2. 甘肃省农业科学院 植物保护研究所,兰州 730070;
3. 兰州大学 药学院 药物化学研究所,兰州 730000
2. Institute of Plant Protection, Gansu Academy of Agricultural Sciences, Lanzhou 730070, China;
3. Institute of Pharmaceutics Chemistry, School of Pharmaceutics, Lanzhou University, Lanzhou 730000, China
米尔贝霉素 (milbemycins,结构式见图式 1),也称弥拜菌素,其结构仅比伊维菌素 (ivermectin) 少了两个糖基,因此其活性在继承伊维菌素稳定性和长效性的同时,还具有比伊维菌素较高的脂溶性[1-2]。对农、林害虫具有广谱活性,且作用迅速,活性高,对人畜安全,不易污染环境,也不易产生抗性,是新型、广谱、无交叉抗性的生物杀虫剂[3]。米尔贝霉素的作用机理[4]与γ-氨基丁酸激动剂阿维菌素不同,可引起谷氨酸门控氯离子通道 (glutamate-gated chloride) 的开放,从而使氯离子内流增加,使正常的动作电位不能释放,导致昆虫麻痹死亡[3]。对已产生抗性的害螨仍有非常好的杀灭效果[5]。
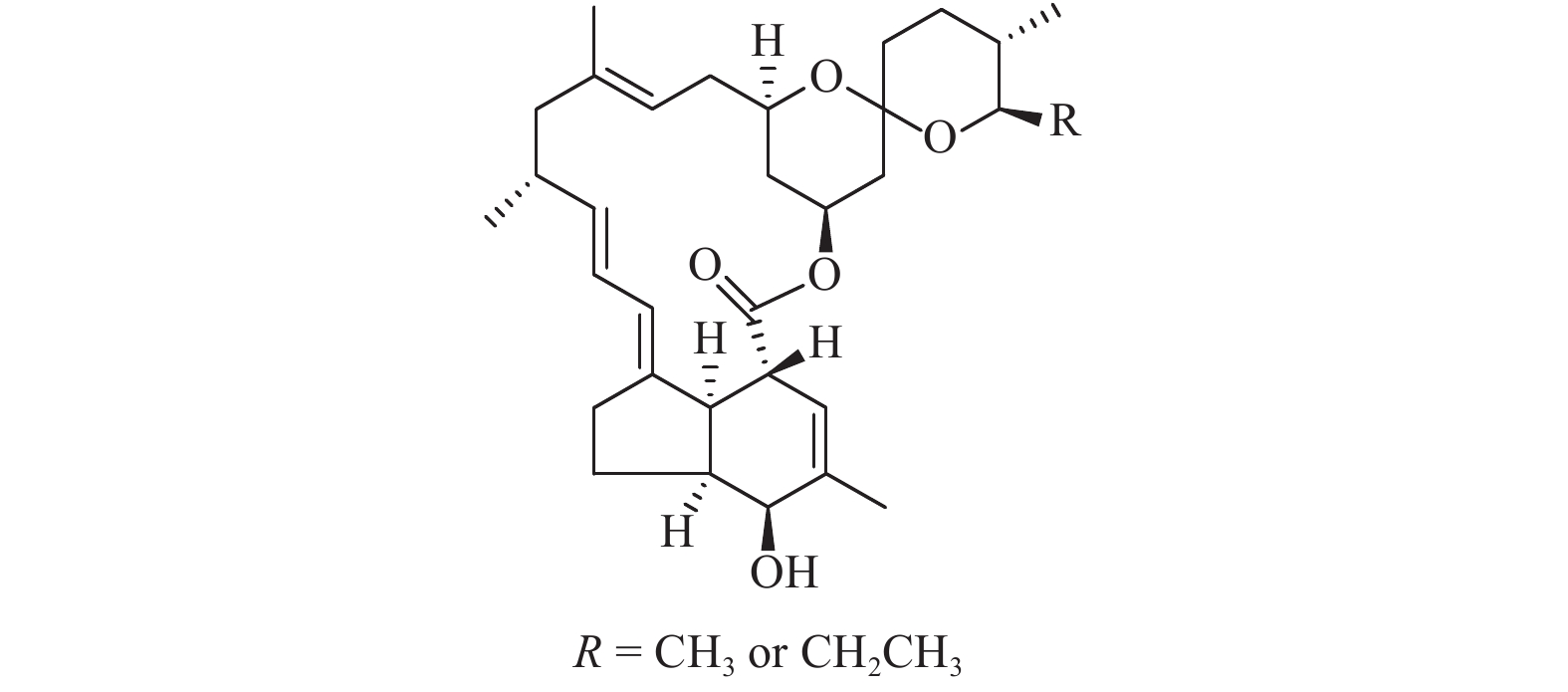
|
图式 1 米尔贝霉素化学结构式 Scheme1 The chemical structure of milbemycin |
目前,围绕米尔贝霉素衍生物的研究已有许多相关报道[6]及专利[7-8]。本研究组发现,磺酰脒类基团是一种具有较好杀虫活性的结构[9]。为了寻找高效、低毒且对抗性害虫具有高活性的米尔贝霉素类衍生物,笔者以廉价、易得的伊维菌素为原料,经脱糖后[10],以磺酰叠氮为氮源,氯化亚铜为催化剂,将其进一步转变为13-氨基米尔贝霉素类似物[11],再通过三组分反应[12]设计合成了一系列结构新颖的米尔贝霉素磺酰脒类化合物 (其合成路线见图式 2)。以朱砂叶螨和豆蚜为供试目标,初步测定了目标化合物的杀虫 (螨) 活性。
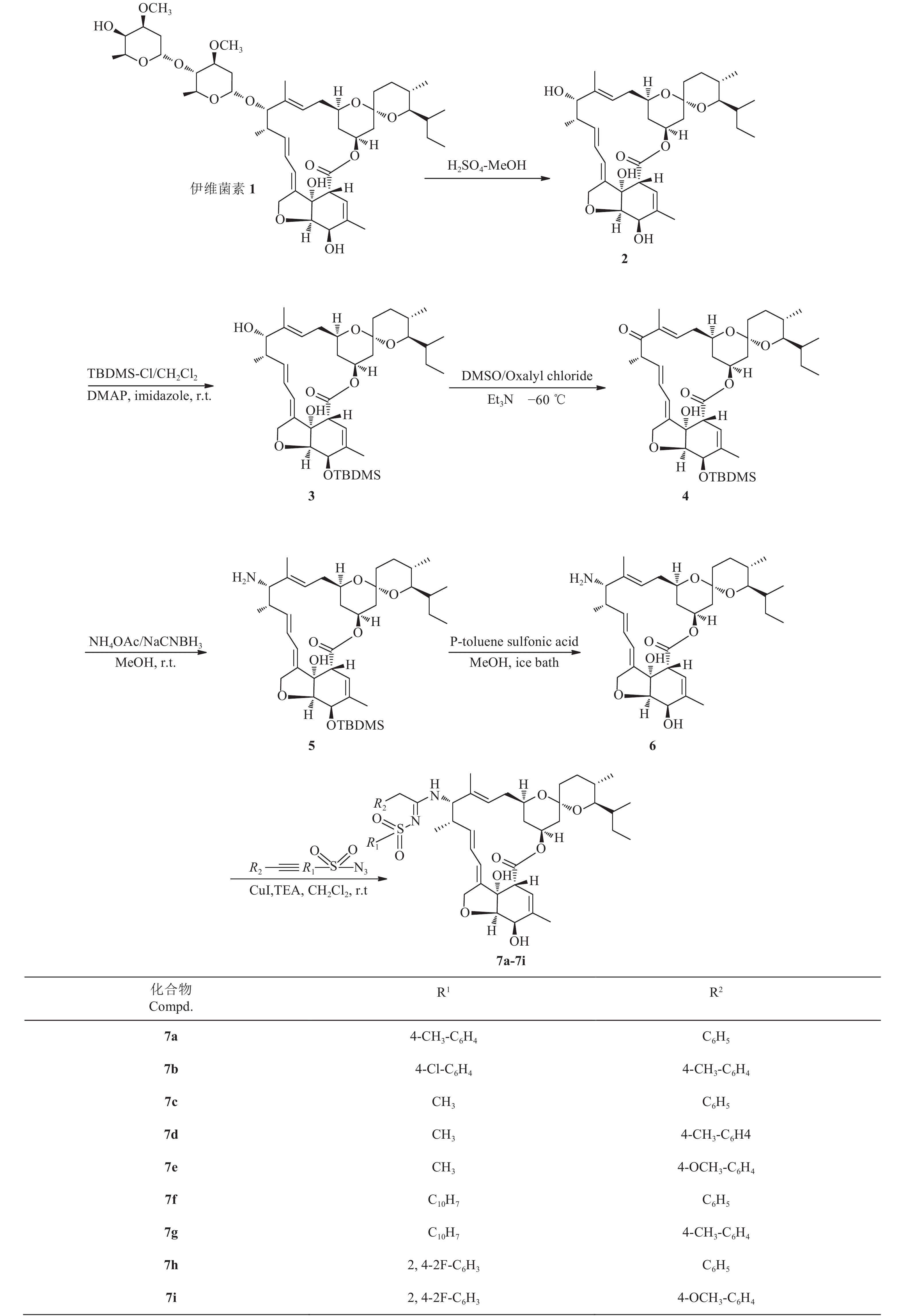
|
2 米尔贝霉素衍生物7a~7i的合成路线 Scheme2 Synthesis route of milbemycins derivatives 7a-7i |
1 实验部分 1.1 仪器与试剂
X-4 数字显微熔点测定仪 (北京泰克仪器有限公司,温度未经校正);Bruker AM-400 核磁共振波谱仪 (德国Bruker公司,以氘代氯仿为溶剂,以四甲基硅烷为内标);Bruker Esquire 6000 液-质联用仪 (德国Bruker公司)。
柱层析用硅胶 (筛孔径48~75 μm,青岛海洋化工有限公司产品);97%伊维菌素 (ivermectin) 原药,四川省江源天然产物有限公司;其他试剂均为分析纯。
1.2 目标化合物7a~7i的合成中间体2、3、4、5、6的合成参照相应文献[14-18]进行。目标化合物的合成以7a为例:将117 mg (0.2 mmol) 中间体6溶于10 mL二氯甲烷中,加入47.3 mg (0.24 mmol) 对甲基苯基磺酰叠氮和26.5 mg (0.26 mmol) 苯乙炔,在氮气保护下,加入3.8 mg (0.02 mmol) 碘化亚铜和0.035 mL (0.25 mmol) 三乙胺,室温搅拌1.5 h。反应结束后,用氯仿稀释,加入饱和氯化铵溶液,在室温下搅拌30 min,然后用氯仿多次萃取。合并有机层,用无水硫酸镁干燥,过滤,减压蒸除溶剂后,粗产物经硅胶柱层析 (200~300目) 纯化,用V (二氯甲烷): V (甲醇) = 20:1洗脱,得目标产物7a。用相同操作方法制得7b~7i。
7a:产率63%. 白色固体,熔点:142~144 ℃. 1H NMR (400 MHz, CDCl3), δ: 7.79~7.82 (m, 2H, Ar-H), 6.97~7.14 (m, 7H, Ar-H), 5.65~5.70 (m, 1H, 10-H), 5.43~5.46 (m, 1H, 9-H), 5.26~5.32 (m, 3H, 3-H, 11-H, 19-H), 4.51~4.64 (m, 3H, 8a-H, 15-H), 4.46~4.49 (m, 1H, -CH2C=N-), 4.30~4.37 (m, 2H, 5-H, -CH2C=N-), 4.29 (s, 1H, 13-H), 3.79 (m, 1H, 6-H), 3.49 (s, 3H, Ar-CH3), 3.33 (s, 1H, 7-OH), 3.27~3.30 (m, 1H, 17-H), 3.20~3.22 (m, 2H, 2-H, 25-H), 2.59~2.61 (m, 1H, 12-H), 2.18~2.44 (m, 4H, 5-OH, 16-H, 24-H), 1.25~1.99 (m, 17H, 4-Me, 14-Me, 20-H, 26-H, 27-H, 22-H, 23-H, 18-H), 0.69~1.07 (m, 12H, 12-Me, 27-Me, 24-Me, 26-Me). MS-ESI, m/z: 857.4[M+H]+.
7b:产率61%. 白色固体,熔点:137~139 ℃. 1H NMR (400 MHz, CDCl3), δ: 7.85 (d, 2H, J=8.4 Hz, Ar-H), 7.43 (d, 2H, J=8.4 Hz, Ar-H), 7.18 (d, 2H, J=8.4 Hz, Ar-H), 7.01 (d, 2H, J=8.4 Hz, Ar-H), 5.67~5.70 (m, 1H, 10-H), 5.52~5.55 (m, 1H, 9-H), 5.30~5.41 (m, 3H, 3-H, 11-H, 19-H), 4.56~4.67 (m, 3H, 8a-H, 15-H), 4.38~4.43 (m, 1H, -CH2C=N-), 4.25~4.30 (m, 2H, 5-H, -CH2C=N-), 4.20 (s, 1H, 13-H), 3.93 (d, 1H, J=6.0 Hz, 6-H), 3.89 (s, 3H, Ar-CH3), 3.78 (s, 1H, 7-OH), 3.51~3.53 (m, 1H, 17-H), 3.20~3.23 (m, 2H, 2-H, 25-H), 2.62~2.63 (m, 1H, 12-H), 2.17~2.31 (m, 4H, 5-OH, 16-H, 24-H), 1.25~1.87 (m, 17H, 4-Me, 14-Me, 20-H, 26-H, 27-H, 22-H, 23-H, 18-H), 0.70~1.19 (m, 12H, 12-Me, 27-Me, 24-Me, 26-Me). MS-ESI, m/z: 891.3[M+H]+.
7c:产率49%. 白色固体,熔点:133~135 ℃. 1H NMR (400 MHz, CDCl3), δ: 7.40~7.49 (m, 2H, Ar-H), 7.32~7.39 (m, 3H, Ar-H), 5.67~5.74 (m, 1H, 10-H), 5.53~5.55 (m, 1H, 9-H), 5.11~5.42 (m, 3H, 3-H, 11-H, 19-H), 4.57~4.67 (m, 3H, 8a-H, 15-H), 4.40~4.48 (m, 1H, -CH2C=N-), 4.27~4.35 (m, 2H, 5-H, -CH2C=N-), 4.11~4.13 (m, 1H, 13-H), 3.92~3.98 (m, 1H, 6-H), 3.50 (s, 3H, -SO2CH3), 3.20~3.23 (m, 1H, 7-OH), 3.07~3.12 (m, 1H, 17-H), 3.02~3.04 (m, 2H, 2-H, 25-H), 2.64~2.65 (m, 1H, 12-H), 2.19~2.32 (m, 4H, 5-OH, 16-H, 24-H), 1.26~1.90 (m, 17H, 4-Me, 14-Me, 20-H, 26-H, 27-H, 22-H, 23-H, 18-H), 0.70~1.05 (m, 12H, 12-Me, 27-Me, 24-Me, 26-Me). MS-ESI, m/z: 781.6[M+H]+.
7d:产率52%. 白色固体,熔点:136~138 ℃. 1H NMR (400 MHz, CDCl3), δ: 7.13~7.31 (m, 4H, Ar-H), 5.67~5.73 (m, 1H, 10-H), 5.49~5.52 (m, 1H, 9-H), 5.13~5.32 (m, 3H, 3-H, 11-H, 19-H), 4.53~4.67 (m, 3H, 8a-H, 15-H), 4.41~4.50 (m, 1H, -CH2C=N-), 4.29~4.38 (m, 2H, 5-H, -CH2C=N-), 4.17~4.24 (m, 1H, 13-H), 3.93~3.97 (m, 1H, 6-H), 3.46~3.56 (m, 3H, Ar-CH3), 3.23~3.27 (m, 3H, 2-H, 25-H, 17-H), 3.20~3.23 (m, 1H, 7-OH), 3.01~3.07 (m, 3H, -SO2CH3), 2.62~2.64 (m, 1H, 12-H), 2.17~2.45 (m, 4H, 5-OH, 16-H, 24-H), 1.26~1.98 (m, 17H, 4-Me, 14-Me, 20-H, 26-H, 27-H, 22-H, 23-H, 18-H), 0.68~1.06 (m, 12H, 12-Me, 27-Me, 24-Me, 26-Me). MS-ESI, m/z: 750.8[M+H]+.
7e:产率56%. 白色固体,熔点:135~137 ℃. 1H NMR (400 MHz, CDCl3), δ: 7.22 (d, 2H, J=8.4 Hz, Ar-H), 7.01 (d, 2H, J=8.0 Hz, Ar-H), 5.68~5.74 (m, 1H, 10-H), 5.53~5.56 (m, 1H, 9-H), 5.20~5.42 (m, 3H, 3-H, 11-H, 19-H), 4.56~4.68 (m, 3H, 8a-H, 15-H), 4.40~4.44 (m, 1H, -CH2C=N-), 4.29~4.31 (m, 2H, 5-H, -CH2C=N-), 4.20~4.22 (m, 1H, 13-H), 3.95~3.97 (m, 1H, 6-H), 3.88 (s, 3H, Ar-CH3), 3.80 (s, 1H, 7-OH), 3.56~3.66 (m, 1H, 17-H), 3.21~3.23 (m, 2H, 2-H, 25-H), 3.03 (s, 3H, -SO2CH3), 2.64~2.65 (m, 1H, 12-H), 2.19~2.32 (m, 4H, 5-OH, 16-H, 24-H), 1.26~1.90 (m, 17H, 4-Me, 14-Me, 20-H, 26-H, 27-H, 22-H, 23-H, 18-H), 0.70~1.05 (m, 12H, 12-Me, 27-Me, 24-Me, 26-Me). MS-ESI, m/z: 811.9[M+H]+.
7f:产率61%. 白色固体,熔点:137~139 ℃. 1H NMR (400 MHz, CDCl3), δ: 8.49 (s, 1H, Ar-H), 7.89~7.96 (m, 4H, Ar-H), 7.59~7.64 (m, 2H, Ar-H), 7.57 (s, 2H, Ar-H), 7.48~7.49 (m, 1H, Ar-H), 7.07~7.16 (m, 2H, Ar-H), 5.65~5.71 (m, 1H, 10-H), 5.51~5.53 (m, 1H, 9-H), 5.24~5.40 (m, 3H, 3-H, 11-H, 19-H), 4.55~4.65 (m, 3H, 8a-H, 15-H), 4.47~4.49 (m, 1H, -CH2C=N-), 4.32~4.40 (m, 2H, 5-H, -CH2C=N-), 4.28 (s, 1H, 13-H), 3.92 (d, 1H, J=6.0 Hz, 6-H), 3.60 (s, 1H, 7-OH), 3.48~3.50 (m, 1H, 17-H), 3.16~3.22 (m, 2H, 2-H, 25-H), 2.61~2.63 (m, 1H, 12-H), 2.15~2.37 (m, 4H, 5-OH, 16-H, 24-H), 1.20~1.91 (m, 17H, 4-Me, 14-Me, 20-H, 26-H, 27-H, 22-H, 23-H, 18-H), 0.71~1.25 (m, 12H, 12-Me, 27-Me, 24-Me, 26-Me). MS-ESI, m/z: 893.3[M+H]+.
7g:产率67%. 白色固体,熔点:140~142 ℃. 1H NMR (400 MHz, CDCl3), δ: 8.49 (s, 1H, Ar-H), 7.90~7.96 (m, 5H, Ar-H), 7.59~7.62 (m, 2H, Ar-H), 6.96~7.14 (m, 3H, Ar-H), 5.65~5.71 (m, 1H, 10-H), 5.47~5.50 (m, 1H, 9-H), 5.29~5.31 (m, 3H, 3-H, 11-H, 19-H), 4.54~4.66 (m, 3H, 8a-H, 15-H), 4.44~4.49 (m, 1H, -CH2C=N-), 4.15~4.19 (m, 2H, 5-H, -CH2C=N-), 4.04~4.13 (m, 1H, 13-H), 3.92 (d, 1H, J=6.0 Hz, 6-H), 3.48~3.49 (m, 4H, 7-OH, Ar-CH3), 3.17~3.23 (m, 3H, 17-H, 2-H, 25-H), 2.60-2.62 (m, 1H, 12-H), 2.16~2.44 (m, 4H, 5-OH, 16-H, 24-H), 1.28~1.97 (m, 17H, 4-Me, 14-Me, 20-H, 26-H, 27-H, 22-H, 23-H, 18-H), 0.67~1.25 (m, 12H, 12-Me, 27-Me, 24-Me, 26-Me). MS-ESI, m/z:907.7[M+H]+.
7h:产率40%. 白色固体,熔点:142~144 ℃. 1H NMR (400 MHz, CDCl3), δ: 7.98~8.06 (m, 1H, Ar-H), 7.50~7.52 (m, 2H, Ar-H), 7.32~7.38 (m, 2H, Ar-H), 6.89~7.20 (m, 3H, Ar-H), 5.65~5.71 (m, 1H, 10-H), 5.51~5.54 (m, 1H, 9-H), 5.24~5.33 (m, 3H, 3-H, 11-H, 19-H), 4.51~4.63 (m, 3H, 8a-H, 15-H), 4.33~4.39 (m, 1H, -CH2C=N-), 4.26~4.28 (m, 2H, 5-H, -CH2C=N-), 4.11~4.13 (m, 1H, 13-H), 3.95~3.97 (m, 1H, 6-H), 3.64 (s, 1H, 7-OH), 3.19~3.22 (m, 3H, 17-H, 2-H, 25-H), 2.58~2.60 (m, 1H, 12-H), 2.13~2.30 (m, 4H, 5-OH, 16-H, 24-H), 1.21~1.98 (m, 17H, 4-Me, 14-Me, 20-H, 26-H, 27-H, 22-H, 23-H, 18-H), 0.67~1.15 (m, 12H, 12-Me, 27-Me, 24-Me, 26-Me). MS-ESI, m/z: 879.8[M+H]+.
7i:产率43%. 白色固体,熔点:144~146 ℃. 1H NMR (400 MHz, CDCl3), δ: 7.97~8.03 (m, 1H, Ar-H), 7.21~7.23 (m, 2H, Ar-H), 6.88~7.04 (m, 4H, Ar-H), 5.65~5.72 (m, 1H, 10-H), 5.51~5.54 (m, 1H, 9-H), 5.23~5.31 (m, 3H, 3-H, 11-H, 19-H), 4.56~4.63 (m, 3H, 8a-H, 15-H), 4.44~4.49 (m, 1H, -CH2C=N-), 4.18~4.28 (m, 2H, 5-H, -CH2C=N-), 4.11~4.13 (m, 1H, 13-H), 3.95 (m, 1H, 6-H), 3.81 (s, 3H, Ar-CH3), 3.66 (s, 1H, 7-OH), 3.21~3.23 (m, 3H, 17-H, 2-H, 25-H), 2.60~2.62 (m, 1H, 12-H), 2.14~2.31 (m, 4H, 5-OH, 16-H, 24-H), 1.25~1.97 (m, 17H, 4-Me, 14-Me, 20-H, 26-H, 27-H, 22-H, 23-H, 18-H), 0.68~1.19 (m, 12H, 12-Me, 27-Me, 24-Me, 26-Me). MS-ESI, m/z: 909.2[M+H]+.
1.3 生物活性测定 1.3.1 杀螨活性测定朱砂叶螨Tetranychus cinnabarinus雌成螨,采自甘肃省农业科学院田间的黄豆苗上,参照FAO (联合国粮农组织) 推荐的玻片浸渍法[19]并加以改进。将米尔贝霉素衍生物7a~7i在预试验的基础上用水稀释成5个不同浓度药液,滴加质量分数为0.1%的吐温-80作为乳化剂。以伊维菌素为药剂对照,以不含伊维菌素及目标化合物的溶液为空白对照。挑选大小一致、体色鲜艳、行动活泼的雌成螨,将其背部粘在双面胶玻璃片一端,每片3行,每行10头。在温度 (25 ± 1)℃、相对湿度85%左右的生化培养箱中放置4 h后用双目镜观察。除掉死亡或不活泼个体,将每板补充至30头。每个玻璃片记为1次重复,每次处理重复3次。将带螨玻璃片的一端浸入溶液中,轻轻摇动5 s后取出,迅速用三角形小滤纸吸干螨体及其周围多余的药液。置于上述生化培养箱中,24 h后用双目镜检查统计结果。
1.3.2 杀蚜活性测定豆蚜Aphis craccivora成虫,采自甘肃省农业科学院试验田的龙葵上。采用点滴法[20]测定。将目标化合物溶解在质量分数为10%的丙酮水溶液中配成1 g/L的药液,加入质量分数为0.1%的吐温-80为乳化剂,在预试验的基础上,用水将配制好的药液稀释成不同浓度供试。每浓度设3次重复。用微量点滴器吸取药液点滴在豆蚜前胸背板上,每虫点滴0.03 μL。以10%丙酮水溶液加0.1%吐温-80为对照。各处理分别放入温度为 (25 ± 1) ℃、相对湿度85%左右的生化培养箱中,24 h后检查统计结果。
1.3.3 数据处理用Abbott公式对处理组死亡率进行校正,试验结果采用SPSS统计软件 (22.0版) 进行分析,计算致死中浓度 (LC50值)。以各衍生物与伊维菌素的LC50比值作相对毒力值。
2 结果与分析毒力测定结果 (表1) 表明:所有米尔贝霉素磺酰脒类化合物对朱砂叶螨和豆蚜均有较强的活性,其中化合物7f、7h和7i对朱砂叶螨24 h的LC50值分别为1.04 × 10–2、9.60 × 10–3和1.44 × 10–2 mg/L,低于伊维菌素的LC50值 (2.76 × 10–2 mg/L );化合物7i对豆蚜24 h的LC50值为7.81 mg/L,也低于伊维菌素的LC50值 (20.8 mg/L),表现出较强的杀蚜活性。
|
|
表 1 化合物7a~7i对朱砂叶螨和豆蚜的毒力 Table 1 The activities of 7a-7i against T. cinnabarinus and A. craccivora |
本研究以廉价易得的伊维菌素为起始原料,以磺酰叠氮为氮源,氯化亚铜为催化剂,经胺化后合成了一系列米尔贝霉素磺酰脒类化合物 (7a~7i),具有原料成本低廉、催化剂价廉、操作简单和产率高等优点。
虽然目前围绕米尔贝霉素衍生物的研究已经取得了很多进展[6],尤其是Takeshiba等[20]研制的lepimectin[13-(α-甲氧亚氨基) 苯乙酰基米尔贝霉素]的成功上市,更加展现出米尔贝霉素类化合物的杀虫前景,但到目前为止,还未见米尔贝霉素磺酰脒类化合物的合成及此类化合物作为农药进行农业虫害防治的应用。本课题组通过三组分反应设计合成了一系列结构新颖的米尔贝霉素磺酰脒类化合物,初步生物活性测定结果表明其杀虫活性比伊维菌素有显著提高。本研究结果可为米尔贝霉素衍生物杀虫活性构效关系研究奠定基础。
| [1] | CAMPBELL W C, BURG R W, FISHER M H, et al. The discovery of ivermectin and other avermectins[M]. Washington, DC: American Chemical Society, 1984. |
| [2] | BANKS B J, BISHOP B F, EVANS N A, et al. Avermectins and flea control: structure-activity relationships and the selection of selamectin for development as an endectocide for companion animals[J]. Bioorg Med Chem, 2000, 8(8): 2017–2025. doi:10.1016/S0968-0896(00)00120-6 |
| [3] |
刘琳琳, 刘翠翠, 王相晶, 等. 阿维菌素类和米尔贝霉素类杀虫剂的特性[J]. 世界农药, 2012, 34(5): 34–39.
LIU L L, LIU C C, WANG X J, et al. The characteristics of biological insecticide avermectins and milbemycins[J]. World Pestic, 2012, 34(5): 34–39. |
| [4] | LUDMERER S W, WARREN V A, WILLIAMS B S, et al. Ivermectin and nodulisporic acid receptors in Drosophila melanogaster contain both γ-aminobutyric acid-gated rdl and glutamate-gated GluClα chloride channel subunits [J]. Biochemistry, 2002, 41(20): 6548–6560. doi:10.1021/bi015920o |
| [5] |
刘翠翠, 刘重喜, 王相晶, 等. 微生物农药米尔贝霉素特性[J]. 世界农药, 2012, 34(1): 35–38.
LIU C C, LIU C X, WANG X J, et al. The characteristics of biological pesticide milbemycin[J]. World Pestic, 2012, 34(1): 35–38. |
| [6] |
陈小龙, 郑裕国, 沈寅初. 生物农药米尔贝霉素的研究进展[J]. 农药, 2003, 42(4): 5–9.
CHEN X L, ZHENG Y G, SHEN Y C. Advances on biological pesticide: milbemycins[J]. Pesticides, 2003, 42(4): 5–9. |
| [7] | SHOOP W L, MROZIK H, FISHER M H. Structure and activity of avermectins and milbemycins in animal health[J]. Vet Parasitol, 1995, 59(2): 139–156. doi:10.1016/0304-4017(94)00743-V |
| [8] | CHABALA J C, FISHER M H. Selective hydrogenation products of C-076 compounds and derivatives thereof: US4199569A[P]. 1980-04-22. |
| [9] | WANG M J, ZHAO X B, WU D, et al. Design, synthesis, crystal structure, insecticidal activity, molecular docking, and QSAR studies of novel N3-substituted imidacloprid derivatives [J]. J Agric Food Chem, 2014, 62(24): 5429–5442. doi:10.1021/jf501108j |
| [10] | MROZIK H, LINN B O, ESKOLA P, et al. Syntheses and biological activities of 13-substituted avermectin aglycons[J]. J Med Chem, 1989, 32(2): 375–381. doi:10.1021/jm00122a015 |
| [11] | MANDAL S, GAUNIYAL H M, PRAMANIK K, et al. ChemInform abstract: glycosylated N-sulfonylamidines: highly efficient copper-catalyzed multicomponent reaction with sugar alkynes, sulfonyl azides, and amines [J]. J Org Chem, 2007, 72(25): 9753–9756. doi:10.1021/jo701565m |
| [12] | TSUKAMOTO Y, SATO K T, KINOTO T, et al. Syntheses of 8,9-epoxy-and 5-O-acyl-8, 9-epoxymilbemycin A4 and their activity against Tetranychus urticae [J]. Biosci, Biotechnol, Biochem, 1995, 59(2): 226–230. doi:10.1271/bbb.59.226 |
| [13] | MROZIK H, CHABALA J C, ESKOLA P, et al. Synthesis of milbemycins from avermectins[J]. Tetrahedron Lett, 1983, 24(48): 5333–5336. doi:10.1016/S0040-4039(00)87861-2 |
| [14] | CVETOVICH R J, SENANAYAKE C H S, AMATO J S, et al. Practical syntheses of 13-O-[(2-methoxyethoxy)methyl]-22,23-dihydroavermectin B1 aglycon [dimedectin isopropanol, MK-324] and 13-epi-O-(methoxymethyl)-22,23-dihydroavermectin B1 aglycon [L-694, 554], flea active ivermectin analogues [J]. J Org Chem, 1997, 62(12): 3989–3993. doi:10.1021/jo970187l |
| [15] | FREI B, HUXLEY P, MAIENFISCH P, et al. Synthesis and configuration of some hydroxymilbemycin derivatives including 22,23-dihydroavermectin B1b aglycone [J]. Helv Chim Acta, 1990, 73(7): 1905–1917. doi:10.1002/(ISSN)1522-2675 |
| [16] | LOEWE M F, CVETOVICH R J, DIMICHELE L M, et al. Glycosidation route to 4″-epi-(methylamino)-4″-deoxyavermectin B1 (MK-244, Emamectin Benzoate) [J]. J Org Chem, 1994, 59(25): 7870–7875. doi:10.1021/jo00104a052 |
| [17] | CVETOVICH R J, AMATO J S, DIMICHELE L, et al. Novel electrochemical reductive amination of 4″-oxo-5-O-(tert-butyl- dimethylsilyl) avermectin B1[J]. J Org Chem, 1997, 62(19): 6697–6698. doi:10.1021/jo970676l |
| [18] | KIM J, STAHL S S. Cu-catalyzed aerobic oxidative three-component coupling route to N-sulfonyl amidines via an ynamine intermediate [J]. J Org Chem, 2015, 80(4): 2448–2454. doi:10.1021/jo5029198 |
| [19] |
师超, 涂锡茂, 冯雪春, 等. 6种杀螨剂对朱砂叶螨不同生测方法的毒力比较[J]. 农药, 2012, 51(3): 223–224.
SHI C, TU X M, FENG X C, et al. Comparison of six acaricides by different bioassay methods to Tetranychus cinnabarinus toxicity [J]. Agrochemicals, 2012, 51(3): 223–224. |
| [20] | HIDEO T, KAZUO S, TOSHIAKI Y, et al. 13-Substituted milbemycin derivatives, their preparation and their use: US5614470A[P]. 1997-03-25. |
 2017, Vol. 19
2017, Vol. 19


