扩展功能
文章信息
- 古再丽努尔·买买提, 王建礼, 詹泊, 刘朝宝, 陈墨轩
- Guzalnur Mammat, WANG Jianli, ZHAN Bo, LIU Chaobao, CHEN Moxuan
- 慢性吗啡处理及戒断对社会新颖性和食物寻求行为的影响
- Effects of Chronic Morphine Exposure and Withdrawal on Social Novelty-and Food-seeking Behaviors
- 四川动物, 2016, 35(1): 44-51
- Sichuan Journal of Zoology, 2016, 35(1): 44-51
- 10.11984/j.issn.1000-7083.20150237
-
文章历史
- 收稿日期: 2015-07-21
- 接受日期: 2015-11-04
Food,water and sexual stimuli are called primary rewards. These stimuli are considered innate because they are essential for survival and reproduction(Walter et al., 2005). Food provides a rewarding effect,which has been reflected in the conditioned place preference(CPP)test(Qi et al., 2011; Duarte et al., 2014; Monclaro et al., 2014). Rats tend to approach and spend more time in an environment paired with another rat(Calcagnetti et al., 1992; Crowder & Hutto,1992; Thiel et al., 2008), and animals spend more time with an unfamiliar individual than a more familiar conspecific(Nadler et al., 2004),showing that social stimuli and social novelty can serve as reinforcing cues. In addition to natural rewards,primary reinforcers also include a variety of drugs,opioids and stimulants. The brain circuitry that mediates behavior essential for survival becomes compromised in individuals that have been exposed to drugs, and affects the drive to acquire natural reinforcers(Kelley,2004; Aragona et al., 2007). For example,chronic exposure to a constant dose of cocaine is sufficient to reduce natural reinforcement(Barnea-Ygael et al., 2014). Opiate and psychostimulant abuse respectively fulfills the need for social comfort and for natural rewards that sustain life,by directly impinging on the underlying emotional substrates(Panksepp et al., 1980). Many literatures indicate that endogenous opioid peptides play a crucial role in controlling the motivation for social interactions and ingestion(Cooper & Kirkham,1993; Moles & Cooper,1995; Panksepp et al., 1997; Nocjar & Panksepp,2007; Bai et al., 2014). The blockade of opioid activity is sufficient to impair the expression of a socially acquired food preference(Moles et al., 1999). Morphine-treated mice consume significantly less food, and the incentives for social and non-social natural rewards increase following withdrawal from intermittent opiate treatment(Nocjar & Panksepp,2007).
Previous work suggests that abstinence from drugs can lead to altered emotional systems. Protracted abstinence is characterized by lowered mood or depression(Goeldner et al., 2011; Lutz et al., 2013). Withdrawal from morphine is associated with an increase in anxiogenic-like behaviors(Castilho et al., 2008; Miladi-Gorji et al., 2012),while morphine exposure is known to induce anxiolytic effects(Shin et al., 2003; Motevasseli et al., 2010; Rezayof et al., 2013). The pharmacologic effect of the drug exposure differs from abstinence. The decreased motivation for a natural reinforcement is thought to be associated with the drug withdrawal-induced depressive state(Zhang et al., 2007). Although it has been widely researched that opiates regulate natural rewards-associated behaviors,including food consumption(Moles & Cooper,1995; Bai et al., 2014),food preference(Nocjar & Panksepp,2007; Kerstette et al., 2012),social transmission of a food preference(Moles et al., 1999), and social interest(Nocjar & Panksepp,2007; Bai et al., 2014),it is not known whether morphine exposure and withdrawal differently affect the motivation to approaching the natural reinforcers. To better underst and the causal relationship between morphine experience and natural reward-seeking behavior,we examined the effects of chronic morphine exposure and withdrawal on the social novelty- and food-seeking behaviors in mice.
1 Materials and methods1.1 SubjectsSix-week old male ICR mice were .: SCXK(宁)2011-0001] obtained from Ningxia Medical University Laboratory Animal Center(Yinchuan,China). The mice were housed in groups of two in st and ard transparent Makrolon cages(l×w×h,32 cm×21.5 cm×17 cm). The colony room was illuminated on a 12∶ 12 light-dark cycle(lights on 2000 h), and the temperature was maintained at 23 ℃±2 ℃. Food and water were available ad libitum. Mice were allowed to adapt to housing conditions for one week, and were h and led daily by the same experimenter for three days prior to testing. All experimental procedures were performed strictly in accordance with the guidelines published in the NIH Guide for the Care and Use of Laboratory Animals.
1.2 General experimental proceduresThe experiences of the mice were divided into two phases: administration and withdrawal phases. In the administration phase,mice received daily subcutaneous injections of 10.0 mg·kg-1 morphine(ME,n=8)or 0.9% saline(SE,n=8)for 14 days. Following 14-day morphine exposure,mice underwent three experimental measurements in sequence: open field test,social novelty-seeking and food-seeking test. In the withdrawal phase,mice that had been treated for 14 days were given seven-day morphine withdrawal(n=8)or saline withdrawal(n=8). During the withdrawal phase,mice were left undisturbed in their home-cages,except for scheduled cage cleaning. Following the seven-day withdrawal,the second round of social novelty-seeking and food-seeking tests were conducted,but not the open field test. Fig. 1 represents a timeline depicting the experimental session.
 |
| Fig. 1 Timeline of the experimental sessionThe boxed text represents the components of the experimental session. Mice were given daily injections of morphine(10 mg·kg-1)or saline for 14 days, then open-field, social novelty-seeking and food-seeking behaviors were assessed. Mice then underwent a seven-day morphine or saline-free period, followed by a second test for social novelty-and food-seeking behavior. |
Morphine-hydrochloride(Northwest Pharmaceutical Co., Ltd. Sinopharm, Xi’an, China)was used in this experiment, and was diluted in saline. During the administration phase, mice received a daily binge injection of morphine or saline for 14 days. The daily binge pattern consisted of two injections of an identical dose of morphine(subcutaneously, 10 mg·kg-1)in 12 h at 08∶ 00 and 14∶ 00. This morphine dose was chosen based on a previous study that demonstrated that chronic administration of 10 mg·kg-1 of morphine increases neuronal activity in the areas of the brain that regulate appetitive behavior for both drugs and natural rewards when tested after six-day withdrawal(Kraus et al., 1997).
1.4 Open field test20 minutes after the last injection, motor activity and anxiety-like behaviors were measured for 5 min in an open field chamber. The chamber was a brightly and evenly illuminated square arena(l×w×h, 50 cm×50 cm×25 cm)made of white glacial polyvinyl chloride and illuminated with four 60 W lamps mounted 1.5 m above the arena. The area was divided into 16 quadrants(4 central and 12 peripheral)(Fiore & Ratti, 2007). A single mouse was placed in the center of the open field and was left to explore for 5 min. To assess anxiety-like behavior, the time spent in the center of the open field was measured during this period. The number of crossings between quadrants was used to assess locomotion. Additionally, rearing(raising on the hind legs and sniffing into the air or the wall of the box) and self-grooming behavior(licking own fur, sometimes using forepaws, passing them over the nose with a series of brief, horizontal movements)were recorded. All focal mice were videotaped for 5 min using a Sony camera. The frequency and total duration of these behaviors were later scored by a researcher blind to experimental treatment using Jwatcher 1.0. After each test was completed, the open-field was thoroughly cleaned with 70% ethanol solution.
1.5 Social novelty-seeking testSocial novelty-seeking test was performed in Makrolon chambers(l×w×h, 46 cm×31.5 cm×20 cm). A cylindrical wire cage containing a familiar mouse that was raised in a cage with the subject mouse was set in one of the chamber's corners. Another cage containing an unfamiliar mouse that had not previously encountered the subject mouse was set in another side corner in parallel. Placement of stimulus-cages within the chamber's corner was counterbalanced between each test. The wire holding cage was stainless steel, 17 cm high, and composed of a solid 11 cm diameter bottom with stainless steel bars spaced at 1 cm intervals. The experimental mice were individually placed in the center of the chamber arena and videotaped for 15 min. Measurement of total approaching or sniffing time at each stimulus-cage(measured by the animal having its nose within 1 cm of cage)was tabulated for each mouse within 15 min. The chamber arena was wiped clean with 70% ethanol between mice to eliminate olfactory cues. Videotapes were viewed by an observer that was blind to the treatment of each mouse.
1.6 Food-seeking testThe food-seeking test was performed in the same conditions as described above except a cylindrical wire cage containing mouse chow was set in the chamber’s corner. Another empty cylindrical wire cage was set in another side corner in parallel. The experimental mice were individually placed in the center of the chamber arena and videotaped for 15 min. The experiments were conducted after mice were food deprived for 2 h prior to the food-seeking test.
1.7 Statistical analysisStatistical analyses were conducted using SPSS 13.0. All data were checked for normality using a one-sample Kolmogorov-Smirnov test and were found to be normally distributed. Independent sample t-tests were used to examine differences in the open-field behaviors. Social novelty-and food-seeking behaviors were compared using One-Way ANOVA. All data were presented as mean±st and ard error(SE) and the alpha was set at 0.05.
2 Results2.1 Behavior in open fieldMorphine-treated mice showed a greater number of total transitions(t14=6.010, P<0.001) and spent more time in the central area than saline-treated mice(t14=4.986, P<0.001)(Fig. 2: A, B). Compared to the saline controls, morphine-treated mice had less self-grooming behavior(t14=-3.849, P=0.002). No significant differences were found in rearing behavior between the two groups(t14=0.672, P=0.512)(Fig. 2: C).
 |
| Fig. 2 The behaviors of ICR mice in open-field test following 14-day saline or morphine exposure(A)the mean number of total transitions, (B)total time spent in the central area, and (C)duration of rearing and self-grooming behavior in mice injected with 0.9% saline(SE)or mice injected with 10 mg·kg-1 morphine(ME); **P≤0.01; Error bars depict st and ard error. |
Saline-administrated mice spent significantly more time approaching the cage containing unfamiliar conspecifics than approaching the cage containing familiar conspecifics(Mean difference=131.37, P=0.032). However, morphine-administered mice spent similar amount of time approaching the cage containing unfamiliar conspecifics and the cage containing familiar conspecifics(Mean difference=114.35, P=0.059)(Fig. 3). Mice undergoing withdrawal from morphine(Mean difference=171.72, P=0.001)or saline(Mean difference=92.67, P=0.044)spent more time approaching the cage containing unfamiliar conspecifics than approaching the cage containing familiar conspecifics. In comparison to the saline controls, there were no significant differences in the approach to the cage containing unfamiliar conspecifics in morphine administration phase(Mean difference=71.642, P=0.229) and withdrawal phase(Mean difference=-11.26, P=0.800)(Fig. 3).
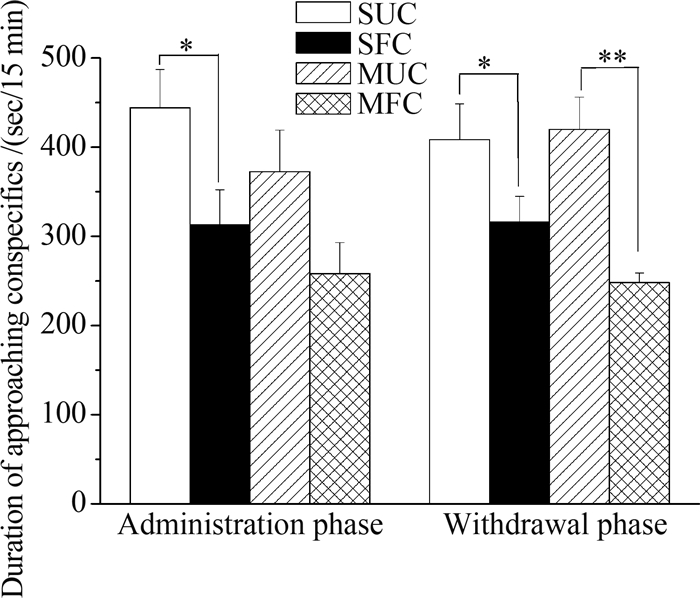 |
| Fig. 3 Time spent approaching conspecifics in saline-and morphine-treated ICR mice following 14-day administration and seven-day withdrawal phasesGraphs depict the mean time spent by saline-treated mice to unfamiliar conspecifics(SUC) and familiar conspecifics(SFC), and morphine-treated mice to unfamiliar conspecifics(MUC) and familiar conspecifics(MFC); *P≤ 0.05, **P≤ 0.01; Error bars depict st and ard error. |
Both saline-administrated(t7=4.556, P=0.003) and morphine-administrated(t7=5.426, P=0.01)mice spent more time approaching the cage containing food rather than empty cage. Compared to saline treated mice, morphine treated mice did not show differences in the time approaching the cage containing food(t14=-0.330, P=0.746)(Fig. 4). Control mice undergoing saline withdrawal spent more time approaching the cage containing food rather than empty cage(t7=3.244, P=0.014). However, morphine-withdrawal mice spent less time approaching the cage containing food(t7=-4.081, P=0.005). Compared to the mice experienced saline withdrawal, the mice experienced morphine withdrawal reduced the time approaching the cage containing food(t14=6.245, P< 0.001)(Fig. 4).
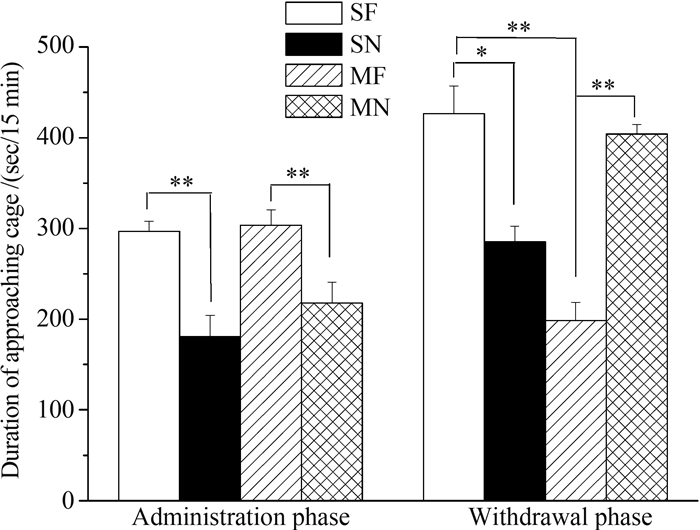 |
| Fig. 4 Time spent approaching food in saline-and morphine-treated ICR mice following 14-day administration and seven-day withdrawal phasesGraphs depict mean time the saline-treated mice approached to the cage with food(SF) and without food(SN), and morphine-treated mice approached the cage with food(MF) and without food(MN); *P≤0.05, **P≤0.01; Error bars depict st and ard error. |
In this study, we assessed the effects of chronic morphine exposure and morphine-withdrawal on social novelty-and food-seeking behaviors. We observed an increase in locomotion in mice that had been administered morphine for 14 days, which suggested that the morphine exposure elicited pharmacologic effects. The morphine exposure decreased the mice's tendency to seek unfamiliar conspecifics, while mice preferred unfamiliar conspecifics after seven-day of morphine with drawal. 14-day morphine administration did not affect the preference to food, but mice undergoing morphine withdrawal showed reduced appetitive behavior for food, indicating that there were different motive behaviors during morphine exposure and withdrawal phases.
3.1 Effects of chronic morphine exposure and withdrawal on social novelty-seeking behaviorSeveral studies indicated that mice exhibited a heightened sensitivity to the effects of morphine(Niu et al., 2013), and repeated morphine exposure decreases self-grooming behavior in female ICR mice(Zhan et al., 2015). We observed that chronic morphine administration enhanced locomotion and attenuated the levels of self-grooming behavior. A previous study showed that repeated morphine exposure did not affect locomotor activity in male ICR mice(Zhan et al., 2015),differing from our present results. This discrepancy may be due to the fact that the mice were exposed to the morphine for four days in Zhan et al.(2015),instead of 14 days,as was done in the present study. The time spent in the central zone,changes in rearing and self-grooming were generally considered to be indices of emotional behavioral processes(To & Bagdy,1999; Carey et al., 2005). The increased time spent in the central area by mice treated with morphine implies a morphine-induced anxiolytic effect,suggesting that these mice experienced morphine induced-sensitization.
Social reward-CPP can be obtained even when rats were separated by wire or mesh partitions with limited physical contact(Kummer et al., 2011; Peartree et al., 2012). In our study,although the stimulus mice were in wire holding cages,saline-treated mice displayed robust approach toward the unfamiliar mice. Mice that were treated with morphine for 14 days did not show significant preference for unfamiliar mice. These results were consistent with a previous report showing diminished social motivation in opiate dependent rats during chronic morphine exposure(Mumford & Kumar,1979). Seven-day morphine withdrawal did not alter the preference for unfamiliar mice,contrary to some results that prior morphine exposure enhances future social interest,with the effect consistently shown after three-day or two-week opiate withdrawal(Barr & Phillips,1999; Nocjar & Panksepp,2002,2007). Opiate induced change in social interest in young rodents may depend on the animal’s social state while under the drug(Van den Berg et al., 1999a,1999b; Broseta et al., 2005; Nocjar & Panksepp,2007). We noted that animals used in some previous work received chronic morphine while housed in isolation(Nocjar & Panksepp,2007). Thus,one possibility for the discrepancies may be due to the animal’s social state in this study compared to previous studies.
3.2 Effects of chronic morphine exposure and withdrawal on food-seeking behaviorFood preference remained high even after 14 days of morphine exposure. However,when mice were undergoing a seven-day morphine withdrawal showed lower food-seeking behavior. Although chronic exposures to drugs induce a complex series of changes in appetitive behavior,there is no consensus on how it affects natural rewards. For example,Vanhille et al.(2015)proposed that drug addiction was associated with a relative devaluation of natural or socially-valued reinforcers that were unable to divert addicts from seeking and consuming the drug. Most rats preferred natural rewards,such as saccharin,over cocaine before protracted drug exposure. Galaj et al.(2013)found repeated heroin exposure decreased the attractiveness of food and reduced motivation to work for natural rewards. Morphine,d-amphetamine or methamphetamine withdrawal results in decreased motivation to obtain the natural reinforcement(Zhang et al., 2007),yet withdrawal from chronic opiates or amphetamine treatments increased food-seeking(Nocjar & Panksepp,2002,2007). These seemingly conflicting results on natural reward processing appear to relate with the nature of the reward-directed behavior(Galaj et al., 2013). Additionally,rats that were treated with morphine for five-days followed by a seven-day morphine-withdrawal period had significantly reduced consumption of 2.5% sucrose solution(Bai et al., 2014); however,the rats appeared to show increased interest in high-fat food after two-week morphine withdrawal,but not after a three-day short-term withdrawal(Nocjar & Panksepp,2007),indicating that food-consuming behavior and food-seeking behavior may be controlled by different neurochemical circuits in the brain. These discrepancies were related with the length of morphine pretreatment and withdrawal as well. Additionally,male rhesus monkeys treated with high cocaine dose showed preference for cocaine over food,therefore the reinforcer values included in the experiment can influence the seeking-behavior(Banks & Negus,2010). In investigating the effect of drugs on animal behavior,we also cannot exclude differences in species(mice vs. rats)because of the importance of genetic variability in opioid modulation of natural reinforcement(Dym et al., 2007).
In the present study,we found that morphine exposure and withdrawal had different effects on social novelty- and food-seeking behavior. These results supported that independent motivational systems mediated the rewarding effects of opioids in the nondependent state, and in the physically dependent/withdrawal state(Bechara et al., 1998). Repeated drug exposure induced short- and long-term neuroadaptations in brain reward circuitries that were normally involved in the regulation of motivation(Barnea-Ygael et al., 2014). Morphine exposure induced an anxiolytic effect as shown in our results,while withdrawal from morphine may lead to the appearance of anxiety-like behavior(Castilho et al., 2008; Miladi-Gorji et al., 2012),though it was not directly measured in the current study. These neuroadaptations were associated with emotional changes that could affect the incentives for social novelty and food.
In conclusion,morphine exposure and withdrawal differently altered the pursuit of social novelty and food,indicating that morphine can significantly impair the appetitive motivations to two types of natural rewards: social-novelty and food. Morphine-induced different neuroadaptations may regulate these motivational changes. Additional research will be required to underst and where and how morphine acts within the brain to alter the incentives for social novelty and food.
| Aragona BJ, Detwiler JM, Wang ZX. 2007. Amphetamine reward in the monogamous prairie vole[J]. Neuroscience Letter, 418(2): 190-194. |
| Bai Y, Li Y, Lv Y, et al. 2014. Complex motivated behaviors for natural rewards following a binge-like regimen of morphine administration: mixed phenotypes of anhedonia and craving after short-term withdrawal[J]. Fronters in Behavavioral Neuroscience, 8: 23. |
| Banks ML, Negus SS. 2010. Effects of extended cocaine access and cocaine withdrawal on choice between cocaine and food in rhesus monkeys[J]. Neuropsychopharmacology, 35(2): 493-504. |
| Barnea-Ygael N, Gal R, Zangen A. 2014. Chronic cocaine administration induces long-term impairment in the drive to obtain natural reinforcers in high- but not low-demanding tasks[J]. Addiction Biology, doi: 10.1111/adb.12196. |
| Barr AM, Phillips AG. 1999. Withdrawal following repeated exposure to damphetamine decreases responding for a sucrose solution as measured by a progressive ratio schedule of reinforcement[J]. Psychopharmacology, 141(1): 99-106. |
| Bechara A, Nader K, van der Kooy D. 1998. A two-separate-motivational-systems hypothesis of opioid addiction[J]. Pharmacology Biochemistry and Behavior, 59(1): 1-17. |
| Broseta I, Rodriguez-Arias M, Aguilar MA, et al. 2005. Isolation decreases physical and motivational aspects of morphine withdrawal[J]. Behavioural Pharmacology, 16(3): 131-138. |
| Calcagnetti DJ, Schechter MD. 1992. Place conditioning reveals the rewarding aspect of social-interaction in juvenile rats[J]. Physiology & Behavior, 51(4): 667-672. |
| Carey RJ, Depalma G, Damianopoulos E. 2005. Evidence for Pavlovian conditioning of cocaine-induced responses linked to emotional behavioral effects[J]. Pharmacology Biochemistry & Behavior, 80(1): 123-134. |
| Castilho VM, Borelli KG, Brandão ML, et al. 2008. Anxiety-like symptoms induced by morphine withdrawal may be due to the sensitization of the dorsal periaqueductal grey[J]. Physiology & Behavior, 94(4): 552-562. |
| Cooper SJ, Kirkham TC. 1993. Opioid mechanisms in the control of food consumption and taste preferences[M].// Her A. Handbook of experimental pharmacology. Berlin: Springer-Verlag, 104(Ⅱ): 239-262. |
| Crowder WF, Hutto CW. 1992. Operant place conditioning measures examined using 2 non-drug reinforcers[J]. Pharmacology Biochemistry and Behavior, 41(4): 817-824. |
| Duarte RB, Patrono E, Borges AC, et al. 2014. Consumption of a highly palatable food induces a lasting place-conditioning memory in marmoset monkeys[J]. Behavioural Processes, 107: 163-166. |
| Dym CT, Pinhas A, Ginzberg M, et al. 2007. Genetic variance contributes to naltrexone-induced inhibition of sucrose intake in inbred and outbred mouse strains[J]. Brain Research, 1135(1): 136-145. |
| Fiore L, Ratti G. 2007. Remote laboratory and animal behaviour: an interactive open field system[J]. Computers & Education, 49(4): 1299-1307. |
| Galaj E, Cruz I, Schachar J, et al. 2013. Differential effects on natural reward processing in rats after repeated heroin[J]. Psychopharmacology (Berl), 229(1): 125-132. |
| Goeldner C, Lutz PE, Darcq E, et al. 2011. Impaired emotional-like behavior and serotonergic function during protracted abstinence from chronic morphine[J]. Biological Psychiatry, 69(3): 236-244. |
| Kelley AE. 2004. Memory and addiction: shared neural circuitry and molecular mechanisms[J]. Neuron, 44(1): 161-179. |
| Kraus MA, Piper JM, Kornetsky C. 1997. Persistent increases in basal cerebral metabolic activity induced by morphine sensitization[J]. Pharmacology Biochemistry and Behavior, 57(1-2): 89-100. |
| Kummer K, Klement S, Eggart V, et al. 2011. Conditioned place preference for social interaction in rats: contribution of sensory components[J]. Fronters in Behavavioral Neuroscience, 5: 80. |
| Lutz PE, Reiss D, Ouagazzal AM, et al. 2013. A history of chronic morphine exposure during adolescence increases despair-like behaviour and strain-dependently promotes sociability in abstinent adult mice[J]. Behavioural Brain Research, 243: 44-52. |
| Miladi-Gorji H, Rashidy-Pour A, Fathollahi Y. 2012. Anxiety profile in morphine-dependent and withdrawn rats: effect of voluntary exercise[J]. Physiology & Behavior, 105(2): 195-202. |
| Moles A, Cooper SJ. 1995. Opioid modulation of sucrose intake in CD-1 mice: effects of gender and housing conditions[J]. Physiology & Behavior, 58(4): 791-796. |
| Moles A, Valsecchi P, Cooper SJ. 1999. Opioid modulation of socially transmitted and spontaneous food preferences in female mice[J]. Behavioural Processes, 44(3): 277-285. |
| Monclaro AV, Sampaio AC, Ribeiro NB, et al. 2014. Time-of-day effect on a food-induced conditioned place preference task in monkeys[J]. Behavioural Brain Research, 259: 336-341. |
| Motevasseli T, Rezayof A, Zarrindast MR, et al. 2010. Role of ventral hippocampal NMDA receptors in anxiolytic-like effect of morphine[J]. Physiology & Behavior, 101(5): 608-613. |
| Mumford L, Kumar R. 1979. Sexual behaviour of morphine-dependent and abstinent male rats[J]. Psychopharmacology, 65(2): 179-185. |
| Nadler JJ, Moy SS, Dold G, et al. 2004. Automated apparatus for rapid quantitation of social approach behaviors in mice[J]. Genes Brain and Behavior, 3(5): 303-314. |
| Niu H, Zheng Y, Huma T, et al. 2013. Lesion of olfactory epithelium attenuates expression of morphine-induced behavioral sensitization and reinstatement of drug-primed conditioned place preference in mice[J]. Pharmacology Biochemistry & Behavior, 103(3): 526-534. |
| Nocjar C, Panksepp J. 2002. Chronic intermittent amphetamine pretreatment enhances future appetitive behavior for drug- and natural-reward: interaction with environmental variables[J]. Behavioural Brain Research, 128(2): 189-203. |
| Nocjar C, Panksepp J. 2007. Prior morphine experience induces long-term increases in social interest and in appetitive behavior for natural reward[J]. Behavioural Brain Research, 181(2): 191-199. |
| Panksepp J, Herman BH, Villberg T, et al. 1980. Endogenous opioids and social behavior[J]. Neuroscience and Biobehavioral Reviews, 4(4): 473-487. |
| Panksepp J, Nelson E, Bekkedal M. 1997. Brain systems for the mediation of social separation-distress and social-reward. Evolutionary antecedents and neuropeptide intermediaries[J]. Annals of the New York Academy of Sciences, 807: 78-100. |
| Peartree NA, Hood LE, Thiel KJ, et al. 2012. Limited physical contact through a mesh barrier is sufficient for social reward-conditioned place preference in adolescent male rats[J]. Physiology & Behavior, 105(3): 749-756. |
| Qi RL, Qu JG, Chen YM, et al. 2011. Differences in morphine-induced and food-induced conditioned place preference between adolescent and adult mice[J]. Zoological Research, 32(5): 528-532. |
| Rezayof A, Assadpour S, Alijanpour S. 2013. Morphine-induced anxiolytic-like effect in morphine-sensitized mice: involvement of ventral hippocampal nicotinic acetylcholine receptors[J]. Pharmacology Biochemistry and Behavior, 103(3): 460-466. |
| Shin IC, Kim HC, Swanson J, et al. 2003. Anxiolytic effects of acute morphine can be modulated by nitric oxide systems[J]. Pharmacology, 68(4): 183-189. |
| Thiel KJ, Okun AC, Neisewander JL. 2008. Social reward-conditioned place preference: a model revealing an interaction between cocaine and social context rewards in rats[J]. Drug and Alcohol Dependence, 96(3): 202-212. |
| To CT, Bagdy G. 1999. Anxiogenic effect of central CCK administration is attenuated by chronic fluoxetine or ipsapirone treatment[J]. Neuropharmacology, 38(2): 279-282. |
| Van den Berg CL, Kitchen I, Gerrits MAFM, et al. 1999a. Morphine treatment during juvenile isolation increases social activity and opioid peptides release in the adult rat[J]. Brain Research, 830(1): 16-23. |
| Van den Berg CL, Pijlman FT, Koning HA, et al. 1999b. Isolation changes the incentive value of sucrose and social behaviour in juvenile and adult rats[J]. Behavioural Brain Research, 106(1-2): 133-142. |
| Vanhille N, Belin-Rauscent A, Mar AC, et al. 2015. High locomotor reactivity to novelty is associated with an increased propensity to choose saccharin over cocaine: new insights into the vulnerability to addiction[J]. Neuropsychopharmacology, 40(3): 577-589. |
| Walter H, Abler B, Ciaramidaro A, et al. 2005. Motivating forces of human actions: neuroimaging reward and social interaction[J]. Brain Research Bulletin, 67(5): 368-381. |
| Zhan B, Ma HY, Wang JL, et al. 2015. Sex differences in morphine-induced behavioral sensitization and social behaviors in ICR mice[J]. Zoological Research, 36(2): 103-108. |
| Zhang D, Zhou X, Wang X, et al. 2007. Morphine withdrawal decreases responding reinforced by sucrose self-administration in progressive ratio[J]. Addiction Biology, 12(2): 152-157. |
 2016, Vol. 35
2016, Vol. 35