2. 浙江省化工研究院有限公司,杭州 310023;
3. 温州医科大学,浙江 温州 325035
2. Zhejiang Institute of Chemical Industry Co. Ltd., Hangzhou 310023, China;
3. Wenzhou Medical University, Wenzhou 325035, Zhejiang Province, China
The highly fluorinated groups have become an important bio-functional moiety in many bioactive compounds, mainly because of its high lipophilicity for crust penetration[1]. The introduction of fluorine atoms into organic molecules has been demonstrated in several widely used pesticides[2-3]. Flubendiamide, the first artificially synthesized insecticide targeting ryanodine receptors (RyRs), has shown extremely high activity against a broad spectrum of lepidopterous insects[4-5]. Since its discovery by Nihon Nohyaku and development with Bayer company in 1998, a series of pesticides containing polyfluorinated groups with high activities was reported, such as pyrifluquinazon[6-7] and pyflubumide[8](Fig. 1).
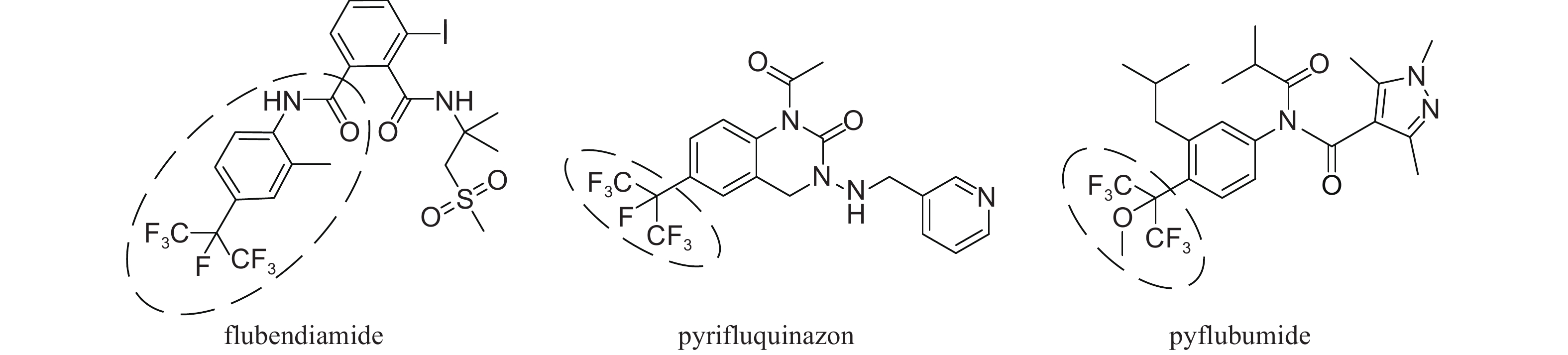
|
Fig. 1 Commercial pesticides with polyfluoroalkyl groups |
Fludioxonil[9-11], a non-systemic fungicide, is the world's biggest seed treatment agent in terms of current unit sales. As a cereal seed treatment, it controls seed- and soil-borne diseases and gives particularly good control of Fusarium roseum and Gerlachia nivalis in small-grain cereals. The key moiety of the fungicide is 2,2-difluoro-1,3-benzodioxole.
In order to find some compounds with high biological activities and new action mode, herein we designed and synthesized a series of novel 2,2-difluoro-1,3-benzodioxol-5-acetamide compounds containing multi-fluorine atoms. As showed in Fig. 2, 2,2-difluoro-1,3-benzodioxole moiety from fludioxonil and heptafluoroisopropyl aniline moiety from flubendiamide were linked with amide group. These new 2,2-difluoro-1,3-benzodioxol-5-acetamide compounds are expected to exhibit desirable biological activities.
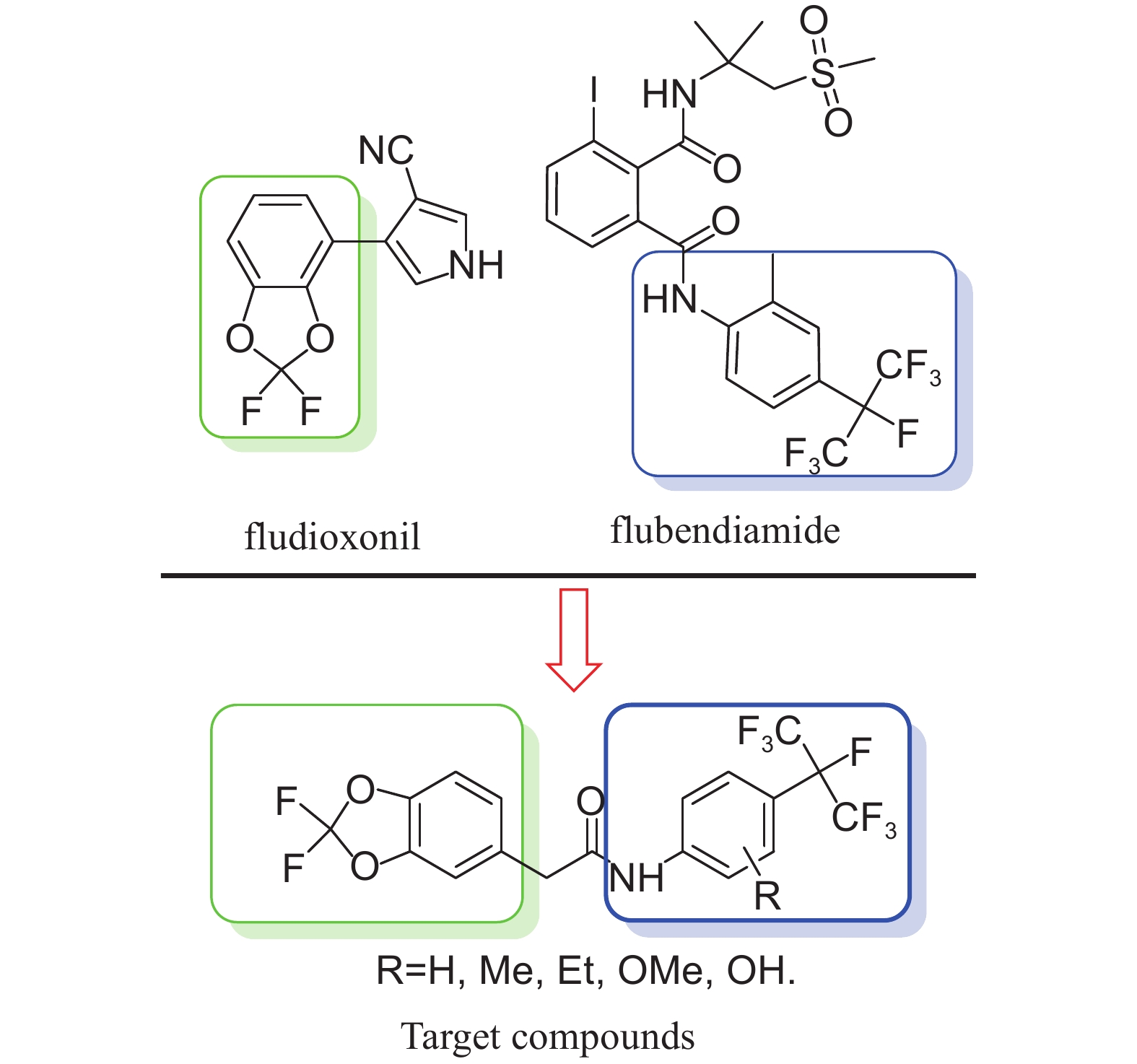
|
Fig. 2 Design of the title compounds |
In addition, organic solvents represent the vast majority of mass consumption and waste generated by the chemical industry. Since most of chemical reactions are conducted in traditional organic solvents, a green protocol with reduced organic waste would be desirable[12-13]. The nanomicelle-forming amphiphile TPGS-750-M (Fig. 3) was reported recently, which can facilitate many reactions under mild conditions in water[14]. Encouraged by this, we have successfully improved the last key step of amidation using HATU as the coupling agent in 2 wt.% TPGS-750-M/H2O under mild conditions[15-22]. Furthmore, a series of novel poly fluorinated 1,3-benzodioxol-5-acetamides (h1-h16) was designed and synthesized (Scheme 1). And the insecticidal activities were evaluated.
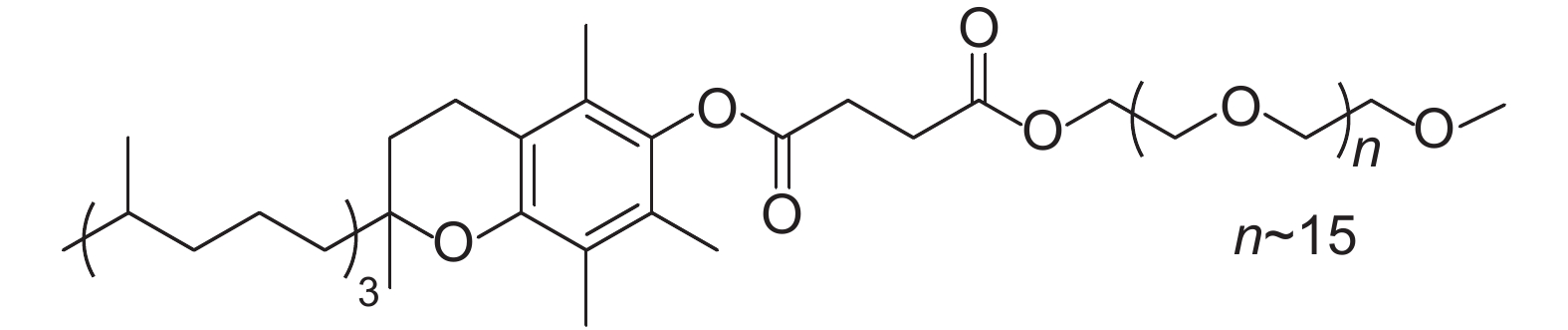
|
Fig. 3 Structural formula of TPGS-750-M |
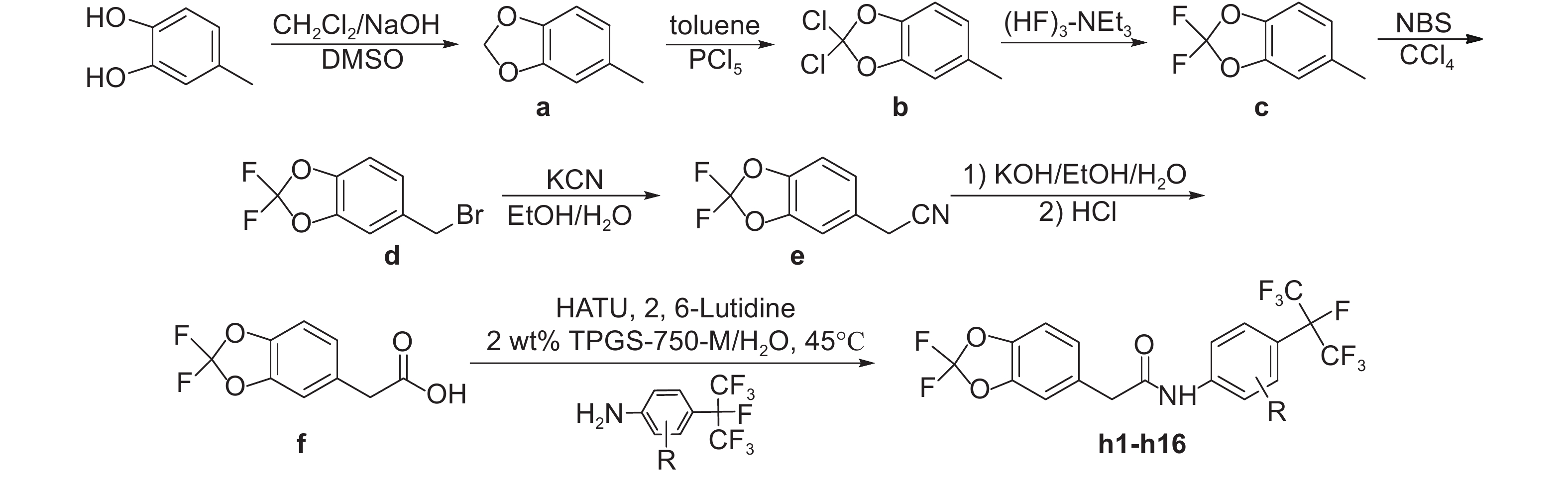
|
Scheme1 General synthetic route of the title compound h1-h16 |
1 Materials and Methods 1.1 Instruments and reagents
All starting materials and reagents used were commercially available and were utilized without further purification (except as indicated). TPGS-750-M was synthesized according to the published procedure[14]. All the melting points were determined with an X-4 melting point apparatus while the thermometer was uncorrected. NMR spectra were recorded on a Varian INOVA spectrometer (400 MHz for 1H NMR), or a Bruker Avance spectrometer (600 MHz for 1H NMR, and 564 MHz for 19F NMR spectroscopy). HRMS (ESI) were performed on an Agilent 6 210 TOF LC/MS instrument. Mass spectra were recorded on a Bruker Esquire 6 000 mass spectrometer (ESI).
1.2 Synthetic procedures 1.2.1 Synthesis of 5-methyl-1,3-benzodioxole (a)Methylene chloride (300.0 g, 3.56 mol) and dimethylsulphoxide (1 000.0 g, 12.80 mol) were added to a flask and stirred under reflux at 90 °C to 100 °C for 1 h. A solution of sodium hydroxide (144.0 g, 3.60 mol) and 4-methylbenzene-1, 2-diol (195.2 g, 1.57 mol) in water (300 mL) were added dropwise in 1 h. At the end of the addition, the mixture was subsequently stirred for a further 2 h under reflux. Then 500 mL of water was added to the reaction solution and steam distillation was conducted. The distillation fractions at 110 °C was collected and separated. Aqueous phase was extracted with dichloromethane (200 mL). The combined organic phases were dried, concentrated and 5-methyl-1,3-benzodioxole (a) was obtained as a colorlesstaine oil, yield: 149.7 g (70%). 1H NMR (400 MHz, CDCl3), δ: 2.30 (s, 3H, -CH3), 6.05 (s, 2H, -CH2-), 6.90-7.05 (m, 3H, Ar-H).
1.2.2 Synthesis of 2,2-dichloro-5-methyl-1,3-benzodioxole (b)5-Methyl-1,3-benzodioxole (13.6 g, 0.10 mol) was added dropwise to phosphorus pentachloride (25.0 g, 0.12 mol) in toluene (50 mL) with stirring. Vigorous evolution of hydrogen chloride started immediately. The mixture was then heated under reflux for 3 h. After distillation, phosphorus trichloride, phosphorus pentachloride and toluene were recovered and 2,2-dichloro-5-methyl-1,3-benzodioxole (b) was obtained as a pale-yellow oil at boiling point: 98-99 °C (2 200 Pa), yield: 17.0 g (82%). 1H NMR (400 MHz, CDCl3), δ: 2.28 (s, 3H, -CH3), 6.70-6.80 (m, 3H, Ar-H).
1.2.3 Synthesis of 2,2-difluoro-5-methyl-1,3-benzodioxole (c)A mixture of 2,2-dichloro-5-methyl-1,3-benzodioxole (16.4 g, 0.08 mol) and triethylamine tris (hydrogen fluoride) (9.6 g, 0.06 mol) was stirred under nitrogen at room temperature. The progress was monitored by TLC (V(ethyl acetate) :V(hexane) = 1 : 20). At the end of the reaction, the mixture was filtered. The filtrate was distilled under reduced pressure to yield 2,2-difluoro-5-methyl-1,3-benzodioxole (c) as a colorless oil at boiling point:60-62 °C(1 600 Pa), yield: 11.7 g (85%). 1H NMR (400 MHz, CDCl3), δ: 2.28 (s, 3H, -CH3), 6.70-6.80 (m, 3H, Ar-H).
1.2.4 Synthesis of 2,2-difluoro-5-bromomethyl-1,3-benzodioxole (d)To a solution of 2,2-difluoro-5-methyl-1,3-benzodioxole (11.5 g, 0.07 mol) in carbon tetrachloride (50 mL) was added N-bromosuccinimide (NBS) (12.3 g, 0.07 mol) and a catalytic amount of benzoyl peroxide (0.2 g, 0.001 mol). After stirred at refluxed for 3 h, the reaction mixture was cooled to room temperature and filtered. The filtrate was concentrated to give 2,2-difluoro-5-bromomethyl-1,3-benzodioxole (d) as a brown oil, yield: 16.0 g (95%). 1H NMR (400 MHz, CDCl3), δ: 4.38 (s, 2H, -CH2-), 6.80-7.40 (m, 3H, Ar-H). The product was used without purification in the next step.
1.2.5 Synthesis of 2-(2,2-difluoro-1,3-benzodioxole-5-yl) acetonitrile (e)A solution of 2,2-difluoro-5-bromomethyl-1,3-benzodioxole ( 12.6 g, 0.05 mol) and potassium cyanide (3.9 g, 0.06 mol) in 80% aqueous ethanol (60 mL) was heated under reflux for 6 h and then cooled to room temperature. After the addition of water (20 mL), the organic product was extracted with ethyl acetate (3 × 25 mL). The combined organic layer was washed with water, dried over anhydrous magnesium sulfate, filtered, and concentrated to afford 2-(2,2-difluoro-1,3-benzodioxole-5-yl) acetonitrile (e) as a pale-yellow liquid that was used directly in the next step, yield: 4.0 g (40%). 1H NMR (400 MHz, CDCl3), δ: 3.78 (s, 2H, -CH2-), 7.01-7.03 (m, 3H, Ar-H).
1.2.6 Synthesis of 2- (2,2-difluoro-1,3-benzodioxole-5-yl) acetic acid (f)A mixture of 2-(2,2-difluoro-1,3-benzodioxole-5-yl) acetonitrile (4.0 g, 0.02 mol), potassium hydroxide pellets (3.4 g, 0.06 mol) in ethanol (20 mL) and water (10 mL) was heated under reflux for 3 h. After cooling, the reaction mixture was poured into ice-water, acidified with dilute hydrochloric acid and extracted with ethyl acetate (3 × 20 mL). The combined extracts were washed with water (2 × 20 mL), dried over sodium sulfate and evaporated under vacuum. Recrystallization of the solid residue from heptane affords 2-(2,2-difluoro-1,3-benzodioxole-5-yl)acetic acid (f) as a pale yellow solid, yield: 8.8 g (82%), m.p. 94-96 °C. 1H NMR (400 MHz, CDCl3), δ: 3.65 (s, 2H, -CH2-), 6.97-7.04 (m, 3H, Ar-H).
1.2.7 General synthetic procedure for 2-(2,2-difluoro-1,3-benzodioxole-5-yl)-N-(2-methyl-4-(perfluoropropan-2-yl) phenyl) acetamide (h1)To a dried 5 mL reaction vial was added a mixture of 2,2-difluoro-1,3-benzodioxole-5-acetic acid (f) (0.2 g, 0.001 mol), 2, 6-lutidine (0.3 g, 0.003 mol) in 2 wt.% TPGS-750-M/H2O (2 mL), HATU (0.418 g, 0.001 mol) and 2-methyl-4-(perfluoropropan-2-yl) aniline (0.5 g, 0.001 mol). The reaction was allowed to stir for 24 hours at 45 °C. The reaction progress was monitored by HPLC. The aqueous reaction mixture was extracted with EtOAc (3 × 2 mL). The organic extracts were then washed with 1 mol/L HCl (3 × 2 mL) and Na2CO3 (3 × 6 mL) saturated solution in water[25]. The organic phase was concentrated under reduced pressure to give a crude product. The product was purified by column chromatography on silica gel (V (ethyl acetate) : V(hexane) = 1 : 1) to give compound h1. The synthetic procedure for compounds h2-h16 is the same as the synthesis of compound h1.
1.3 Insecticidal activityThe preliminary bioactivity tests were performed on representative test organisms reared in the laboratory. All compounds were dissolved in N, N-dimethylformamide (DMF) and diluted with distilled water containing 0.1% tween-80 to achieve the testing solution with the desired concentration. For insecticidal activity test, flubendiamide and abamectin were tested under the same conditions as the control.
1.3.1 Insecticidal activity against Homoptera pest—Aphis craccivoraThe foliar contact activity against bean aphid (A. craccivora) was tested. Tender shoots of soybean with 60 insects of each species were dipped in the diluted solutions of the chemicals for 5 s. Then the superfluous liquor was removed, and they were kept in the conditioned room for normal cultivation. The mortality was evaluated by the number of live larvae in the treated bottles relative to that in the untreated controls after 48 h. Controls were performed under the same conditions.
1.3.2 Insecticidal activity against Lepidopteran pest—Mythimna separataThe insecticidal activity against armyworms (M. separata) was also tested by foliar application. Individual corn leaves were placed on moistened pieces of filter paper in petri-dishes. The leaves were then sprayed with the testing solution and exposed to dry. The dishes were infested with 10 third-instar larvae and maintained in the conditioned room. The mortality rates were evaluated 48 h after treatment.
2 Results and Discussion 2.1 Synthesis of the title compoundsAs shown in Scheme 1, 2,2-difluoro-5-methyl-1,3-benzodioxole (c) is the key intermediate for the synthesis of title compounds and was prepared starting from 4-methylbenzene-1, 2-diol by cyclization, chlorination and fluorination reaction [23-26]. During the fluorination reaction, triethylamine trihydrogenfluoride was employed as the fluorinating agent and high yields were obtained under ambient reaction conditions. Then the intermediate c was converted to another key intermediate 2,2-difluoro-1,3-benzodioxole-5-acetic acid (f) by bromination, cyanation and hydrolysis. In traditional synthetic procedure, the intermediate f was converted to compound h1 by acylchlorination and acylation reaction in 45% total yield[27](Scheme 2, Route A). In the improved route, compound h1 was prepared in 60% yield by coupling intermediate f and 2-methyl-4-heptafluoroisopropyl aniline using TPGS-750-M in water (Scheme 2, Route B). With the above mentioned environment-friendly and easy operative method, title compounds h2-h16 were synthesized with moderate yields. Physical and chemical data of the title compounds h1-h16 were listed in Table 1. 1H NMR, 19F NMR and HRMS spectra were listed in Table 2.
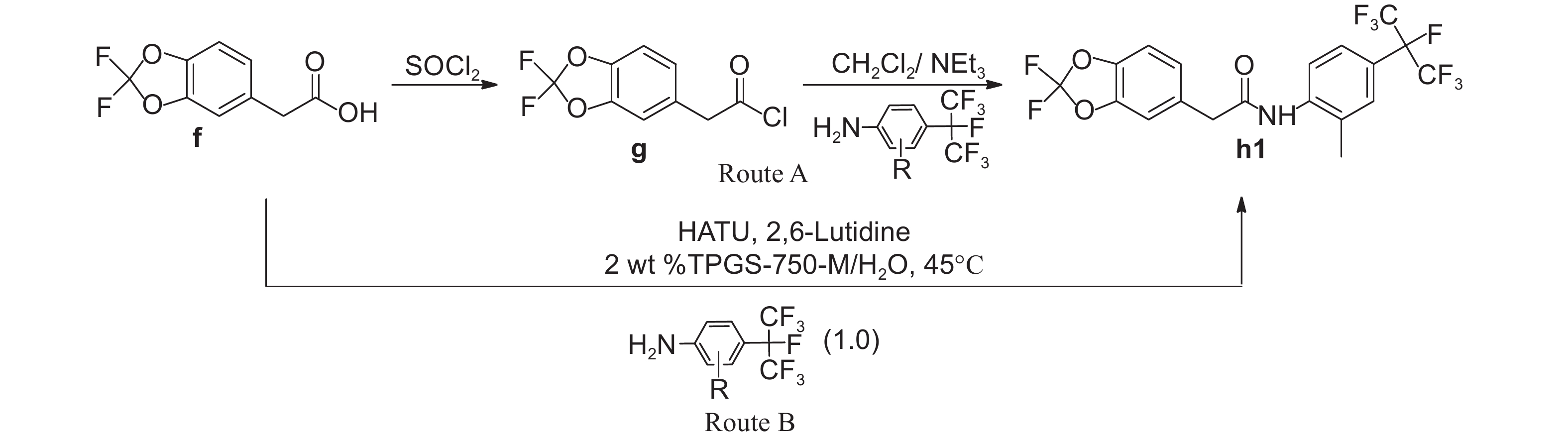
|
Scheme2 Route improvement for the synthesis of the title compounds h1 |
|
|
Table 1 Physical and chemical data of title compounds h1-h16 |
|
|
Table 2 The 1H NMR, 19F NMR and HRMS of the title compounds h1-h16 |
2.2 Insecticidal activity
The insecticidal activity of the above compounds against bean aphids (A. craccivora) and armyworms (M. separata) in vivo was evaluated. The results were listed in Table 3. h5, h8, h10 and h16 exhibited 100% mortality against A. craccivora at the concentration of 500 mg/L. h1, h7, h12 and h15 also showed high activities, and displayed mortalities higher than 80%. As for the insecticidal activities against M. separata, compounds h2, h4 and h14 exhibited mortalities higher than 80% at the concentration of 500 mg/L.
|
|
Table 3 Insecticidal activity of title compounds h1 to h16 |
Preliminary structure-activity relationship studies suggested that the insecticidal activities were significantly influenced by the substituent on the benzene ring of p-heptafluoroisopropyl aniline. For example, alkyl and alkoxy substituted compounds (h1-h8, h10-h16) showed good insecticidal activities, whereas hydroxyl substituted compound h9 exhibited no insecticidal activity. The target compounds displayed high selectivity between A. craccivora and M. separate, and compounds with substituent on 2-position of heptafluoroisopropyl aniline exhibited obvious insecticidal effect against A. craccivora. Whereas 3-position substituted compounds (h4 and h6) and unsubstituted compound h2 showed insecticidal activities against M. separata. Further studies on structure optimization are currently in progress.
| [1] | DOLBIER JR W R. Guide to fluorine NMR for organic chemists[M]. Hoboken, New Jersey: John Wiley & Sons Inc, 2009. |
| [2] | MÜLLER K, FAEH C, DIEDERICH F. Fluorine in pharmaceuticals: looking beyond intuition[J]. Science, 2007, 317 (5846):1881–1886. doi:10.1126/science.1131943 |
| [3] | JESCHKE P. The unique role of halogen substituents in the design of modern agrochemicals[J]. Pest Manag Sci, 2010, 66 (1):10–27. doi:10.1002/ps.v66:1 |
| [4] | TOHNISHI M, NAKAO H, KOHNO E, et al. Phthalamide derivatives, or salt thereof agrohorticultural insecticide, and method for using the same: EP1006107A2[P]. 2000-06-07. |
| [5] | TOHNISHI M, NAKAO H, FURUYA T, et al. Flubendiamide, a novel insecticide highly active against lepidopterous insect pests[J]. J Pestic Sci, 2005, 30 (4):354–360. |
| [6] | UEHARA M, WATANABE M, KIMURA M, et al. Substituted aminoquinazolinone(thione)derivatives or salts thereof, intermediates thereof, and pest controllers and a method for using the same: EP1097932A1[P]. 2001-05-09. |
| [7] | SANPEI O, UEHARA M, NIINO N, et al. Process for producing substituted aminoquinazolinone derivative, intermediate therefor, and pest control agent: WO2004099184A1[P]. 2004-11-18. |
| [8] | FURUYA T, KANNO H, MACHIYA K, et al. Substituted pyrazolecarboxylic acid anilide derivative or salt thereof, intermediate thereof, agent for agricultural and horticultural use, and use thereof: US2009105325A1[P]. 2009-04-23. |
| [9] | NYFELER R, EHRENFREUND J. Difluorbenzodioxyl cyanopyrrole microbicidal compositions: US4705800A[P]. 1987-11-10. |
| [10] | SUTTER M. 3-Aryl-4-cyano-pyrrole derivatives, process for their preparation and microbiocidal agents containing them: EP0386681A1[P]. 1990-09-12. |
| [11] | LEADBEATER A J, NEVILL D J, STECK B, et al. CGA 173506: a novel fungicide for seed treatment[C]//Brighton Crop Protection Conference, Pests and Diseases. Thornton Heath, UK: British Crop Protection Council, 1990: 825-830. |
| [12] | LIPSHUTZ B H. The ‘Nano-to-Nano’ effect applied to organic synthesis in water[J]. Johnson Matthey Technol Rev, 2017, 61 (3):196–202. doi:10.1595/205651317X695785 |
| [13] | LIPSHUTZ B H, GALLOU F, HANDA S. Evolution of solvents in organic chemistry[J]. ACS Sustainable Chem Eng, 2016, 4 (11):5838–5849. doi:10.1021/acssuschemeng.6b01810 |
| [14] | LIPSHUTZ B H, GHORAI S, ABELA A R, et al. TPGS-750-M: a second-generation amphiphile for metal-catalyzed cross-couplings in water at room temperature[J]. J Org Chem, 2011, 76 (11):4379–4391. doi:10.1021/jo101974u |
| [15] | CARPINO L A, IMAZUMI H, EL-FAHAM A, et al. The uronium/guanidinium peptide coupling reagents: finally the true uronium salts[J]. Angew Chem Int Ed, 2002, 41 (3):441–445. doi:10.1002/1521-3773(20020201)41:3<>1.0.CO;2-9 |
| [16] | CARPINO L A. 1-Hydroxy-7-azabenzotriazole. An efficient peptide coupling additive[J]. J Am Chem Soc, 1993, 115 (10):4397–4398. doi:10.1021/ja00063a082 |
| [17] | CARPINO L A, EL-FAHAM A, ALBERICIO F. Racemization studies during solid-phase peptide synthesis using azabenzotriazole-based coupling reagents[J]. Tetrahedron Lett, 1994, 35 (15):2279–2282. doi:10.1016/0040-4039(94)85198-0 |
| [18] | FENNIE M W, ROTH J M. Comparing amide-forming reactions using green chemistry metrics in an undergraduate organic laboratory[J]. J Chem Educ, 2016, 93 (10):1788–1793. doi:10.1021/acs.jchemed.6b00090 |
| [19] | DUNETZ J R, MAGANO J, WEISENBURGER G A. Large-scale applications of amide coupling reagents for the synthesis of pharmaceuticals[J]. Org Process Res Dev, 2016, 20 (2):140–177. doi:10.1021/op500305s |
| [20] | GABRIEL C M, KEENER M, GALLOU F, et al. Amide and peptide bond formation in water at room temperature[J]. Org Lett, 2015, 17 (16):3968–3971. doi:10.1021/acs.orglett.5b01812 |
| [21] | PARMENTIER M, WAGNER M K, MAGRA K, et al. Selective amidation of unprotected amino alcohols using surfactant-in-water technology: a highly desirable alternative to reprotoxic polar aprotic solvents[J]. Org Process Res Dev, 2016, 20 (6):1104–1107. doi:10.1021/acs.oprd.6b00133 |
| [22] | KRAUSE N. New surfactants for chemistry in water[J]. Current Opinion in Green and Sustainable Chemistry, 2017, 7 :18–20. doi:10.1016/j.cogsc.2017.06.009 |
| [23] | LEIMGRUBER W, WICK A E. Process for the methylenation of catechols: US3922285A[P]. 1975-11-25. |
| [24] | BERKELHAMMER G, KAMESWARAN V. Insecticidal and acaricidal m-phenoxybenzyl esters of 2,2-difluoro-1,3-benzodioxole-5-(α-alkyl)acetic acids: US4105780A[P]. 1978-08-08. |
| [25] | FRANCKOWIA G, MARHOLD A, BECHEM M, et al. Circulation-active dioxyalkylenearyl-dihydropyridines: US5344944A[P]. 1994-09-06. |
| [26] | SCHLOSSER M, GORECKA J, CASTAGNETTI E. A homologous series of O- and N-functionalized 2,2-difluoro1,3-benzodioxoles: an exercise in organometallic methodology [J]. Eur J Org Chem, 2003 :452–462. |
| [27] | ONISHI M, YOSHIURA A, KOHNO E, et al. Process for the production of perfluoroalkylated aniline derivatives: EP1006102B1[P]. 2006-08-30. |
 2019, Vol. 21
2019, Vol. 21


