鱼类饲料的糖蛋白质比是一个重要的营养参数,一方面,适当增加饲料中糖含量可以减少蛋白质用于能量的消耗,起到节约蛋白质、减少氮排放和降低饲料成本的作用;另一方面,饲料适宜的糖蛋白质比还能促进鱼体正常代谢和生长,提高饲料转化效率,减少体脂沉积,改善鱼肉品质 [1]。投喂率与水温、鱼体大小是影响鱼类生长的3个最主要因素[2]。投喂不足,鱼体生长缓慢,规格不齐;反之,则鱼类摄入能量过高,造成鱼体肥胖、浪费饲料、污染养殖环境[3]。研究表明,由于鱼类饲料的营养水平不同,其适宜的投喂率也存在差异[4, 5, 6],二者共同决定了营养物质的摄入总量,进而影响鱼体生长、代谢和饲料利用效率等。因此,研究鱼类饲料适宜的糖蛋白质比和投喂率,对了解该鱼的糖、蛋白质营养需求水平,饲料糖节约蛋白质的效应,以及为制定其合理的投喂策略,降低养殖成本等都具有重要作用。
异育银鲫(Carassius auratus gibelio)是我国养殖产量最大的鲫鱼品系[7]。近十多年来,对该鱼的糖[8, 9, 10]、蛋白质[11, 12]营养需求及投喂方法[13, 14, 15] 的研究较多,但有关饲料糖蛋白质比及投喂率对该鱼生长和代谢的影响还未见报道。为此,作者开展本实验研究,以期为异育银鲫的饲料配制和投喂管理提供参考。
1 材料与方法 1.1 实验鱼实验鱼为异育银鲫“中科3号”,由安徽省农业科学院水产研究所实验基地提供。实验鱼用质量浓度为5%的食盐水消毒后,驯养于3个规格为4.00 m × 2.00 m × 1.20 m的长方形水泥池中,驯养期15 d,期间投喂破碎的商品饲料。
1.2 实验饲料实验饲料以进口鱼粉、豆粕为蛋白源,鱼油为脂肪源,玉米淀粉为糖源,微晶纤维素调节至100%。设计了3组糖(以无氮浸出物表示,NFE)/蛋白质水平分别为24%/42%、32%/36%、40%/30%的等能等脂饲料(表1)。饲料原料均过60目筛,按配比称重,经逐级充分混合后,用小型饲料成型机挤压成型,自然条件下晾干后于-20 ℃冰箱贮藏,投喂时人工将其剪切成粒长1.0~1.5 mm,粒径约1.0 mm的颗粒。

|
表1 实验饲料组成及营养水平(% 风干基础) Tab. 1 Composition and nutrient levels of experimental diets (% air-dry basis) |
每组饲料的投喂率分别设定为鱼体湿质量的2%、3%和4%3个水平,实验共9组,分别命名为C24/P42/FR2、C24/P42/FR3、C24/P42/FR4、C32/P36/FR2、C32/P36/FR3、C32/P36/FR4、C40/P30/FR2、C40/P30/FR3 和C40/P30/FR4(C-糖,P-蛋白质,FR-投喂率),每组设3个重复,每个重复放养24尾初始体质量为(2.40±0.10)g的健康异育银鲫。实验鱼放养于27个220 L的玻璃缸养殖桶中。实验期间每10 d测定1次各桶鱼的质量并按照设定的投喂率调整投喂量。
实验采用静水连续充气养殖,期间每天投喂2次,投喂时间为08:30、16:30,每次持续20~40 min。每天投喂结束后采用虹吸法吸出各桶内污物,然后通过独立的进排水管道换水,日换水量约为3/5。实验用水为曝气后的自来水,水温(27.0 ±3.0)℃,pH 6.8~7.6,水中溶解氧大于6.5 mg/L,氨氮小于0.1 mg/L。正式实验期50 d,期间无实验鱼死亡。
1.4 生长指标测定正式实验结束后,实验鱼饥饿24 h,测定各重复内鱼的总重和尾数,计算特定生长率(special growth rate,SGR)、饲料转化效率(feed conversion efficiency,FCE)、蛋白效率比(protein efficiency ratio,PER)。从每个重复内随机取6尾鱼,用纱布擦干鱼体表水分,依次测定体长、体重、计算肥满度(condition factor,CF);解剖后取肝胰脏,测定肝胰脏重,计算肝体指数(hepatosomatic index,HSI),胴体-20 ℃保存用于组分测定。相关指标计算公式如下:

从每个重复内随机取3尾鱼,于冰盘上解剖,快速分离出肝胰脏,用4 ℃预冷的0.85%生理盐水冲洗,然后用滤纸吸干水分,-20 ℃冷冻保存,备用。测定时,按质量体积比1∶9加入预冷的0.85%生理盐水,以FLOKE高速组织匀浆机匀浆(10 000 r/min,2 min),4 ℃条件下4 000 r/min离心10 min,取上清液待测。
1.5.2 生理生化指标测定肝糖原含量、肝酯酶(hepatic lipase,HL)和脂蛋白酯酶(lipoprotein lipase,LPL)活性、脂质过氧化物(lipid peroxidation,LPO)由南京建成生物工程研究所提供的试剂盒测定;总酯酶(total lipase,TLP)是HL和LPL的总和。苹果酸酶(malic enzyme,ME)的活性由苏州科铭生物技术有限公司提供的试剂盒测定。具体操作详见试剂盒说明书。肝脏和肌肉组织中蛋白质含量采用考马斯亮蓝法测定。
1.6 常规营养成分测定每桶取15尾鱼作为1个混合样,用于全鱼成分测定,其中粗蛋白质含量采用凯氏定氮法测定;粗脂肪含量采用索氏抽提法测定;粗灰分含量采用550 ℃马福炉灼烧法测定;水分含量采用105 ℃恒温干燥法测定;粗纤维含量按照GB/T 6434—2006中方法测定。胴体、饲料成分每组测3个重复值,测定方法同上。
1.7 数据处理实验数据采用平均数±标准差(mean±SD)表示,采用Excel 2007和SAS 9.1.4 统计软件进行分析处理,处理间差异用单因子方差分析,差异显著时再用邓肯氏复极差法进行多重比较。饲料种类、投喂率及其交互作用用双因子方差分析。P< 0.05为差异显著。
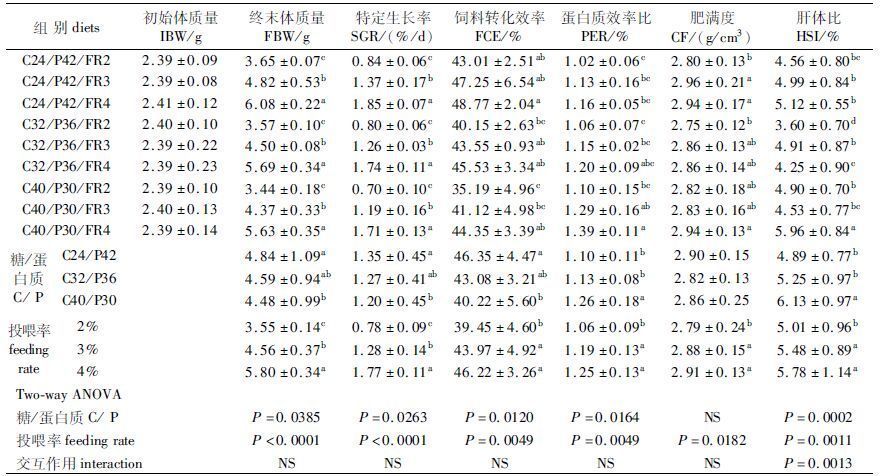
2 结果 2.1 异育银鲫的生长性能由表2可知,饲料C/P水平和投喂率显著影响异育银鲫的FBW、SGR、FCE、PER和HSI,CF仅受投喂率显著影响(P<0.05)。随着饲料C/P水平上升,C40/P30组FBW、SGR、FCE显著低于C24/P42组(P<0.05),C32/P36与C24/P42组间差异不显著(P>0.05);C40/P30组PER、HSI显著高于C24/P42与C32/P36组(P<0.05),C24/P42与C32/P36组间差异不显著(P>0.05)。随着投喂率增加,3%与4%组FBW、SGR、FCE、PER、CF、HSI显著高于2%组(P<0.05),4%组除FBW、SGR显著高于3%组外(P<0.05),两组间FCE、PER、CF、HSI无显著差异(P>0.05)。饲料C/P水平和投喂率对HSI有交互作用(P<0.05)。
|
表2 异育银鲫的生长性能 Tab. 2 Growth performance of Carassius auratus gibelio |
由表3可知,全鱼蛋白质、肝脏脂肪含量未受饲料C/P水平和投喂率影响(P>0.05),全鱼水分、脂肪、灰分,胴体水分、脂肪和肝脏糖原含量仅受投喂率显著影响(P<0.05)。随着投喂率增加,3%与4%组全鱼水分、灰分和胴体水分显著低于而脂肪含量显著高于2%组(P<0.05),3%与4%组间差异不显著(P>0.05);3%组肝糖原含量显著高于2%组(P<0.05),3%与4%组、2%与4%组间差异不显著(P>0.05)。饲料C/P水平和投喂率对鱼体组成无交互作用。

|
表3 异育银鲫的体组成(% 湿重基础) Tab. 3 Body composition of Carassius auratus gibelio (% wet weight basis) |
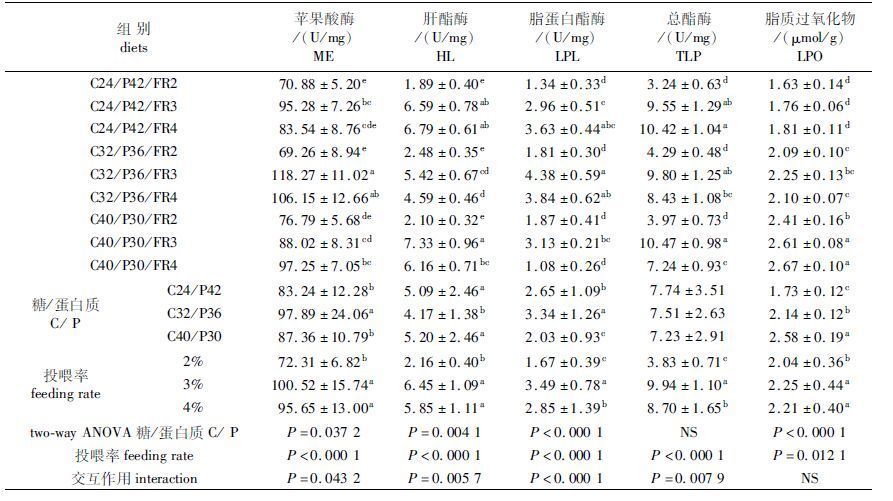
由表4可知,饲料C/P水平和投喂率显著影响异育银鲫肝脏ME、HL、LPL活性及LPO含量,TLP活性仅显著受投喂率影响(P<0.05)。随着饲料C/P水平上升,ME活性C32/P36组显著高于C24/P42与C40/P30组(P<0.05),C24/P42与C40/P30组间差异不显著(P>0.05);HL与LPL活性变化趋势相反,但TLP活性无显著变化;C40/P30组LPO含量显著高于C24/P42与C32/P36组(P<0.05)。随着投喂率增加,3%与4%组ME、HL、LPL、TLP活性及LPO含量显著高于2%组(P<0.05),4%组LPL与TLP活性显著低于3%组(P<0.05),两组间ME、HL活性及LPO含量差异不显著(P>0.05)。饲料C/P水平和投喂率对肝脏ME、HL、LPL、TLP活性均有交互作用。
|
表4 异育银鲫肝脏脂质代谢指标 n=6 Tab. 4 Lipid metabolism indices of Carassius auratus gibelio |
糖类是廉价且易得的能源物质,饲料中适宜的C/P水平对降低生产成本等具有重要意义[17]。本实验中,C40/P30与C32/P36组、C32/P36与C24/P42组FBW、SGR、FCE差异不显著,随着饲料C/P水平上升,C40/P30组PER显著高于C24/P42组,但FBW、SGR、FCE较后者显著下降。结果表明,当饲料中糖替代蛋白质的水平适宜时,鱼体生长和饲料利用效率无明显差异,表现出较好的节约蛋白质的作用;而当替代水平过高时,饲料能量蛋白质比失衡,较高的糖及较低的蛋白质水平致使饲料转化效率下降,鱼体生长受阻,相似的结果见于对奥尼罗非鱼[18](Oreochromis niloticus×O. aureus)和大菱鲆[19](Scophthalmus maximus)的研究中。本实验中,就生长和饲料利用指标而言,C32/P36水平的饲料可满足异育银鲫的生长需求,这与TAN等[10]和叶文娟等[12]的研究结果一致。
研究表明,当鱼类营养摄入水平低于维持其日常水平时,鱼体将会负增长;随着食物可得性增加,鱼类摄食量也逐步增加,在达到其最适摄食量之前,SGR与摄食量间呈线性增长关系;当投喂量超过最适摄食量时,鱼体消化和吸收效率降低,导致SGR下降,饲料系数虚高[20]。本实验中,随着投喂率增加,鱼体FBW、SGR显著增大,FCE、PER、CF先上升后趋于稳定,这与朱晓鸣等[13]的研究结论一致。4%组FBW、SGR显著高于3%组,两组间FCE、PER虽无显著差异,但4%组FCE、PER均高于3%组,因此,4%的投喂率接近实验鱼的最大摄食水平,仍适宜于其生长需求。
本实验中,饲料C/P水平和投喂率对HSI有交互作用(P<0.0001)。C40/P32组HSI显著高于C24/P42与C32/P36组,3%与4%组HSI显著高于2%组(P<0.05),这可能是随着饲料C/P水平和投喂率增加,异育银鲫摄入的糖或能量水平较高,引起肝脏代谢负荷增大所致。
3.2 饲料C/P水平和投喂率对异育银鲫体组成的影响在研究饲料C/P水平对鱼类的影响时,研究者常采用两因子交叉实验法,饲料设计若干个蛋白质水平,每个蛋白质水平内再设定若干个糖水平,对饲料的能量未做一致性要求,结果显示,饲料C/P水平对鱼体组成、肝糖原含量等有显著影响[21, 22]。本实验中,在等能等脂条件下,异育银鲫全鱼、胴体及肝脏组成不受饲料C/P水平影响,实验结果与GAYE等[23]对吉富罗非鱼(Oreochromis niloticus)的研究结论相类似。这表明,在本实验条件下,由于C/P水平在一定范围内变化导致饲料中增加的糖或蛋白质主要用于鱼体代谢所需的能量消耗,抑或不能被充分利用而浪费,并未转化成额外的能源物质贮积于体内。不同研究者获得的结论各异,除受饲料组成影响外,还与实验对象、实验条件等不同有关。
随着投喂率增加,全鱼、胴体水分含量显著下降,脂肪含量显著升高,全鱼灰分含量变化与水分一致,相同的结果见于对鲤[24](Cyprinus carpio)、卡特拉鲃[25](C.catle)和褐牙鲆[26](Paralichthys olivaceus)的研究。3%、4%组肝脏脂肪含量与2%组无显著变化,而全鱼及胴体脂肪含量较2%组显著增加,实验结果提示,3%与4%组促进了异育银鲫体脂的合成,但由于实验鱼规格较小,处于生长初期阶段,加上定期测量体重的影响等,肝脏代谢速率加剧,合成的脂肪主要转移到腹腔或肌肉组织储存[27],并未造成肝脏脂肪的蓄积。
3.3 饲料C/P水平和投喂率对异育银鲫肝脏脂质代谢指标的影响苹果酸酶(ME)、葡萄糖-6-磷酸脱氢酶(G6PDH)、磷酸葡萄糖脱氢酶(PGDH)等通过催化不同反应生成NADPH,为合成脂肪酸提供必需的原料,是参与脂肪合成的重要酶[28]。饲料C/P水平对鱼类脂肪合成相关酶的活性影响还存在争议,大量研究表明,ME、G6PDH、PGDH等酶的活性随饲料C/P水平升高而增大[28, 29, 30],高糖饲料具有抑制糖异生和氨基酸降解、促进脂肪合成的作用[31];而不同的观点则认为,与NADPH生成相关的酶的活性不受饲料C/P水平影响[32, 33, 34],饲料中高水平糖主要用于糖原合成或者能量消耗,并不能增加脂肪合成[35]。HL和LPL是参与脂肪降解的关键酶,这两种酶合称为TLP[36, 37]。本实验中,饲料C/P水平显著影响异育银鲫肝脏ME活性,但不同C/P水平组鱼体及肝脏脂肪含量并无显著差异,推测是NADPH的生成不仅与ME活性有关,还受其他代谢途径的影响,各C/P水平组生成的NADPH总量相当,同时,实验结果显示,尽管饲料C/P水平显著影响HL和LPL活性,但对TLP活性无显著影响,因此脂肪合成与分解处于动态平衡状态,饲料C/P水平的变化并未导致鱼体及肝脏脂肪含量的差异。
有关投喂率对鱼类脂质代谢指标的影响还少有报道。本实验中,与2%组相比,3%、4%组肝脏ME、HL、LPL、TLP活性显著上升,全鱼及胴体脂肪含量也显著增加,脂肪代谢酶活性与投喂率间呈正相关,增加投喂率促进了体脂的合成,实验结果与SHIMENO等[24]的研究结论一致。LPO是细胞毒性物质,其含量反映了机体脂质过氧化水平和细胞损伤程度[38],本实验中,肝脏LPO含量随饲料C/P水平和投喂率增加而显著升高,这可能是鱼体糖代谢负荷增大而加速了自由基的生成所致。就本实验而言,4%的投喂率虽有增大体脂沉积和细胞脂质过氧化损伤的趋势,但显著促进了异育银鲫的生长。
综上所述,C32/P36水平的饲料能够满足异育银鲫幼鱼的生长需求,其适宜的投喂率为4%。
| [1] | HEMER G I, MOMMSEN T P, KROGDAHL Å. Carbohydrates in fish nutrition: effects on growth, glucose metabolism and hepatic enzymes [J]. Aquaculture Nutrition, 2002, 8(3): 175-194. |
| [2] | DENG D F, KOSHIO S, YOKOYAMA S, et al. Effects of feeding rate on growth performance of white sturgeon (Acipenser transmontanus) larvae [J]. Aquaculture, 2003, 217(1/4): 589-598. |
| [3] | ROBINSON E H, LI M H. Effect of dietary protein concentration and feeding rate on weight gain, feed efficiency, and body composition of pond-raised channel catfish Ictalurus punctatus [J]. Journal of the World Aquaculture Society, 1999, 30(3): 311-318. |
| [4] | ROBINSON E H, LI M H, MANNING B B, et al. Effects of dietary protein and feeding rate on channel catfish Ictalurus punctatus production, composition of gain, processing yield, and water quality [J]. Journal of the World Aquaculture Society, 2004, 35(4): 468-477. |
| [5] | 孙丽华, 陈浩如, 黄洪辉, 等. 摄食水平和饵料种类对军曹鱼幼鱼生长及氮收支的影响[J]. 热带海洋学报, 2010, 29(4): 94-101. SUN L H, CHEN H R, HUANG H H, et al. Effects of ration level and feed type on growth and nitrogen budget of young cobia (Rachycentron canadum)[J]. Journal of Tropical Oceanography, 2010, 29(4): 94-101. |
| [6] | 孙耀, 张波, 陈超, 等. 摄食水平和饵料种类对3种海洋鱼类生长和生长效率的影响[J]. 中国水产科学, 2000, 7(3): 41-45. SUN Y, ZHANG B, CHEN C, et al. Effect of ration and feed species on growth and ecological conversion efficiency of 3 species of sea fishes[J]. Journal of Fishery Sciences of China, 2000, 7(3): 41-45. |
| [7] | 陈学年, 郭玉娟, 王忠卫, 等. 异育银鲫“中科3号”与丰产鲫形态特征及生长的比较研究[J]. 淡水渔业, 2011, 41(5): 83-87. CHEN X N, GUO Y J, WANG Z W, et al. Comparison of morphometric attribute and growing performance between Allogynogenetic gibel carp (Carassius auratus gibelio) “CAS Ⅲ” and Pengze crucian carp (Carassius auratus Var.Pengzenensis)[J]. Freshwater Fisheries, 2011, 41(5): 83-87. |
| [8] | 蔡春芳, 陈立侨, 吴萍, 等. 饲料糖种类和水平对异育银鲫肝糖原代谢的影响[J]. 中国水产科学, 2003, 10(1): 55-59. CAI C F, CHEN L Q, WU P, et al. Effects of types and levels of dietary carbohydrate on liver glycogen metabolism of allogynogenetic silver crucian carp[J]. Journal of Fishery Sciences of China, 2003, 10(1): 55-59. |
| [9] | TAN Q, XIE S, ZHU X, et al. Effect of dietary carbohydrate sources on growth performance and utilization for gibel carp (Carassius auratus gibelio) and Chinese longsnout catfish (Leiocassis longirostris Günther) [J]. Aquaculture Nutrition, 2006, 12(1): 61-70. |
| [10] | TAN Q S, WANG F, XIE S Q, et al. Effect of high dietary starch levels on the growth performance, blood chemistry and body composition of gibel carp (Carassius auratus var. gibelio) [J]. Aquaculture Research, 2009, 40(9): 1011-1018. |
| [11] | 蔡春芳, 吴康, 潘新法, 等. 蛋白质营养对异育银鲫生长和免疫力的影响[J]. 水生生物学报, 2001, 25(6): 590-596. CAI C F, WU K, PAN X F, et al. The effects of protein nutrition on growth and immunoloical activity of allogynogenetic silver crucian carp[J]. Acta Hydrobiologica Sinica, 2001, 25(6): 590-596. |
| [12] | 叶文娟, 朱晓鸣, 韩冬, 等. 异育银鲫的蛋白需求研究[C]//2012年中国水产学会学术年会论文摘要集. 北京: 中国水产学会, 2012: 143. YE W J, ZHU X M, HAN D, et al. The protein requirement of gibel carp (Carassius auratus gibelio)[C]//China Society of Fisheries Abstracts of the Annual Meeting 2012. Beijing: China Society of Fisheries, 2012: 143. |
| [13] | 朱晓鸣, 解绶启, 崔奕波. 摄食水平对异育银鲫生长及能量收支的影响[J]. 海洋与湖沼, 2000, 31(5): 471-479. ZHU X M, XIE S Q, CUI Y B. Effect of ration level on growth and energy budget of the gibel carp, Carassius auratus gibelio[J]. Oceanologia et Limnologia Sinica, 2000, 31(5): 471-479. |
| [14] | ZHOU Z, CUI Y, XIE S, et al. Effect of feeding frequency on growth, feed utilization, and size variation of juvenile gibel carp (Carassius auratus gibelio)[J]. Journal of Applied Ichthyology, 2003, 19(4): 244-249. |
| [15] | ZHAO S B, HAN D, ZHU X M, et al. Effects of feeding frequency and dietary protein levels on juvenile allogynogenetic gibel carp (Carassius auratus gibelio) var. CAS III: growth, feed utilization and serum free essential amino acids dynamics[J]. Aquaculture Research, 2016, 47(1): 209-303, doi: 10.1111/are.12491. |
| [16] | SEILIEZ I, PANSERAT S, LANSARD M, et al. Dietary carbohydrate-to-protein ratio affects TOR signaling and metabolism-related gene expression in the liver and muscle of rainbow trout after a single meal [J]. American Journal of Physiology-Regulatory Integrative and Comparative Physiology, 2011, 300(3): R733-R743. |
| [17] | WINFREE R A, STICKNEY R R. Effects of dietary protein and energy on growth, feed conversion efficiency and body composition of Tilapia aurea [J]. The Journal of Nutrition, 1981, 111(6): 1001-1012. |
| [18] | SHIAU S Y, PENG C Y. Protein-sparing effect by carbohydrate in diets for tilapia, Oreochromis niloticus×O. aureus [J]. Aquaculture, 1993, 117(3/4): 327-334. |
| [19] | ZENG L, LEI J L, AI C X, et al. Protein-sparing effect of carbohydrate in diets for juvenile turbot Scophthalmus maximus reared at different salinities [J]. Chinese Journal of Oceanology and Limnology, 2015, 33(1): 57-69. |
| [20] | DE SILVA S S, ANDERSON T A. Fish nutrition in aquaculture[M]. London: Chapman & Hall, 1995: 319. |
| [21] | SHIAU S Y, LIN Y H. Carbohydrate utilization and its protein-sparing effect in diets for grouper (Epinephelus malabaricus) [J]. Animal Science, 2001, 73(1): 299-304. |
| [22] | MOHANTA K N, MOHANTY S N, JENA J K. Protein-sparing effect of carbohydrate in silver barb, Puntius gonionotus fry [J]. Aquaculture Nutrition, 2007, 13(4): 311-317. |
| [23] | GAYE-SIESSEGGER J, FOCKEN U, BECKER K. Effect of dietary protein/carbohydrate ratio on activities of hepatic enzymes involved in the amino acid metabolism of Nile tilapia, Oreochromis niloticus (L.) [J]. Fish Physiology and Biochemistry, 2006, 32(4): 275-282. |
| [24] | SHIMENO S, SHIKATA T, HOSOKAWA H, et al. Metabolic responses to feeding rates in common carp, Cyprinus carpio [J]. Aquaculture, 1997, 151(1/4): 371-377. |
| [25] | ABIDI S F, KHAN M A. Evaluation of feeding rate based on growth, feed conversion, protein gain and carcass quality of fingerling Indian major carp, Catla catla (Hamilton)[J]. Aquaculture Research, 2012, 45(3): 439-447. |
| [26] | CHO S H, LEE S M, PARK B H, et al. Effect of feeding ratio on growth and body composition of juvenile olive flounder Paralichthys olivaceus fed extruded pellets during the summer season [J]. Aquaculture, 2006, 251(1): 78-84. |
| [27] | SHERIDAN M A. Regulation of lipid metabolism in poikilothermic vertebrates [J]. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry, 1994, 107(4): 495-508. |
| [28] | DIAS J, ALVAREZ M J, DIEZ A, et al. Regulation of hepatic lipogenesis by dietary protein/energy in juvenile European seabass (Dicentrarchus labrax) [J]. Aquaculture, 1998, 161(1/4): 169-186. |
| [29] | LIN J H, SHIAU S Y. Hepatic enzyme adaptation to different dietary carbohydrates in juvenile tilapia Oreochromis niloticus×O. aureus [J]. Fish Physiology and Biochemistry, 1995, 14(2): 165-170. |
| [30] | METÓN I, MEDIAVILLA D, CASERAS A, et al. Effect of diet composition and ration size on key enzyme activities of glycolysis-gluconeogenesis, the pentose phosphate pathway and amino acid metabolism in liver of Gilthead sea bream (Sparus aurata) [J]. British Journal of Nutrition, 1999, 82(3): 223-232. |
| [31] | SHIMENO S, KHEYYALI D, SHIKATA T. Metabolic response to dietary carbohydrate to protein rations in Carp [J]. Fisheries Science, 1995, 61(2): 277-281. |
| [32] | SÁ R, POUSÃO-FERREIRA P, OLIVA-TELES A. Growth performance and metabolic utilization of diets with different protein: carbohydrate ratios by white sea bream (Diplodus sargus, L.) juveniles [J]. Aquaculture Research, 2007, 38(1): 100-105. |
| [33] | SUAREZ M D, SANZ A, BAZOCO J, et al. Metabolic effects of changes in the dietary protein: carbohydrate ratio in eel (Angilla anguilla) and trout (Oncorhynchus mykiss) [J]. Aquaculture International, 2002, 10(2): 143-156. |
| [34] | ENES P, PANSERAT S, KAUSHIK S, et al. Effect of normal and waxy maize starch on growth, food utilization and hepatic glucose metabolism in European sea bass (Dicentrarchus labrax) juveniles [J]. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 2006, 143(1): 89-96. |
| [35] | SÁ R, POUSÃO-FERREIRA P, OLIVA-TELES A. Effect of dietary starch source (normal versus waxy) and protein levels on the performance of white sea bream Diplodus sargus (Linnaeus) juveniles [J]. Aquaculture Research, 2008, 39(10): 1069-1076. |
| [36] | SANTAMRINA-FOJO S, HAUDENSCHILD C, AMAR M. The role of hepatic lipase in lipoprotein metabolism and atherosclerosis [J]. Current Opinion in Lipidology, 1998, 9(3): 211-219. |
| [37] | CHOI S Y, GOLDBERG I J, CURTISS L K, et al. Interaction between ApoB and hepatic lipase mediates the uptake of ApoB-containing lipoproteins [J]. The Journal of Biological Chemistry, 1998, 273(32): 20456-20462. |
| [38] | GARCÍA-GONZÁLEZ A, HERRERA-ABARCA J, OCHOA J L. Effect of superoxide dismutase from bovine erythrocytes on different activity parameters in adjuvant-induced arthritis [J]. Archives of Medical Research, 1999, 30(2): 132-137. |
 2016, Vol. 25
2016, Vol. 25


