Oncorhynchus mykiss)的生长[4];卢清等[5]和李红艳等[6]的研究均表明饲料中添加5%的浒苔(Enteromorpha prolifera)可显著提高大菱鲆(Scophthalmus maximus)幼鱼的生长。
海藻粉作为添加剂还能提高养殖动物的免疫能力。许多体外实验均表明海藻粉具有抗菌、抗病毒和抗氧化活性[7-9]。在饲料中添加提取自泡叶藻的黏合剂提高了大西洋鲑(Salmo salar)的溶菌酶水平[10];以海带粉饲喂牙鲆(Paralichthys olivaceus)也发现提高了其溶菌酶水平[11];以马尾藻饲喂鲻鱼(Mugil cephalus)并对其进行荧光假单胞菌攻毒也发现增强了其溶菌酶活性[12];在饲料中添加5%的浒苔粉显著提高了大菱鲆幼鱼的头肾巨噬细胞的吞噬活力和呼吸爆发活力[13]。除了免疫保护活性外,还发现海藻中多酚的含量与其抗氧化能力呈正相关[14],作用机理主要是通过清除ROS和抑制脂类过氧化[15]。例如,预先以来自巨藻(Macrocystis pyritera)的褐藻酸钠和来自角叉菜(Chondrus ocellatus)的卡拉胶饲喂鲑点石斑鱼(Epinephelus coicoides),再使用弧菌进行攻毒,鲑点石斑鱼表现出增强的呼吸爆发、超氧化物歧化酶和吞噬活性[16]。
鼠尾藻(Sargassum thunbergii )是我国沿海常见的野生暖温带海藻,隶属于褐藻门(Ochrophyta)、无孢子纲(Phaeophyceae)、马尾藻科(Sargassaceae)、马尾藻属(Sargassum)。鼠尾藻营养丰富,蛋白含量较高,目前作为优质饵料在海参养殖中被广泛使用[17];鼠尾藻还能增强免疫能力,《中国海洋药物辞典》曾报道鼠尾藻具有软坚散结、利尿消肿、清热化痰的功效[18]。近年来随着鼠尾藻育种技术的突破和野生马尾藻资源的扩张,其资源量得到一定程度的恢复。大菱鲆作为我国海水鱼养殖的代表性品种,迫切需要转换养殖方式,开展集约化生态养殖,其中研发具有提高免疫和抗应激能力的配合饲料是重要的环节。本文尝试在大菱鲆饲料中添加鼠尾藻粉,充分利用其营养和免疫特性,评估其作为大菱鲆饲料原料的可行性,以期为鼠尾藻资源的规模化开发和利用提供理论依据。
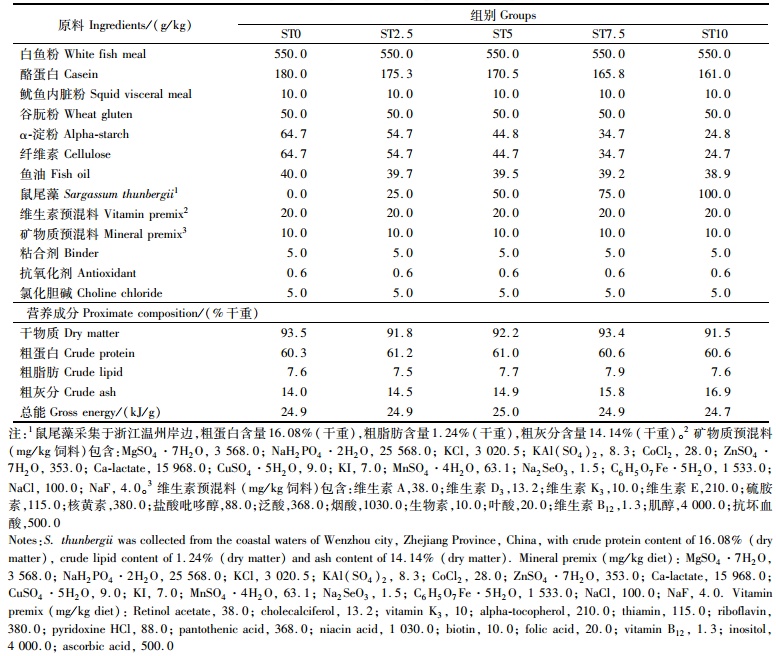
1 材料与方法 1.1 实验饲料以鱼粉、酪蛋白为主要蛋白源,鱼油为主要脂肪源,配制粗蛋白含量为60.3%,粗脂肪含量为7.6%的基础饲料。在此基础上分别添加0、2.5%、5.0%、7.5%、10.0%的鼠尾藻粉,配制成5组等氮等能的实验饲料,命名为ST0、ST2.5、ST5、ST7.5、ST10,饲料配方及营养组成见表 1。饲料原料粉碎过80目筛,按比例称量混匀后加鱼油及适量蒸馏水均匀混合,制成粒径为3.5 mm的颗粒饲料,阴凉处风干后-20 ℃冰箱保存备用。
|
表 1 实验饲料的组成与营养成分 Tab.1 Ingredients and proximate composition of experimental diets |
养殖实验在山东省海洋资源与环境研究院养殖实验室进行,采用全封闭水循环系统。实验鱼购自蓬莱宗哲养殖场,种质来源相同、大小均匀、健康无病,平均体质量约为(66.38 ± 0.71)g。正式实验前,大菱鲆幼鱼在养殖系统中驯养2周,其间投喂对照组饲料,然后随机分为5组,每组设3个重复,每个重复25尾鱼,放养于绿色圆柱形(直径80 cm,高70 cm,水深约50 cm)养殖桶中,每种饲料随机投喂3个桶。每天定时(8:00,15:30)定量投喂2次,投喂量为鱼体质量的1%~2 %,根据摄食情况调整投喂量,投喂结束半小时左右排残饵,数颗粒,计算残饵量。养殖过程控制水温在(15.9 ± 0.5) ℃,pH 7.8~8.2,盐度28~30,保证溶氧>5 mg/L,氨氮、亚硝酸氮均 < 0.1 mg/L。养殖周期56 d。
养殖实验结束后,饥饿24 h,记录每桶鱼的数量并称重,计算增重率,从每桶随机取10尾实验鱼,测量体长、体质量,计算肥满度。随机选取3尾实验鱼用于全鱼体组成常规分析。剩下7尾进行尾静脉取血,血样在4 ℃冰箱静置4 h,离心分离(4 000 r/min,10 min),取上清液,-80 ℃保存,用于测定血清生理生化指标;取血后的实验鱼分离内脏和肝脏,并分别称重,计算肝体比和脏体比。
1.3 测定指标和方法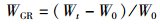 (1)
(1)
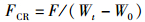 (2)
(2)
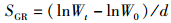 (3)
(3)
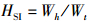 (4)
(4)
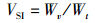 (5)
(5)
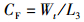 (6)
(6)
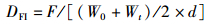 (7)
(7)
式中:WGR为增重率; FCR为饲料系数; SGR为特定生长率; HSI为肝体比; VSI为脏体比; CF为肥满度; DFI为日摄食率; Wt为实验结束时鱼末体质量,g; W0为实验开始鱼初体质量,g; F为摄食干饲料重,g; d为养殖天数; Wh为肝脏质量; Wv为内脏质量; L为实验结束鱼末体长。
饲料及组织样品中,水分采用105 ℃烘干恒重法(GB/T 6435—2006)测定;粗蛋白采用凯氏定氮法(GB/T 6432—2006)测定;饲料中粗脂肪和组织样品的粗脂肪,采用索氏抽提法(GB/T 6433—2006)测定;粗灰分采用马弗炉550 ℃灼烧法(GB/T 6438—2007)测定;能量采用燃烧法(IKA,C6000,德国)测定。
血清中葡萄糖(GLU)、三酰甘油(TG)、高密度脂蛋白胆固醇(HDL-C)、低密度脂蛋白胆固醇(LDL-C)和胆固醇(CHO)采用全自动生化分析仪(7020,日立,日本)进行测定。血清碱性磷酸酶(ACP)、总超氧化物歧化酶(T-SOD)、过氧化氢酶(CAT)和丙二醛(MDA)均采用南京建成的试剂盒测定。
1.4 数据统计分析采用SPSS 11.5软件对数据进行单因素方差分析(One-Way ANOVA),当处理之间差异显著(P < 0.05)时,用Duncan’s检验进行多重比较,实验结果以平均值±标准差(Means±SD)形式表示。
2 结果与分析 2.1 饲料中添加鼠尾藻粉对大菱鲆生长指标的影响如表 2所示,随着鼠尾藻粉添加量的升高,增重率(WGR)和特定生长率(SGR)呈现出逐渐下降的趋势,ST2.5组与对照组相比无显著差异(P>0.05),其余各组均显著低于对照组(P<0.05)。日摄食率(DFI)表现出逐渐下降的趋势,其中ST10组显著低于对照组(P<0.05)。饲料系数(FCR)、成活率(SR)、脏体比(VSI)、肝体比(HSI)和肥满度(CF)各组之间均无显著性差异(P>0.05)。

|
表 2 实验8周后大菱鲆幼鱼的生长表现与形体指数 Tab.2 Growth performance and somatic parameters of juvenile turbot fed experimental diets for 8 weeks |
如表 3所示,随着鼠尾藻粉添加量的提高,全鱼粗蛋白含量有逐渐升高的趋势,粗脂肪含量有逐渐下降的趋势,但各组之间无显著差异(P>0.05)。粗灰分含量有逐渐升高的趋势,试验组均显著高于对照组(P<0.05),ST5、ST7.5和ST10组显著高于ST2.5组(P<0.05)。

|
表 3 实验8周后大菱鲆幼鱼的全鱼体成分(%干重) Tab.3 Whole body composition (% dry matter) of juvenile turbot fed the experimental diets for 8 weeks |
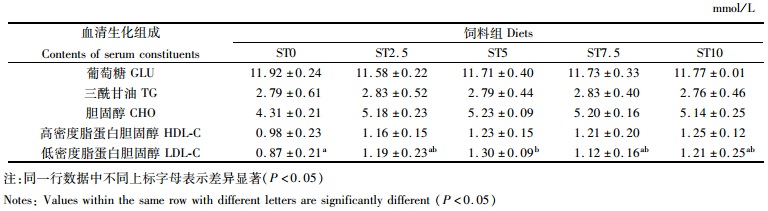
如表 4所示,各组之间的血清葡萄糖(GLU)、甘油三酯(TG)、胆固醇(CHO)浓度、高密度脂蛋白胆固醇(HDL-C)无显著差异(P>0.05);血清低密度脂蛋白胆固醇(LDL-C)浓度表现出先升高后下降的趋势,ST5组最高,并显著高于对照组(P < 0.05)。
|
表 4 实验8周后大菱鲆幼鱼的血清生化组成 Tab.4 Contents of serum constituents of juvenile turbot fed Experimental diets for 8 weeks |
如表 5所示:大菱鲆酸性磷酸酶(ACP)活性随着鼠尾藻粉添加量的提高表现出先升高后下降的趋势,其中ST5组最高;总超氧化物歧化酶(T-SOD)活性各组之间无显著差异(P>0.05);过氧化氢酶(CAT)活性呈现出先升高后下降的趋势,其中ST5组最高;ST10组的丙二醛(MDA)浓度显著高于其他各组(P<0.05),其他各组之间无显著差异(P>0.05)。

|
表 5 实验8周后大菱鲆幼鱼的血清抗氧化和非特异性免疫酶活性 Tab.5 Activities of serum antioxidation and nonspecific immune enzymes of juvenile turbot fed experimental diets for 8 weeks |
本研究在饲料中添加2.5%的鼠尾藻粉对大菱鲆的生长无负面影响,然而添加2.5%以上水平的鼠尾藻粉降低了大菱鲆幼鱼的增重率和特定生长率(表 2)。目前主要报道了3类海藻在饲料中的添加情况:(1)绿藻,PEIXOTO等[19]在饲料中添加7.5%的石莼对欧洲鲈鱼(Dicentrarchus labrax)的生长无负面影响,MARINHO等[21]、SILVA等[22]和VALENTE等[23]分别在尼罗罗非鱼(Oreochromis niloticus)中添加10%的石莼(Ulva sp.)对其生长无负面影响,VALENTE等[24]研究表明在欧洲鲈鱼饲料中石莼添加到10%对生长无负面影响,LINARES等[25]也发现在塞内加尔鳎饲料中添加10%的石莼对其生长无负面影响;(2)红藻,SILVA等[22]发现在饲料中添加10%的江蓠(Gracilaria vermiculophylla)对尼罗罗非鱼有负面影响,而VALENTE等[24]研究了两种江蓠(G. bursa-pastoris和G. cornea)对欧洲鲈鱼的影响,结果表明添加10% G. bursa-pastoris对生长无负面影响,而添加10% G. cornea显著降低了生长;(3)褐藻,QUEIROZ等[20]发现在金头鲷(Sparus aurata)饲料中添加7.5%的褐藻对其生长无显著影响,PEIXOTO等[19]在饲料中添加7.5%的墨角藻(Fucus spp.)对欧洲鲈鱼的生长无影响,LINARES等[25]也发现在塞内加尔鳎饲料中添加10%的裙带菜(Undaria pinnatifida)对其生长无影响。这些研究表明,饲料中海藻粉的最适添加量因鱼种的不同而不同,不同海藻粉在同一鱼种的饲料最适添加量也不尽相同。本研究所获得的最适添加量(2.5%)小于上述研究,主要原因有:采用的藻种和实验动物与上述研究均不同;上述研究最多只设置了3个海藻梯度,而本研究设置了5个海藻梯度,因此能更精确地反映出最适添加量。饲料中添加过多的海藻粉通常会造成鱼类生长的下降,这与海藻粉的风味和含有抗营养因子有关。海藻粉含有皂苷、鞣酸和植酸等苦味物质,降低了饲料的诱食性,导致摄食率的下降,并且会干扰饲料脂肪和胆汁盐的吸收[26-27],本研究随着鼠尾藻添加量的增加,日摄食率呈现出逐渐下降的趋势,表明鼠尾藻添加造成了饲料风味的改变,从而引起摄食和生长的下降[28]。此外,海藻中还含有多糖(木聚糖、琼脂和海藻酸盐)等抗营养因子,这些抗营养因子降低了其营养品质,而且影响了消化吸收效率,从而也会导致生长速率的下降[29-30],PERNADO等[31]曾报道尼罗罗非鱼对G. vermiculophylla, Porphyra dioica和Ulva spp.消化利用率好于Sargassum muticum(与鼠尾藻同属),这也可能是本研究鼠尾藻最适添加量低于其他海藻的原因。
与对照组相比,随着鼠尾藻粉添加量的提高,实验组的全鱼粗灰分含量也逐渐升高(表 3),这与饲料中粗灰分含量的变化趋势一致。海藻粉含有较高矿物质含量,如钙的含量比玉米、麸皮分别高出2.29%、2.39%,磷比玉米高出0.29%,还含有丰富的碘剂[32],因此造成饲料中粗灰分含量高于对照组。钙磷等矿物质被鱼体吸收后可提高骨骼灰分含量[33],因此添加鼠尾藻粉提高了大菱鲆全鱼的粗灰分含量。
血清中高密度脂蛋白负责将血液中的胆固醇转运到肝脏,而低密度脂蛋白负责将胆固醇和三酰甘油转运到血液,二者共同影响了血清中CHO和TG的含量[34]。因此,LDL-C/HDL-C被认为是动脉硬化性病变的表征[35]。在本实验中,与对照组相比,实验组的LDL-C和HDL-C均有所升高,但二者的比值呈现出下降趋势,且与对照组逐渐接近,并且CHO和TG与对照组相比均无显著变化,这表明添加鼠尾藻粉对大菱鲆的血脂健康无负面影响。
鱼类血清中的超氧化物歧化酶、酸性磷酸酶、碱性磷酸酶和过氧化氢酶等是评价非特异性免疫作用的重要指标,在提高鱼体的抗病力以及抗应激力中发挥了促进作用[36]。与对照组相比,添加鼠尾藻粉后酸性磷酸酶和过氧化氢酶呈现出先上升后下降的趋势,在ST5组达到最高,王鹏等[37]在大菱鲆鱼苗的饲料中添加褐藻低聚糖也提高了其酸性磷酸酶和过氧化氢酶活性。非特异性免疫防御是鱼类的第一道保护屏障,在鱼类的免疫系统中起着至关重要的作用,添加5%的鼠尾藻粉可明显提高大菱鲆的非特异性免疫能力。
4 结论在本实验条件下,添加2.5%的鼠尾藻粉对大菱鲆幼鱼的生长无显著影响,添加5%的鼠尾藻粉提高了大菱鲆幼鱼的非特异性免疫能力。
| [1] |
JIMÉNEZ-ESCRIG A, GÓMEZ-ORDÓHEZ E, RUPÉREZ P. Seaweed as a source of novel nutraceuticals: sulfated polysaccharides and peptides[M]//KIM S K. Advances in Food and Nutrition Research. New York: Academic Press, 2011: 325-337.
|
| [2] |
EL-TAWIL N E. Effects of green seaweeds (Ulva sp.) as feed supplements in red tilapia (Oreochromis sp.) diet on growth performance, feed utilization and body composition[J]. Journal of the Arabian Aquaculture Society, 2010, 5(2): 179-194. |
| [3] |
MUSTAFA G, WAKAMATSU S, TAKEDA T A, et al. Effects of algae meal as feed additive on growth, feed efficiency, and body composition in Red Sea Bream[J]. Fisheries Science, 1995, 61(1): 25-28. DOI:10.2331/fishsci.61.25 |
| [4] |
SOLER-VILA A, COUGHLAN S, GUIRY M D, et al. The red alga Porphyra dioica as a fish-feed ingredient for rainbow trout (Oncorhynchus mykiss):effects on growth, feed efficiency, and carcass composition[J]. Journal of Applied Phycology, 2009, 21(5): 617-624. DOI:10.1007/s10811-009-9423-z |
| [5] |
卢青, 杨宁, 王正丽, 等. 饲料中添加浒苔对大菱鲆生长和非特异性免疫力的影响[J]. 青岛农业大学学报(自然科学版), 2015, 32(1): 62-66. LU Q, YANG N, WANG Z L, et al. The effects of dietary Enteromorpha prolifera on the growth and non-specific immunity of juvenile turbot (Scophthalmus maximus)[J]. Journal of Qingdao Agricultural University (Natural Science), 2015, 32(1): 62-66. DOI:10.3969/J.ISSN.1674-148X.2015.01.014 |
| [6] |
李红艳, 刘天红, 王璇璇, 等. 不同饲料配方中浒苔添加量对大菱鲆生长的影响[J]. 水产科学, 2016, 35(4): 334-339. LI H Y, LIU T H, WANG X X, et al. Effect of dietary green alga Enteromorpha prolifera in different formulations on growth of turbot Scophthalmus maximus[J]. Fisheries Science, 2016, 35(4): 334-339. |
| [7] |
HEMMINGSON J A, FALSHAW R, FURNEAUX R H, et al. Structure and antiviral activity of the galactofucan sulfates extracted from Undaria pinnatifida (Phaeophyta)[J]. Journal of Applied Phycology, 2006, 18(2): 185-193. DOI:10.1007/s10811-006-9096-9 |
| [8] |
COX S, ABU-GHANNAM N, GUPTA S. An assessment of the antioxidant and antimicrobial activity of six species of edible Irish seaweeds[J]. International Food Research Journal, 2010, 17: 205-220. |
| [9] |
NARASIMHAN M K, PAVITHRA S K, KRISHNAN V, et al. In vitro analysis of antioxidant, antimicrobial and antiproliferative activity of Enteromorpha antenna, Enteromorpha linza and Gracilaria corticata extracts[J]. Jundishapur Journal of Natural Pharmaceutical Products, 2013, 8(4): 151-159. DOI:10.5812/jjnpp. |
| [10] |
GABRIELSEN B O, AUSTRENG E. Growth, product quality and immune status of Atlantic salmon, Salmo salar L., fed wet feed with alginate[J]. Aquaculture Research, 1998, 29(6): 397-401. DOI:10.1111/j.1365-2109.1998.tb01146.x |
| [11] |
KIM S S, LEE K J. Effects of dietary kelp (Ecklonia cava) on growth and innate immunity in juvenile olive flounder Paralichthys olivaceus (Temminck et Schlegel)[J]. Aquaculture Research, 2008, 39(15): 1687-1690. |
| [12] |
KANIMOZHI S, KRISHNAVENI M, DEIVASIGMANI B, et al. Immunomo-stimulation effects of Sargassum whitti on Mugil cephalus against Pseudomonas fluorescence[J]. Journal of Current Microbiology and Applied Sciences, 2013, 2(7): 93-103. |
| [13] |
郭中帅, 杨宁, 王正丽, 等. 大菱鲆幼鱼饲料中浒苔适宜添加量的应用研究[J]. 水产科学, 2015, 34(7): 423-427. GUO Z S, YANG N, WANG Z L, et al. Optimal dietary Enteromorpha prolifera in turbot (Scophthalmus maximus)[J]. Fisheries Science, 2015, 34(7): 423-427. |
| [14] |
DEVI G K, MANIVANNAN K, THIRUMARAN G, et al. In vitro antioxidant activities of selected seaweeds from Southeast coast of India[J]. Asian Pacific Journal of Tropical Medicine, 2011, 4(3): 205-211. DOI:10.1016/S1995-7645(11)60070-9 |
| [15] |
HEO S J, PARK EJ, LEE K W, et al. Antioxidant activities of enzymatic extracts from brown seaweeds[J]. Bioresource Technology, 2005, 96(14): 1613-1623. DOI:10.1016/j.biortech.2004.07.013 |
| [16] |
CHENG A C, TU C W, CHEN Y Y, et al. The immunostimulatory effects of sodium alginate and iota-carrageenan on orange-spotted grouper Epinephelus coicoides and its resistance against Vibrio alginolyticus[J]. Fish & Shellfish Immunology, 2007, 22(3): 197-205. |
| [17] |
吴海歌, 于超, 姚子昂, 等. 鼠尾藻营养成分分析[J]. 大连大学学报, 2008, 29(3): 84-85, 93. WU H G, YU C, YAO Z A, et al. Analysis of the nutrient component in Sargassum thunbergii[J]. Journal of Dalian University, 2008, 29(3): 84-85, 93. DOI:10.3969/j.issn.1008-2395.2008.03.019 |
| [18] |
姜凤梧, 张玉顺. 中国海洋药物辞典[M]. 北京: 海洋出版社, 1994: 387. JIANG F W, ZHANG Y S. Chinese dictionary of marine drugs[M]. Beijing: China Ocean Press, 1994: 387. |
| [19] |
PEIXOTO M J, SALAS-LEITÓN E, PEREIRA L F, et al. Role of dietary seaweed supplementation on growth performance, digestive capacity and immune and stress responsiveness in European seabass (Dicentrarchus labrax)[J]. Aquaculture Reports, 2016, 3: 189-197. DOI:10.1016/j.aqrep.2016.03.005 |
| [20] |
QUEIROZ A C, PEREIRA R, DOMINGUES A F, et al. Effect of seaweed supplementation on growth performance, immune and oxidative stress responses in gilthead seabream (Sparus aurata)[C]//International Meeting on Marine Research. Peniche, 2014.
|
| [21] |
MARINHO G, NUNES C, SOUSA-PINTO I, et al. The IMTA-cultivated Chlorophyta Ulva spp. as a sustainable ingredient in Nile tilapia (Oreochromis niloticus) diets[J]. Journal of Applied Phycology, 2013, 25(5): 1359-1367. DOI:10.1007/s10811-012-9965-3 |
| [22] |
SILVA D M, VALENTE L M P, SOUSA-PINTO I, et al. Evaluation of IMTA-produced seaweeds (Gracilaria, Porphyra, and Ulva) as dietary ingredients in Nile tilapia, Oreochromis niloticus L., juveniles. Effects on growth performance and gut histology[J]. Journal of Applied Phycology, 2015, 27(4): 1671-1680. DOI:10.1007/s10811-014-0453-9 |
| [23] |
VALENTE L M P, ARAU'JO M, BATISTA S, et al. Carotenoid deposition, flesh quality and immunological response of Nile tilapia fed increasing levels of IMTA-cultivated Ulva spp.[J]. Journal of Applied Phycology, 2016, 28(1): 691-701. DOI:10.1007/s10811-015-0590-9 |
| [24] |
VALENTE L M P, GOUVEIA A, REMA P, et al. Evaluation of three seaweeds Gracilaria bursa-pastoris, Ulva rigida and Gracilaria cornea as dietary ingredients in European sea bass (Dicentrarchus labrax) juveniles[J]. Aquaculture, 2006, 252(1): 85-91. DOI:10.1016/j.aquaculture.2005.11.052 |
| [25] |
LINARES F, PELETEIRO J B, LVAREZ-BLÁZQUEZ B, et al. Dietary inclusion of 10% seaweeds in Senegalese sole (Solea senegalensis) juveniles[C]//San Sebastián: Aquaculture Europe, 2014.
|
| [26] |
TACON A G J. Fishmeal replacers:review of antinutrients within oilseeds and pulses-a limiting factor for the aquafeed green revolution[J]. Coniers Options Mediterraneennes, 1997, 22: 153-182. |
| [27] |
FRANCIS G, MAKKAR H P S, BECKER K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish[J]. Aquaculture, 2001, 199(3/4): 197-227. |
| [28] |
YILDIRIM Ö, ERGVN S, YAMAN S, et al. Effects of two seaweeds (Ulva lactuca and Enteromorpha linza) as a feed additive in diets on growth performance, feed utilization, and body composition of rainbow trout (Oncorhynchus mykiss)[J]. Kafkas Vniversitesi Veteriner Fakültesi Dergisi, 2009, 15(3): 455-460. |
| [29] |
HORIE Y, SUGASE K, HORIE K. Physiological differences of soluble and insoluble dietary fibre fractions of brown algae and mushrooms in pepsin activity in vitro and protein digestibility[J]. Asia Pacific Journal of Clinical Nutrition, 1995, 4(2): 251-255. |
| [30] |
DALLAIRE V, LESSARD P, VANDENBERG G, et al. Effect of algal incorporation on growth, survival and carcass composition of rainbow trout (Oncorhynchus mykiss) fry[J]. Bioresource Technology, 2007, 98(7): 1433-1439. DOI:10.1016/j.biortech.2006.05.043 |
| [31] |
PEINADO M J, RUIZ R, ECHÁVARRI A, et al. Garlic derivative propyl propane thiosulfonate is effective against broiler enteropathogens in vivo[J]. Poultry Science, 2012, 91(9): 2148-2157. DOI:10.3382/ps.2012-02280 |
| [32] |
陈琴. 海藻在渔用饲料中的应用[J]. 水产养殖, 2002(6): 36-38. CHEN Q. Application of seaweeds in aquaculture feed[J]. Journal of Aquaculture, 2002(6): 36-38. DOI:10.3969/j.issn.1004-2091.2002.06.016 |
| [33] |
ANDREWS J W, MURAI T, CAMPBELL C. Effects of dietary calcium and phosphorus on growth, food conversion, bone ash and hematocrit levels of catfish[J]. The Journal of Nutrition, 1973, 103(5): 766-771. DOI:10.1093/jn/103.5.766 |
| [34] |
纪利芹, 蒋克勇, 韩龙江, 等. 连续降温对大菱鲆成鱼代谢机能的影响[J]. 海洋科学, 2014, 38(5): 46-53. JI L Q, JIANG K Y, HAN L J, et al. Effect of continuous cooling on metabolic function of adult Scophthalmus maximus L.[J]. Marine Sciences, 2014, 38(5): 46-53. |
| [35] |
GORDON T, CASTELLI W P, HJORTLAND M C, et al. High density lipoprotein as a protective factor against coronary heart disease:the framingham study[J]. The American Journal of Medicine, 1977, 62(5): 707-714. DOI:10.1016/0002-9343(77)90874-9 |
| [36] |
宋晓玲, 杨绪彤, 思瀚文, 等. 双歧杆菌细胞壁肽聚糖的分离及其对二种海产动物免疫活性的影响[J]. 水产学报, 2005, 29(3): 350-355. SONG X L, YANG X T, SI H W, et al. Peptidoglycan isolation from Bifidobacterium thermophilum and its effect on immuno-enzymetic activity of Penaeus japonicus and Paralichthys olivaceus[J]. Journal of Fisheries of China, 2005, 29(3): 350-355. |
| [37] |
王鹏, 江晓路, 江艳华, 等. 褐藻低聚糖对提高大菱鲆免疫机能的作用[J]. 海洋科学, 2006, 30(8): 6-9. WANG P, JIANG X L, JIANG Y H, et al. Immunoregulatory effect of alginate oligosaccharides on Scophthalmus maximus[J]. Marine Sciences, 2006, 30(8): 6-9. DOI:10.3969/j.issn.1000-3096.2006.08.003 |
2. College of Life Sciences, Ludong University, Yantai 264025, Shandong, China;
3. College of Fisheries and Life Science, Shanghai Ocean University, Shanghai 201306, China
 2019,
Vol. 28
2019,
Vol. 28


