The Chinese Meteorological Society
Article Information
- ZHANG, Yangmei, Yaqiang WANG, Xiaoye ZHANG, et al., 2018.
- Chemical Components, Variation, and Source Identification of PM1 during the Heavy Air Pollution Episodes in Beijing in December 2016. 2018.
- J. Meteor. Res., 32(1): 1-13
- http://dx.doi.org/10.1007/s13351-018-7051-8
Article History
- Received June 12, 2017
- in final form October 14, 2017
2. Center for Excellence in Regional Atmospheric Environment, Institute of Urban Environment, Chinese Academy of Sciences, Xiamen 361021;
3. College of Earth Science, University of Chinese Academy of Sciences, Beijing 100049
Beijing, the capital city of China, experienced severe air pollution for decades. The frequent hazy events that usually occur during winter have impaired China’s atmospheric visibility, public health, and international image. In winter 2016/17, Beijing experienced heavy air pollution episodes (HPEs) that lasted for 7 days. To reduce pollution, various sections of Beijing and its vicinity carried out a series of measures. From 16 to 21 December 2016, Beijing issued a red alert for smog–haze and implemented the following measures. Classes in elementary and middle schools, kindergartens, and extracurricular training schools were suspended. Cars were allowed on the roads on alternating days depending on whether their license plates end in odd or even numbers. Outdoor operations of construction sites were banned. Some industrial plants were required to limit or stop production (http://www.zhb.gov.cn/gkml/). Although these strict measures were enforced during the HPEs, the air quality of Beijing and Hebei Province remained unsatisfactory. Establishing effective measures and strategies to control pollution continues as a considerable challenge for the government. As a result, identifying the main factors that contribute to pollution and implementing an effective pollution control strategy are greatly needed.
Fine particles are well known to strongly affect atmospheric visibility and human health (Kanakidou et al., 2005; Kroll and Seinfeld, 2008; Liang et al., 2016). Generally speaking, submicron aerosol occupies 70%–90% of PM2.5 in polluted situations (Zhang et al., 2009). Furthermore, the diameter of submicron aerosol is very close to the wavelength of visible light and is hence efficient in scattering sunlight. Thus, the submicron aerosol could worsen the visibility more severely than larger particles (Chow et al., 2002). Characterizing submicron aerosol could help estimate the haze sources, compositions, and formation mechanisms.
A variety of instruments have been widely employed to study aerosol features. Aerodyne aerosol mass spectrometer (AMS) is a unique device that can provide both chemical compositions and size distributions of NR (non-refractory)-PM1 species, including organics, sulfate, nitrate, ammonium, and chloride (Canagaratna et al., 2007). Given the high sensitivity and capability in aerosol composition detection, AMS measurements have gradually become the focus of recent studies (Allan et al., 2003; Sun et al., 2009, 2010; Zhang Y. M. et al., 2014; Canagaratna et al., 2015). A number of studies and findings have been obtained to improve our understanding of aerosol sources, new particle formation, and related processes in China (Zhang et al., 2011, 2013; Sun et al., 2013; Tan et al., 2014). However, the real formation mechanism, evolution process, and solid proofs of the pollution sources remain insufficient to date.
The present study aims to characterize the chemical components, size distribution, and evolution of the specific heavy haze episodes that occurred in Beijing in December 2016. The roles of different species during the entire event, formation mechanism, and evolution process of chemical compositions are discussed. In particular, organics as a major component of fine particles accounts for more than half of the total mass. Evaluation of the subtypes of organics is highly significant in clarifying the possible sources and their contributions to the organic aerosol (OA). The primary organic aerosol (POA) from traffic, cooking, and coal combustion and secondary OA (SOA) are distinguished.
2 Experimental description 2.1 Aerosol measurementsAerosol chemical composition analyses were performed in a tank settled at the top (13th floor) of the Chinese Academy of Meteorological Sciences (CAMS) building, located in the China Meteorological Administration (CMA) campus in Beijing. In this study, the entire observation experiment was conducted from 15 to 23 December 2016, covering the periods before, during, and after the red alert for smog–haze that was issued by the National Alert Center. An aerodyne mass spectrometer (Q-AMS; Aerodyne Research, Inc., Boston, MA, USA) was employed to measure the mass concentrations (30 nm–1 μm) of organics, sulfate, nitrate, ammonium, and chloride (OSNAC) in mass spectrometry (MS) mode and their size distributions in the time-of-flight (TOF) mode with a 5-min time resolution (Jayne et al., 2000; Zhang et al., 2013). The AMS combined with other kinds of aerosol instruments that shared a common sampling inlet was installed in the tank. The room temperature of the tank was controlled at 25°C, 40%–60% for the relative humidity (RH), and atmospheric air was sampled through a PM10 cyclone (16.7 L min–1) to remove the particles larger than 10 μm. To maintain the consistency and comparability of this aerosol study with other works, the atmospheric air was dried to RH < 30% with an automatic aerosol dryer unit ( Tuch et al., 2009). This step would ensure that the studied aerosol was dry.
Particle number size distribution (PNSD) measurements were performed. In the process, Twin Differential Mobility Particle Sizer systems (TDMPS, TROPOS, Germany), including a Differential Mobility Analyzer (DMA) and an ultrafine DMA, were used to select sampled aerosol particles of a specific size. Meanwhile, a Condensation Particle Counter (CPC, TSI, USA) was used to measure the particle size range (3–800 nm) every 10 min (Shen et al., 2015). The PNSD data were used to verify the collection efficiency (CE) of AMS (Zhang et al., 2013, 2014).
The concentrations of the pollutant matters (NO2, SO2, CO, O3, and PM2.5) and meteorological hourly data were loaded from the Guanyuan station dataset in the environmental protection agency website (http://zx.bjmemc. com.cn/). The Guanyuan station was about 2 km away from the study site. The distance between these two sites was sufficiently small, such that the data were representative to a certain degree.
2.2 Data assurance and processesThe details in running the AMS and associated calibration processes, including flow rate calibration, ionization efficiency calibration, and velocity calibration, have been described by many works (Jayne et al., 2000; Allan et al., 2003). Given that the correlation between AMS and PNSD data with a linear regression formula is 0.975, and the correlation coefficient r2 = 0.869, the CE in this study was chosen as 1. Positive matrix factorization (PMF) analyses were performed by combining the PMF2.exe algorithm (Paatero, 1997) and the Igor-Pro-based PMF Evaluation Tool (PET, v2.04) (Ulbrich et al., 2009) in robust mode on the Q-AMS unit mass spectra to deconvolve OA into different factors (Zhang Q. et al., 2011; Hu et al., 2013; Sun et al., 2013).
To investigate the dominant variation of the polluted episodes, the mass concentration of PM2.5 and the size-resolved mass concentration of chemical components were accounted for so as to identify different pollution stages comprehensively. Five heavy polluted episodes (HPEs) and two non-polluted episodes (NPEs) were chosen and discussed. HPEs are the periods with rapid rise in PM2.5 levels as well as chemical mass concentrations, and NPEs are the durations with dramatic drops of PM2.5 and low chemical mass concentrations. The durations of these episodes were 2200 Beijing Time (BT) 15–0300 BT 16 December (HPE1), 1300–2230 BT 16 December (HPE2), 1200 BT 17–0030 18 December (HPE3), 1900 BT 19–1000 BT 20 December (HPE4), 1400 BT 20–2000 BT 21 December (HPE5), 0800–1600 BT 15 December (NPE1), and 0900 BT 22–0000 BT 23 December (NPE2). These episodes are also marked in Fig. 2.
3 Results 3.1 General information on the HPEsThe time series of meteorological and aerosol data is as follows: wind direction (WD), wind speed (WS), relative humidity (RH), gaseous pollutants (NO2, SO2, O3, and CO), and chemical components (organics, sulfate, nitrate, ammonium, and chloride). The average mass concentration of PM2.5 and PM1 was 225 and 101 μg m–3, respectively. The maximum mass loading of PM2.5 (524 μg m–3) and PM1 (250 μg m–3) happened on 20 December, which was about 7 times that of the China National Ambient Air Quality Standard (24-h mean of 75 μg m–3) and 20 times that of the World Health Organization Standard (24-h mean of 25 μg m–3). Compared with the severe air pollution cases in January 2013 (680 μg m–3) and winter 2015 (626 μg m–3) (Sun et al., 2016), the PM2.5 concentration in the current case was a little lower than that observed during those severe haze episodes. Although the maximum concentration for this case was not higher than that in 2013 and 2015, the severe air pollution lasting for 7 days forced the Beijing municipal to issue the red alert for smog–haze. The meteorological conditions were stagnant during the entire HPEs, as shown in Fig. 1. WS was constantly low, less than 2 m s–1, while the RH remained above 60% and even higher than 80% during the most severe pollution period. The stagnant conditions together with high RH were likely the primary external factor facilitating formation of severe pollution in winter time of Beijing. The same conclusion was also obtained by Sun et al. (2016).
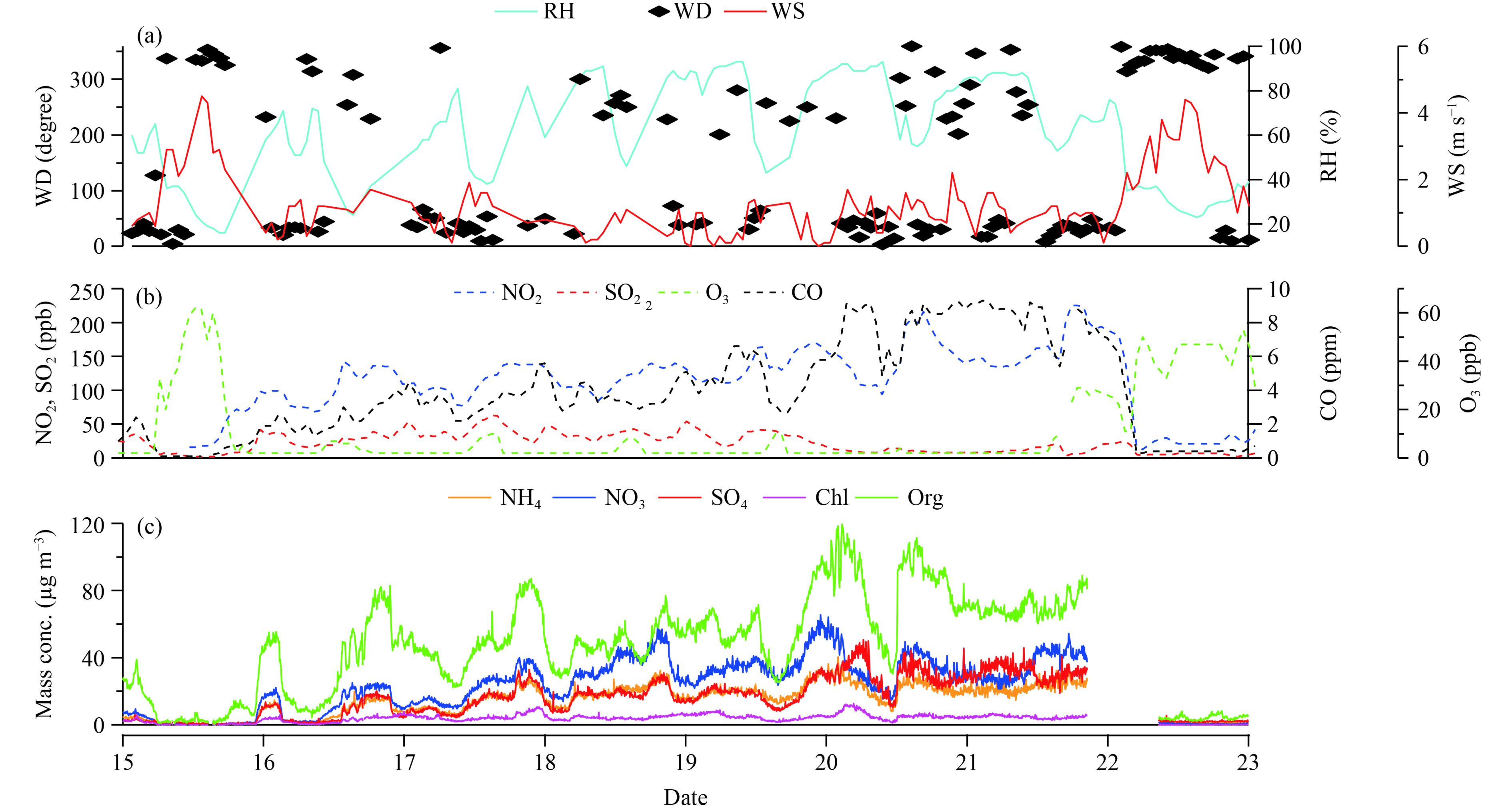
|
| Figure 1 Temporal evolutions of (a) meteorological data, (b) gas pollutants, and (c) particle chemical components in Beijing during 15–23 December 2016. |
Comprehensive factors such as the meteorological situations, variations of gaseous pollutants, photochemical reactions, local emissions, and regional transport played different roles in the evolution of HPEs. At the beginning and end of the observation experiment, wind speed reached 4 m s–1, which swept the particles and pollutions away and led to very low mass concentrations for the chemical species and gaseous pollutants (NO2, SO2, and CO) except for higher O3 during these two periods. On the contrary, the chemical components PM1, NO2, and CO demonstrated an increasing trend, and PM1 reached a maximum level on 20 December 2016 under the low wind speed. The upward trends of CO and NO2 resulted from the constant primary emission as well as the stagnant environment. Amounts of particles scattered much more solar radiation back to space, weakening the photochemical reaction. Furthermore, the weak photochemical reaction slowed down O3 production, lessened the NO2 participation into O3 production, and resulted in low levels of O3 and continuous increase in NO2 concentrations. The growing concentrations of chemical components were linked with the accumulation of existing particles by coagulation processes, gas–particle transformation processes, and endless condensation processes from the low-volatility components or gas phase. In a word, interactions between photochemical reactions, gas–particle transformation, and meteorological conditions made the entire pollution process complex.
We noted that the SO2 concentration was relatively stable during the observation, and even decreased on 19 December. This trend was accompanied by increased sulfate concentration. The reversed variations of SO2 and sulfate indicate that additional SO2 was involved in the gas–particle transformation and into the particle phase.
The RH in the whole observation period exhibited fluctuations from 30% to 90%, and the increase of RH was always accompanied by particulate mass concentration increments. Furthermore, the maximum NR-PM1 concentration occurred under the highest RH. RH showed a stronger effect on sulfate formation in wintertime (Sun et al., 2013), which is often associated with fog events and the ability of the precursor SO2 to form sulfate. Additional details on sulfate formation are discussed in later sections.
3.2 Analysis of HPEs and NPEs 3.2.1 Mass loadings and chemical compositionsAccording to the PM2.5 mass concentration and the temporal evolution of size distributions of organics, sulfate, nitrate, and ammonium, seven different episodes including two non-polluted episodes (NPE1 and NPE2) and five heavy polluted episodes (HPE1–5) were identified. The average mass concentrations of the five main components, sum of organics, sulfate, nitrate, ammonium, and chloride (OSNAC), and PM2.5 in the seven cases are listed in Table 1. The pie charts for the five main components in the seven episodes are also displayed in Fig. 2.
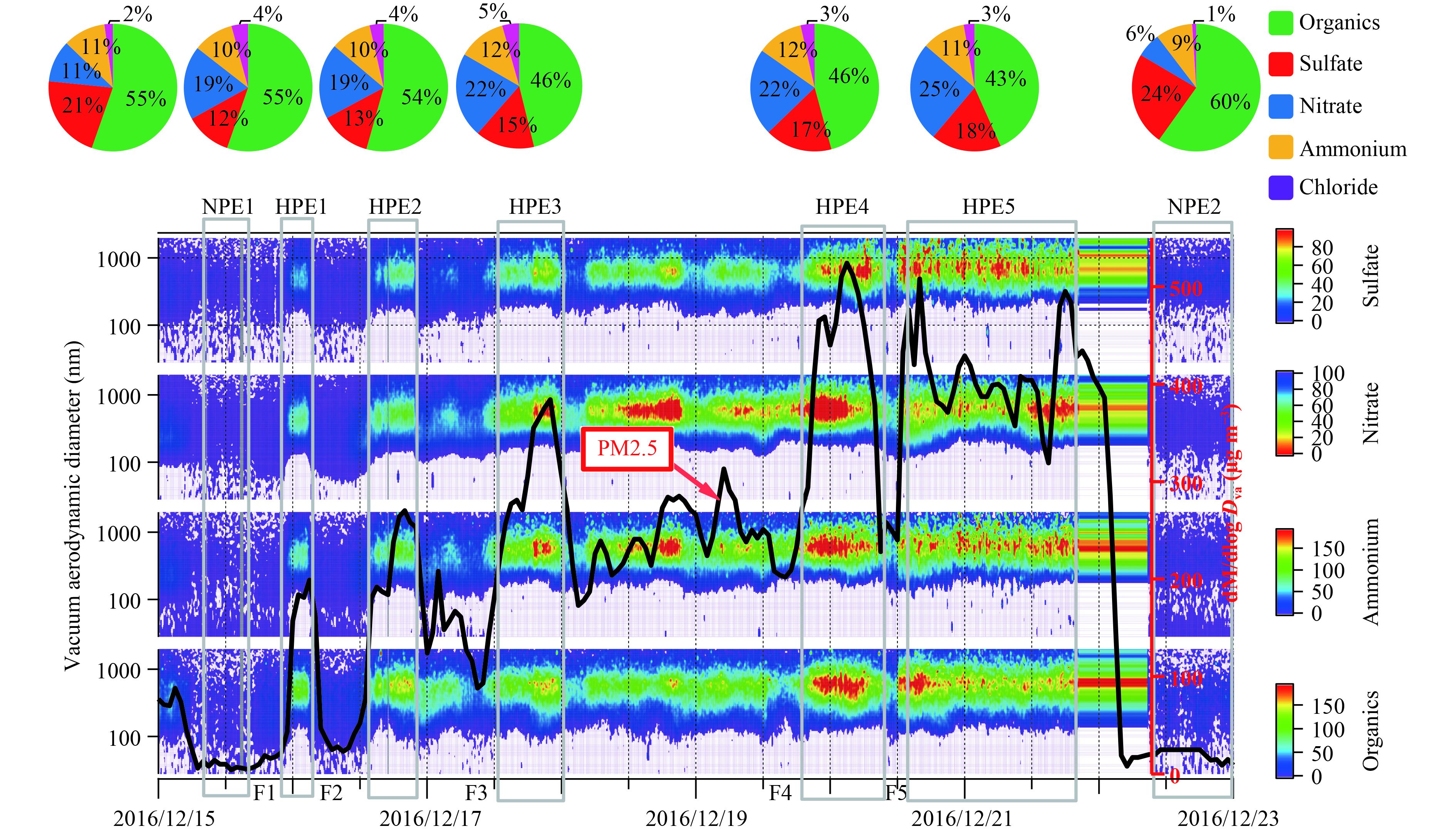
|
| Figure 2 Temporal evolution of main chemical components and PM2.5 mass concentration during HPEs and NPEs. |
Table 1 shows that the concentrations of OSNAC in the five HPE cases are 71.6, 111.0, 136.0, 188.2, and 173.6 μg m–3, respectively, while those in the two NPE cases were 4.7 and 9.7 μg m–3 at the beginning and end of the pollution events. HPE4 is the most serious polluted episode, being about 2.6 times that of HPE1 and even 40 times that of NPE1.
| Case | Organics | Sulfate | Nitrate | Ammonium | Chloride | ΣOSNAC | PM2.5 |
| HPE1 | 39.6 | 8.3 | 13.4 | 7.3 | 3.0 | 71.6 | 154.5 |
| HPE2 | 60.3 | 14.2 | 21.2 | 11.4 | 3.9 | 111.0 | 221.4 |
| HPE3 | 63.0 | 20.7 | 30.0 | 16.8 | 5.9 | 136.4 | 309.2 |
| HPE4 | 86.2 | 31.8 | 41.6 | 22.0 | 6.6 | 188.2 | 432.7 |
| HPE5 | 75.3 | 30.7 | 43.9 | 19.0 | 4.7 | 173.6 | 409.9 |
| NPE1 | 2.6 | 1.0 | 0.5 | 0.5 | 0.1 | 4.7 | 8.6 |
| NPE2 | 5.8 | 2.3 | 0.6 | 0.9 | 0.1 | 9.7 | 20.7 |
The mass concentration of PM2.5 varied widely among the evolution processes. Five formation rates of PM2.5 were calculated based on the mass increments of PM2.5 from the lowest value to the highest value during the five stages, marked as F1, F2, F3, F4, and F5 in Fig. 2. The PM2.5 formation rates in the five stages were 26, 22, 22, 32, and 67 μg m–3 h–1, respectively. Similar formation rates at the first three stages were obtained, indicating a similar secondary aerosol formation mechanism. But the formation rates in F4 and F5 were higher than those in the former stages, and reached the highest in F5. It is noted that the concentrations of CO and NO2 in F4 and F5 also increased much more than those in other three stages. As primary emission makers are coal combustion burning and traffic emission, these two gaseous pollutants are worthy being considered. Meanwhile, the sulfate exhibited dramatic increases during F4 and F5 stages, too. The aqueous-phase reaction of sulfate particularly under high NO2 conditions would be the main reason for this higher formation rate (Wang et al., 2016). On the other hand, the formation rates in F4 and F5 in this study were much higher than those observed during severe haze episodes in October 2014 (2.0–4.4 μg m–3 h–1) (Yang et al., 2015), but lower than that during the January 2013 haze episodes (88 μg m–3 h–1) (Wang et al., 2014; Zhang et al., 2015), and the highest formation rates in winter 2015 were 115 and 165 μg m–3 h–1 (Sun et al., 2016). The lower formation rates in this case compared with those in 2013 and 2015 can be mainly attributed to the weak primary source emission, weak photochemical reactions with lower O3 concentration, and less regional transport under the stagnant meteorological condition.
The proportions of the five main species in OSNAC are plotted in pie charts (Fig. 2). Totally, no dramatic difference in chemical composition was noted among the five HPEs, whereas about half was obtained from organics, 20% nitrate, 15% sulfate, 10% ammonium, and 4% chloride. The chemical constitution pattern was similar to the amount mentioned in former literatures (Huang et al., 2010; Sun et al., 2010, 2013, 2016; Zhang et al., 2011, 2013). The pattern infers the same source types, major atmospheric processes, and formation mechanisms. Organics corresponded to 43%–55% in the polluted cases and even 60% in the non-polluted episodes. Another significant species (nitrate) was around 20% of OSNAC in the polluted events, but only 10% in the clean episodes. Sulfate was found to be more important in the NPEs than in the HPEs, with a proportion of 20% in NPEs and 10% in HPEs. The clean period was more inclined to new particle formation process, with sulfate and organics as the necessary species involved in the formation (Kulmala et al., 2004; Wu et al., 2007; Shen et al., 2011; Zhang Y. M. et al., 2011). This situation resulted in the higher contribution to the NR-PM1. Compared with organics, sulfate, and nitrate, the ammonium played a relatively stable role in chemical compositions; its percentages in both HPEs and NPEs were 11%.
3.2.2 Chemical size distributionThe relationship between mass concentration and diameter follows the cubic formula. A slight growth of small particles in size would significantly increase the mass concentration. To investigate the variations of particle size throughout the polluted periods, examination of HPEs is helpful for understanding the heavy haze and its formation mechanism. Figure 3 displays the average mass-resolved size distributions of organics, sulfate, nitrate, and ammonium of the selected seven episodes. The black dashed lines indicate the peak positions of nitrate. The size distributions of organics were broader than those of the other inorganic species, and organics dominated a large part of the small particles. The peak sizes of the chemical species displayed an accumulation mode of around 400–600 nm. This result indicates that most particles were aged in the atmosphere.
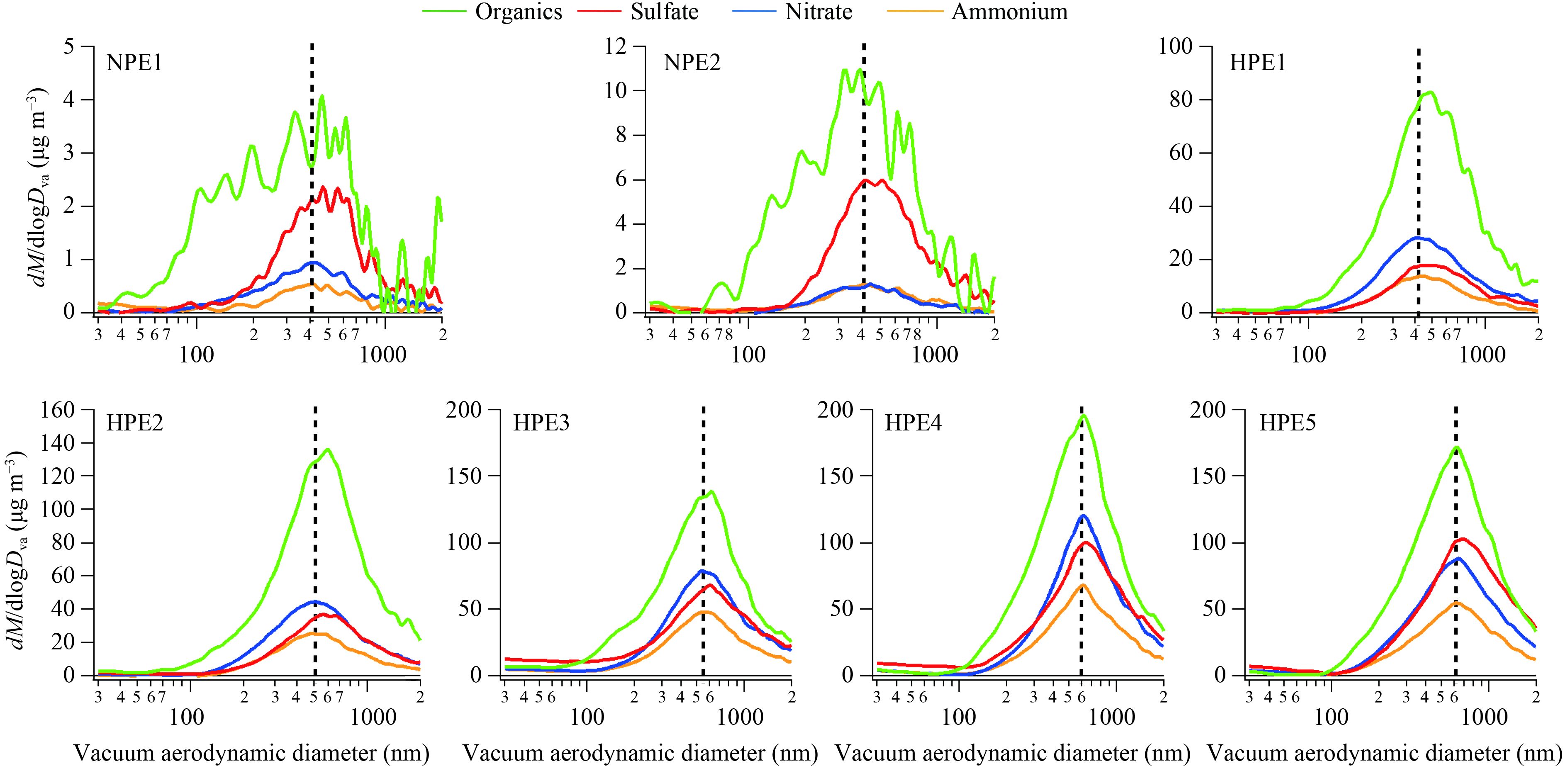
|
| Figure 3 Average size distributions of organics, sulfate, nitrate, and ammonium particles during HPEs and NPEs. The black dashed lines denote the peak positions of nitrate. |
Different size distribution patterns were also found between NPEs and HPEs (Fig. 3) . In the two NPEs, all the particles less than 100 nm consisted largely of organics, whereas organics, sulfate, nitrate, and ammonium contributed to the particle composition measured between 200 and 800 nm. No sulfate but merely organics was monitored for small particles. These results suggest that the newly acquired small particles may have been derived from primary organic aerosol (POA) emission, such as vehicles or/and the secondary transformation process of volatile organic components (VOCs). However, sulfate is seldom involved in the original stage of the new particle formation. Accumulation mode sulfate, nitrate, and ammonium particles might be formed by condensation processes of their precursor gases SO2, NO2, and NH3 that were condensed on the surface of existing organic particles.
In terms of the five HPEs, the peak sizes of all the species grew gradually (Fig. 3). For example, the peak size of nitrate in HPE1 was 400 nm, with an average mass concentration of 13.4 μg m–3. In HPE2, the peak size grew to around 500 nm, and the mass loading was 21.2 μg m–3. Meanwhile, the size peaks and mass concentrations in HPE3, HPE4, and HPE5 were 580 nm and 30 μg m–3, 600 nm and 41.6 μg m–3, and 600 nm and 43.9 μg m–3, respectively. Oxidization, condensation, and coagulation processes, as well as heterogeneous reactions might be significant factors for particle growth. This topic remains a considerable challenge for intercepting the formation mechanism of heavily polluted episodes (Holmes, 2007; Chan and Yao, 2008; Yue et al., 2010; Liang et al., 2016; Wang et al., 2016).
3.3 Diurnal cycle of the HPEsThe above section mentioned that the mass concentrations of the NR-PM1 and other species increased continuously during the whole HPEs. In fact, daily variations in the mass concentrations were also evident. The diurnal patterns of different NR-PM1 species are influenced by multiple factors, such as boundary layer development, photochemical activities, local emissions, regional transport, and meteorological conditions (Aiken et al., 2008; Jimenez et al., 2009; Zhang et al., 2009, 2013; Tie et al., 2015). Thus, examination of diurnal cycle is useful in the study of the particle formation mechanism.
The average diurnal cycles of main chemical species, precursor gases, and RH are plotted in Fig. 4. Organics exhibited a pronounced tri-modal diurnal pattern, which peaked from midnight to early morning for 7 h (2300–0500 BT), at noon, and in the evening. The development of boundary layer resulted in the descent of organics from 0600 BT in the morning. The noon peak might be linked to enhanced cooking emissions during lunch, and the evening peak was likely related to multiple factors such as dinner cooking and extensive traffic emission during the rush hours. This diurnal cycle of organics is fairly similar to those reported in 2006 in Beijing (Sun et al., 2010) and the winter diurnal cycles (Zhang et al., 2012).
For sulfate and chloride, obvious peaks in the early morning and evening were observed, and the concentrations decreased to the lowest during noon time. The decreased boundary layer and the oxidization process cannot intercept the early morning peak. To clarify the possible reasons for this pattern, the diurnal cycles of sulfate, SO2, O3, and RH were compared in Fig. 4b. Sulfate followed the diurnal cycles of RH but appeared an inverse variation to SO2 and O3. This phenomenon infers three things. First, the SO2 is not emitted locally but transported from distant locations by some mechanisms. Second, the aqueous reaction in the atmosphere might be a critical means to form particulate sulfate because of the relative similar patterns of sulfate and RH. Most of SO2 is transformed to a particulate sulfate. Third, the oxidization process is not important to the sulfate. For instance, nitrate began to increase at 1000 BT and climbed to the first peak at noon, lasting for 5 h. Then, the process reached a second peak at 2000 BT. The noon peak was related to the oxidization reactions, and the evening peak was linked with the rush hour. Figure 4c displays the diurnal cycles of nitrate and NO2. It is revealed that the nitrate, NO2, and O3 involved similar diurnal patterns. The oxidization process of NO2 was the main approach to nitrate formation. Although the “even–odd tag forbidden rule” was implemented, the total traffic number remained large. Traffic was considered as the main local emission source of NO2, and NO2 was emitted continuously. The severely polluted environment weakened the solar radiation and lessened the amount of NO2 involved in O3 formation. This condition resulted in increased NO2 participants in the oxidization process and thus produced a large nitrate amount.

|
| Figure 4 Diurnal cycles of chemical species mass concentrations in (a) NR-PM1; (b) sulfate, relative gases, and RH; and (c) nitrate and nitrogen dioxide. |
Organic species were apparently an important aerosol component in the studied haze event. AMS mass spectral matrix of OA with Positive Matrix Factorization (PMF;Paatero and Tapper, 1994) uses the analysis and evaluation tool described by Ulbrich et al. (2009). The PMF analysis based on the Q-AMS MS dataset in our experiment was first investigated for 1–7 factors and the rotational parameter (FPEAK) varying from –1 to 1 (step = 0.5) (see supplemental material Fig. S1, available online). The scaled residual values were also employed to make decision for the factor numbers (Fig. S2). The rotation was not applied in our data because there was no improvement for the Q/Qexp values (Fig. S2). More detailed information for the final decision of optimum factor numbers were discussed in supplement files (Figs. S3–S7).
Based on the complex consideration and analysis, five distinct OA components were identified in this experiment in accordance with their unique MS profiles, correlation with tracers, and diurnal variations (Ulbrich et al., 2009). Cooking organic aerosol (COA), hydrocarbon organic aerosol (HOA), and coal combustion organic aerosol (CCOA) can be regarded as surrogates of primary organic aerosol (POA), whereas the two oxygenated organic aerosol (OOA I and OOA II) are SOA (Jimenez et al., 2009; Ng et al., 2010).
Cooking emission is a major organic aerosol source in Beijing because of unique Chinese cooking habits and culture (He et al., 2004). A high m/z 55/57 ratio is widely used as an indicator for the presence of COA at various urban sites on the basis of previous studies (Mohr et al., 2009; Sun et al., 2010, 2013; Xu et al., 2016; Zhang et al., 2017). In this study, the high ratio of m/z 55/57 and high fraction of m/z 55 in identified COA mass spectra were observed. As a kind of local emission, two pronounced peaks occurred at approximately 1200 and 1800 BT, consistent with the traditional meal times, in the diurnal cycle of this component, especially during the clean periods when the peaks were very obvious in the time series of COA (Fig. 5). On average, the COA concentration during the whole experiment was 8.9 μg m–3, accounting for 18% of the total organic mass. This percentage is similar to the result in Beijing in 2006 (Sun et al., 2010) and 2008 (Huang et al., 2010; Zhang et al., 2013). The result also coincides with those in other cities, such as Lanzhou (Zhang et al., 2017).
HOA has been widely identified by several previous studies in Beijing (Sun et al., 2010, 2013; Zhang J. K. et al., 2014). Most of these studies showed similar MS patterns to those of primary combustion sources, such as gasoline and diesel vehicle exhausts (Mohr et al., 2009; Canagaratna et al., 2010). In this study, the MS pattern of HOA was primarily characterized by high m/z 43. Other m/z 29, 41, 43 55, and 57 were also distinguishable (Fig. 5a). The mean mass loading of HOA in this experiment was 7.8 μg m–3 and about 16% of OA. This percentage is very close to the values in the previous studies in Beijing (Huang et al., 2010; Sun et al., 2013). In the haze red-alert period, all the traffic flows worse than European III were banned. Furthermore, the odd–even tag limitation regulation reduced emissions and resulted in the decreased proportions of HOA in OA. On the other hand, in the time series, the diurnal cycle of another oxygenated organics aerosol (OOA II) displayed a similar pattern to that of nitrate and NO2, indicating that OOA II would be a kind of oxygenated organics from aged HOA.
Coal combustion is the most important source of winter heating in northern China. CCOA, another important POA factor, accounted for 23% of the total organic mass in this study. The mean mass concentration was 11.4 μg m–3. The mass spectrum of CCOA contained high-intensity signals at m/z 41, 43, 44, 55, 57, 69, 91, and 115, similar to those in other previous studies (Hu et al., 2013; Zhang J. K. et al., 2014), especially for the distinct signals at the latter two ion fragments, which had been used as good tracer ions for CCOA (Fig. 5). Another m/z 60 was also prominent in the CCOA MS profile, as the typical tracer of biomass burning emission. In this case, our reported CCOA might be a kind of mixtures of both coal combustion and biomass burning organic aerosols. This mixture requires more profound research. The temporal variation of CCOA showed a similar trend with the time series of chloride. Same results were also discussed in other works (Xu et al., 2016; Zhang et al., 2017). As CO is another common marker of coal combustion, diurnal cycles of CO in gaseous phase and chloride displayed higher mass loading at night and lower at daytime, which was similar to this resolved factor. Thus, taking this factor as coal combustion organic aerosol (CCOA) was reasonable (Fig. 5c).
The two OOA subtypes were distinguished by prominent contributions at m/z 43 and 44 in the MS profiles. These contributions indicate prevalence of the oxidized organic compounds. A relatively higher signal at m/z 43 corresponded to the less oxidized OA components and was referred to as OOA I (Fig. 5) (Sun et al., 2010; Ng et al., 2011). A relatively higher signal at m/z 44 than that at m/z 43 generally corresponded to the more oxidized OA component and was referred to as OOA II. The time series of the two OOA components correlated well with those of the inorganic secondary species (sulfate and nitrate). OOA I correlated well with sulfate, whereas OOA II correlated well with nitrate in this study. On average, OOA I accounted for 19% of the total OA mass, with 9.7 μg m–3 in the mass concentration. OOA II occupied 24% of the total OA, which was also the highest contributor of the total OA. Overall, the two OOA components were 43% of the total OA. This result is comparable to some other findings during wintertime (Sun et al., 2013; Zhang et al., 2013, 2017; Xu et al., 2016).
As discussed previously, aqueous reaction became a dominant process for the sulfate formation, whereas the oxidized reactions considerably influenced the behavior of nitrate. The diurnal cycle of OOA II matched well with that of nitrate and NO2, exhibiting a higher concentration between 1500 and 2000 BT. Nitrate and NO2 as markers of traffic source were widely accepted, and the good correlations among them imply that the OOA II was likely a kind of aged/oxygenated organic of HOA from traffic emission. It is well known that Beijing is surrounded on three sides by mountains , and on one side by a plain. Traffic emission, as a dominant local source, was continuous. During the heavy polluted episodes, stagnant wind brought the local traffic emitted pollution to and from the urban area and mountain sides; the particles were also aged or oxygenated during the entire polluted process, resulting in the transformation of HOA to OOA II. In all, during the HPEs, the majority of OA was derived from primary organics, including COA, HOA, and CCOA, and the different kinds of secondary OA transformed from CCOA and HOA, which also originated from the POA.

|
| Figure 5 (a) Mass profiles, (b) time series and related species (right axis in grey lines), and (c) diurnal cycles of subtype organics. |
Given the PMF results, the OAs were separated into five subtypes of components, to infer different sources or evolution processes. During the entire polluted period, different OAs would perform in different ways. To survey the roles of these OAs, average mass concentrations of the five organics during the seven periods and the whole period are shown in Table 2. The percentages of these five organic subtypes in OA are plotted in Fig. 6.
Table 2 shows that all the OA components display an increasing trend from HPE1 to HPE5. The POAs (including COA, HOA, and CCOA) reached the highest values in HPE4 and decreased in HPE5. The stagnant meteorological conditions from HPE1 to HPE4 and continuous emissions from local sources drived the accumulations of COA, HOA, and CCOA. In HPE5, a gentle wind blew some of the accumulations away. This occurrence reduced their mass loadings. For the two types of SOAs, the maximum concentrations were noted at HPE5, indicating that the oxidization process from VOCs or POAs to SOAs was advanced constantly. For the OA increasing rates, the COA, HOA, and OOA II amounts increased faster from HPE1 to HPE2. Moreover, the CCOA and OOA I grew much further from HPE3 to HPE4. The OOA II amount was relatively constant from HPE2 to HPE5. During the HPEs, O3 remained at a low level and weakened the atmosphere’s oxidizing ability. The OOA II was generally considered as a representative of additional oxidized organic aerosols. Thus, we concluded that the oxidization or aging process during the HPEs was insignificant.
| COA | HOA | CCOA | OOA I | OOA II | |
| HPE1 | 5.3 | 7.4 | 14.4 | 3.6 | 11.1 |
| HPE2 | 10.4 | 10.5 | 16.2 | 6.6 | 17.1 |
| HPE3 | 13.9 | 10.3 | 13.2 | 9.9 | 16.8 |
| HPE4 | 15.7 | 13.3 | 23.2 | 18.6 | 17.5 |
| HPE5 | 11.7 | 11.8 | 15.5 | 22.1 | 18.0 |
| NPE1 | 1.0 | 0.3 | 0.3 | 0.4 | 0.8 |
| NPE2 | 1.2 | 0.5 | 0.5 | 0.6 | 1.7 |
| All | 8.9 | 7.8 | 11.4 | 9.7 | 11.7 |
During the different episodes, the five OA components played distinct roles (Fig. 6). In HPE1, CCOA was the dominant aerosol, occupying about 35% of OA. By contrast, OOA I occupied the largest proportion in HPE5. The high concentration of CCOA in HPE1 possibly came from the regional transport at the beginning of the haze red-alert event. As the meteorological conditions stagnated further, the pollution worsened. The regional transport of CCOA also slowed down. As a result, the source of CCOA reduced in the later HPEs. With the pollution being serious, continuous emissions of COA and HOA from local cooking and traffic activities occurred because of their increasing contribution to the total OA. On the two clean periods, COA and OOA II were about 35% and 30%, respectively, in NPE1, and 25% and 38%, respectively, in NPE2. Low particulate matter concentration, intensive solar radiation, and high O3 concentration may provide an optimum environment for the photochemical aging process during NPE2. This aspect led to a high contribution of OOA II.
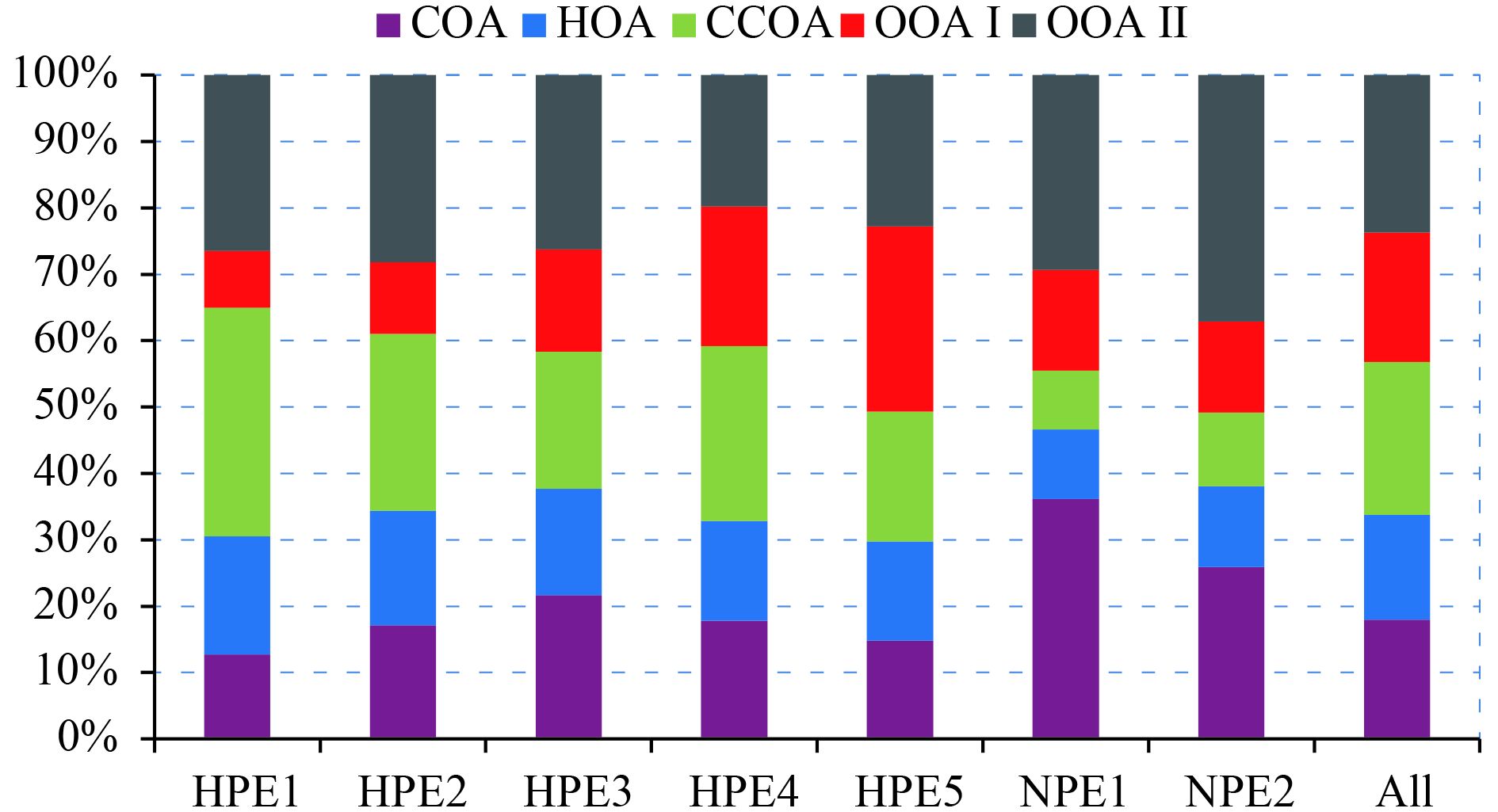
|
| Figure 6 Proportions of OA components in different cases. |
We conducted a detailed analysis on the chemical components, variations, and sources of PM1 during the heavy air pollution episodes in Beijing in December 2016. The levels of NR-PM1 species, including organics, sulfate, nitrate, ammonium, and chloride, were measured in situ from 15 to 22 December 2016 with an Aerodyne Q-AMS. In this case, a red alert for smog–haze was issued between 16 and 21 December 2016 by the Beijing municipal government. Five HPEs and two NPEs were identified, and the features of submicron aerosols in the different pollution episodes were discussed and revealed.
Overall, during the NPEs, wind speed was high. The RH, mass concentrations of the chemical species, and gaseous pollutions (NO2, SO2, and CO) were low, whereas the O3 concentration was high. However, different conditions were found in HPEs. That is, the wind speed was very low, RH was even higher than 90%, and the concentrations of chemical components, NO2 and CO, increased continuously to the maximum level on 20 December 2016.
The average mass concentration of PM2.5 and PM1 was 225 and 101 μg m–3, respectively. The maximum mass loading of PM2.5 (524 μg m–3) and PM1 (250 μg m–3) happened on 20 December, which were about 7 times that of the China National Ambient Air Quality Standard (24-h mean of 75 μg m–3) and 20 times that of the World Health Organization Standard (24-h mean of 25 μg m–3). The mean concentrations of NR-PM1 in the five HPE cases were 71.6, 111.0, 136.0, 188.2, and 173.6 μg m–3, respectively. By contrast, their concentrations in the NPE cases were 4.7 and 9.7 μg m–3 at the beginning and end of the pollution events. HPE4 was the most seriously polluted episode, with pollutant concentration about 2.6 times that of HPE1 and even 40 times that of NPE1. The formation rates of PM2.5 in the five stages were 26, 22, 22, 32, and 67 μg m–3 h–1, respectively. Similar formation rates at the first three stages were observed, indicating that the secondary aerosol formation mechanisms were similar. While the formation rates in F4 and F5 increased, the highest rate reached in F5. Totally, no dramatic difference in chemical composition was noted among the five HPEs. About half from organics, 20% from nitrate, 15% from sulfate, 10% from ammonium, and 4% from chloride were ascribed to NR-PM1. Nitrate was around 20% of NR-PM1 in the polluted events. By contrast, only 10% was observed in the clean episodes. Sulfate was more important in the NPEs than in the HPEs, with a proportion of 20% in the NPEs and 10% in the HPEs.
The peak sizes of the chemical species displayed an accumulation mode of around 400–600 nm. This result indicates that most particles were aged in the atmosphere. Different size distribution patterns were also found between the NPEs and HPEs. In the two NPEs, all the particles less than 100 nm in size consisted largely of organics, whereas organics, sulfate, nitrate, and ammonium contributed to the particles between 200 and 800 nm in size. No sulfate, but merely organics was monitored in small particles. This result suggests the important roles of POA or/and the secondary transformation process of VOCs in new particle formation. For the five HPEs, the peak sizes of all the species grew gradually. Nitrate can grow from 400 nm in HPE1 to 500 nm in HPE2. Oxidization, condensation, coagulation processes, and heterogeneous reactions may serve as the significant factors for particle growth.
Diurnal cycles in mass concentrations of chemical species were investigated. Organics exhibited a pronounced tri-modal diurnal pattern. This pattern peaked from midnight to early morning for 7 h (2300–0500 BT), at noon, and in the evening. This was due to the boundary layer development, enhanced cooking emissions during meal time, and extensive traffic emission in rush hours. For the sulfate and chloride, obvious peaks in the early morning and evening were observed, and the concentrations decreased to their lowest at noon time. The diurnal cycles of sulfate indicate that SO2 was emitted regionally and transported to the site. Moreover, the aqueous reaction in the atmosphere may be a critical means for forming particulate sulfate because of the relatively similar patterns of sulfate and RH. Nitrates, NO2, and O3 involve similar diurnal patterns. The oxidization process of NO2 was the main approach for nitrate formation.
PMF analysis was performed on AMS mass spectra to investigate the sources and processes of OA during the HPEs. Five components were identified, including three POA factors associated with traffic (HOA), cooking (COA), and coal combustion (CCOA) emissions, and two oxygenated organic aerosol (OOA I and OOA II). On average, the COA concentration during the whole campaign was 8.9 μg m–3, which accounted for 18% of the total organic mass. The mean mass loading of HOA was 7.8 μg m–3 and about 16% of OA. CCOA, another important POA factor, accounted for 23% of the total organic mass in this study, and the mean mass concentration was 11.4 μg m–3. On average, OOA I accounted for 19% of the total OA mass, with 9.7 μg m–3 in the mass concentration. OOA II occupied 24% of the total OA, which was also the highest contributor to the total OA and would be an aged/oxygenated component of HOA. Overall, the two OOA components were 43% of the total OA. The POAs reached the highest values in HPE4 and decreased in HPE5. The stagnant meteorological situation from HPE1 to HPE4 and continuous emissions from local sources drived the accumulations of COA, HOA, and CCOA. For the two types of SOAs, the maximum concentrations appeared in HPE5. This result indicates that the oxidization process from VOCs or POAs to SOAs was constantly advanced. The COA, HOA, and OOA II increased faster from HPE1 to HPE2. Meanwhile, the CCOA and OOA I grew much more from HPE3 to HPE4. OOA II was relatively constant from HPE2 to HPE5. The accumulation of organic particles, and not the oxidation or aging process, played a significant role in the concentration increase. In HPE1, CCOA was the dominant aerosol and comprised about 35% of the OA. By contrast, OOA I occupied the largest proportion in HPE5. In the two clean periods, COA and OOA II were about 35% and 30%, respectively, in NPE1, but 25% and 38%, respectively, in NPE2.
| Aiken, A. C., P. F. DeCarlo, J. H. Kroll, et al., 2008: O/C and OM/OC ratios of primary, secondary, and ambient organic aerosols with high-resolution time-of-flight aerosol mass spectrometry. Environ. Sci. Technol., 42, 4478–4485. DOI:10.1021/es703009q |
| Allan, J. D., M. R. Alfarra, K. N. Bower, et al., 2003: Quantitative sampling using an Aerodyne aerosol mass spectrometer. 2: Measurements of fine particulate chemical composition in two U.K. cities. J. Geophys. Res., 108, 4091. DOI:10.1029/2002JD002359 |
| Canagaratna, M. R., J. T. Jayne, J. L. Jimenez, et al., 2007: Chemical and microphysical characterization of ambient aerosols with the aerodyne aerosol mass spectrometer. Mass Spectrom. Rev., 26, 185–222. DOI:10.1002/mas.20115 |
| Canagaratna, M. R., T. B. Onasch, E. C. Wood, et al., 2010: Evolution of vehicle exhaust particles in the atmosphere. J. Air Waste Manage. Assoc., 60, 1192–1203. DOI:10.3155/1047-3289.60.10.1192 |
| Canagaratna, M. R., J. L. Jimenez, J. H. Kroll, et al., 2015: Elemental ratio measurements of organic compounds using aerosol mass spectrometry: Characterization, improved calibration, and implications. Atmos. Chem. Phys., 15, 253–272. DOI:10.5194/acp-15-253-2015 |
| Chan, C. K., and X. H. Yao, 2008: Air pollution in mega cities in China. Atmos. Environ., 42, 1–42. DOI:10.1016/j.atmosenv.2007.09.003 |
| Chow, J. C., J. D. Bachmann, S. S. G. Wierman, et al., 2002: Visibility: Science and regulation. J. Air Waste Manage. Assoc., 52, 973–999. DOI:10.1080/10473289.2002.10470844 |
| He, L. Y., M. Hu, X. F. Huang, et al., 2004: Measurement of emissions of fine particulate organic matter from Chinese cooking. Atmos. Environ., 38, 6557–6564. DOI:10.1016/j.atmosenv.2004.08.034 |
| Holmes, N. S., 2007: A review of particle formation events and growth in the atmosphere in the various environments and discussion of mechanistic implications. Atmos. Environ., 41, 2183–2201. DOI:10.1016/j.atmosenv.2006.10.058 |
| Hu, W. W., M. Hu, B. Yuan, et al., 2013: Insights on organic aerosol aging and the influence of coal combustion at a regional receptor site of central eastern China. Atmos. Chem. Phys., 13, 10809–10858. DOI:10.5194/acpd-13-10809-2013 |
| Huang, X. F., L. Y. He, M. Hu, et al., 2010: Highly time-resolved chemical characterization of atmospheric submicron particles during 2008 Beijing Olympic Games using an Aerodyne High-Resolution Aerosol Mass Spectrometer. Atmos. Chem. Phys., 10, 8933–8945. DOI:10.5194/acp-10-8933-2010 |
| Jayne, J. T., D. C. Leard, X. F. Zhang, et al., 2000: Development of an aerosol mass spectrometer for size and composition analysis of submicron particles. Aerosol Sci. Technol., 33, 49–70. DOI:10.1080/027868200410840 |
| Jimenez, J. L., M. R. Canagaratna, N. M. Donahue, et al., 2009: Evolution of organic aerosols in the atmosphere. Science, 326, 1525–1529. DOI:10.1126/science.1180353 |
| Kanakidou, M., J. H. Seinfeld, S. N. Pis, et al., 2005: Organic aerosol and global climate modeling: A review. Atmos. Chem. Phys., 5, 1053–1123. DOI:10.5194/acp-5-1053-2005 |
| Kroll, J. H., and J. H. Seinfeld, 2008: Chemistry of secondary organic aerosol: Formation and evolution of low-volatility organics in the atmosphere. Atmos. Environ., 42, 3593–3624. DOI:10.1016/j.atmosenv.2008.01.003 |
| Kulmala, Vehkamäki, Petäjä M., et al., 2004: Formation and growth rates of ultrafine atmospheric particles: A review of observations. J. Aerosol Sci., 35, 143–176. DOI:10.1016/j.jaerosci.2003.10.003 |
| Liang, C. S., F. K. Duan, K. B. He, et al., 2016: Review on recent progress in observations, source identifications and countermeasures of PM2.5. Environ. Intl., 86, 150–170. DOI:10.1016/j.envint.2015.10.016 |
| Mohr, C., A. Huffman, M. J. Cubison, et al., 2009: Characterization of primary organic aerosol emissions from meat cooking, trash burning, and motor vehicles with high-resolution aerosol mass spectrometry and comparison with ambient and chamber observations. Environ. Sci. Technol., 43, 2443–2449. DOI:10.1021/es8011518 |
| Ng, N. L., M. R. Canagaratna, J. L. Jimenez, et al., 2010: Real-time methods for estimating organic component mass concentrations from aerosol mass spectrometer data. Environ. Sci. Technol., 45, 910–916. DOI:10.1021/es102951k |
| Ng, N. L., M. R. Canagaratna, J. L. Jimenez, et al., 2011: Changes in organic aerosol composition with aging inferred from aerosol mass spectra. Atmos. Chem. Phys., 11, 6465–6474. DOI:10.5194/acp-11-6465-2011 |
| Paatero, P., 1997: Least squares formulation of robust non-negative factor analysis. Chemometrics and Intelligent Laboratory Systems, 37, 23–35. DOI:10.1016/S0169-7439(96)00044-5 |
| Paatero, P., and U. Tapper, 1994: Positive matrix factorization: A non-negative factor model with optimal utilization of error estimates of data values. Environmetrics, 5, 111–126. DOI:10.1002/env.3170050203 |
| Shen, X., J. Y. Sun, Y. M. Zhang, et al., 2011: First long-term study of particle number size distributions and new particle formation events of regional aerosol in the North China Plain. Atmos. Chem. Phys., 11, 1565–1580. DOI:10.5194/acp-11-1565-2011 |
| Shen, J., Y. Sun X., Y. J., et al., 2015: Characterization of submicron aerosols and effect on visibility during a severe haze-fog episode in Yangtze River Delta, China. Atmos. Environ., 120, 307–316. DOI:10.1016/j.atmosenv.2015.09.011 |
| Sun, J. Y., Q. Zhang, M. R. Canagaratna, et al., 2010: Highly time- and size-resolved characterization of submicron aerosol particles in Beijing using an Aerodyne Aerosol Mass Spectrometer. Atmos. Environ., 44, 131–140. DOI:10.1016/j.atmosenv.2009.03.020 |
| Sun, Y., Q. Zhang, A. M. Macdonald, et al., 2009: Size-resolved aerosol chemistry on Whistler Mountain, Canada with a high-resolution aerosol mass spectrometer during INTEX-B. Atmos. Chem. Phys., 9, 3095–3111. DOI:10.5194/acp-9-3095-2009 |
| Sun, Y. L., Z. F. Wang, P. Q. Fu, et al., 2013: Aerosol composition, sources and processes during wintertime in Beijing, China. Atmos. Chem. Phys., 13, 4577–4592. DOI:10.5194/acp-13-4577-2013 |
| Sun, Y. L., C. Chen, Y. J. Zhang, et al., 2016: Rapid formation and evolution of an extreme haze episode in Northern China during winter 2015. Scientific Reports, 6, 27151. DOI:10.1038/srep27151 |
| Tan, J. H., J. C. Duan, F. H. Chai, et al., 2014: Source apportionment of size segregated fine/ultrafine particle by PMF in Beijing. Atmos. Res., 139, 90–100. DOI:10.1016/j.atmosres.2014.01.007 |
| Tie, X. X., Q. Zhang, H. He, et al., 2015: A budget analysis of the formation of haze in Beijing. Atmos. Environ., 100, 25–36. DOI:10.1016/j.atmosenv.2014.10.038 |
| Tuch, M., Haudek T., Müller A., et al., 2009: Design and performance of an automatic regenerating adsorption aerosol dryer for continuous operation at monitoring sites. Atmos. Meas. Tech. Discuss., 2, 1143–1160. DOI:10.5194/amtd-2-1143-2009 |
| Ulbrich, I. M., M. R. Canagaratna, Q. Zhang, et al., 2009: Interpretation of organic components from Positive Matrix Factorization of aerosol mass spectrometric data. Atmos. Chem. Phys., 9, 2891–2918. DOI:10.5194/acp-9-2891-2009 |
| Wang, G. H., R. Y. Zhang, M. E. Gomez, et al., 2016: Persistent sulfate formation from London Fog to Chinese haze. Proc. Natl. Acad. Sci. USA, 113, 13630–13635. DOI:10.1073/pnas.1616540113 |
| Wang, Y. S., L. Yao, L. L. Wang, et al., 2014: Mechanism for the formation of the January 2013 heavy haze pollution episode over central and eastern China. Sci. China Earth Sci., 57, 14–25. DOI:10.1007/s11430-013-4773-4 |
| Wu, Z. J., M. Hu, S. Liu, et al., 2007: New particle formation in Beijing, China: Statistical analysis of a 1-year data set. J. Geophys. Res., 112, D09209. DOI:10.1029/2006JD007406 |
| Xu, J. Z., J. S. Shi, Q. Zhang, et al., 2016: Wintertime organic and inorganic aerosols in Lanzhou, China: Sources, processes, and comparison with the results during summer. Atmos. Chem. Phys., 16, 14937–14957. DOI:10.5194/acp-16-14937-2016 |
| Yang, Y. R., X. G. Liu, Y. Qu, et al., 2015: Characteristics and formation mechanism of continuous extreme hazes in China: A case study in autumn of 2014 in the North China Plain. Atmos. Chem. Phys. Discuss., 15, 10987–11029. DOI:10.5194/acpd-15-10987-2015 |
| Yue, D. L., M. Hu, R. Y. Zhang, et al., 2010: The roles of sulfuric acid in new particle formation and growth in the mega city of Beijing. Atmos. Chem. Phys., 10, 4953–4960. DOI:10.5194/acp-10-4953-2010 |
| Zhang, J. K., Y. Sun, Z. R. Liu, et al., 2014: Characterization of submicron aerosols during a month of serious pollution in Beijing, 2013. Atmos. Chem. Phys., 14, 2887–2903. DOI:10.5194/acp-14-2887-2014 |
| Zhang, Q., J. L. Jimenez, M. R. Canagaratna, et al., 2011: Understanding atmospheric organic aerosols via factor analysis of aerosol mass spectrometry: A review. Anal. Bioanal. Chem., 401, 3045–3067. DOI:10.1007/s00216-011-5355-y |
| Zhang, X. H., Y. M. Zhang, J. Y. Sun, et al., 2017: Chemical characterization of submicron aerosol particles during wintertime in a northwest city of China using an Aerodyne aerosol mass spectrometry. Environ. Pollut., 222, 567. DOI:10.1016/j.envpol.2016.11.012 |
| Zhang, X. Y., Y. Q. Wang, W. L. Lin, et al., 2009: Changes of atmospheric composition and optical properties over Beijing—2008 Olympic monitoring campaign. Bull. Amer. Meteor. Soc., 90, 1633–1651. DOI:10.1175/2009BAMS2804.1 |
| Zhang, Y. M., X. Y. Zhang, J. Y. Sun, et al., 2011: Characterization of new particle and secondary aerosol formation during summertime in Beijing, China. Tellus B, 63, 382–394. DOI:10.1111/j.1600-0889.2011.00533.x |
| Zhang, Y. M., J. Y. Sun, X. Y. Zhang, et al., 2013: Seasonal characterization of components and size distributions for submicron aerosols in Beijing. Sci. China Earth Sci., 56, 890–900. DOI:10.1007/s11430-012-4515-z |
| Zhang, Y. M., X. Y. Zhang, J. Y. Sun, et al., 2014: Chemical composition and mass size distribution of PM1 at an elevated site in central East China. Atmos. Chem. Phys., 14, 12237–12249. DOI:10.5194/acp-14-12237-2014 |
| Zhang, Y. W., X. Y. Zhang, Y. M. Zhang, et al., 2015: Significant concentration changes of chemical components of PM1 in the Yangtze River Delta area of China and the implications for the formation mechanism of heavy haze–fog pollution. Sci. Total Environ., 538, 7–15. DOI:10.1016/j.scitotenv.2015.06.104 |
 2018, Vol. 32
2018, Vol. 32


