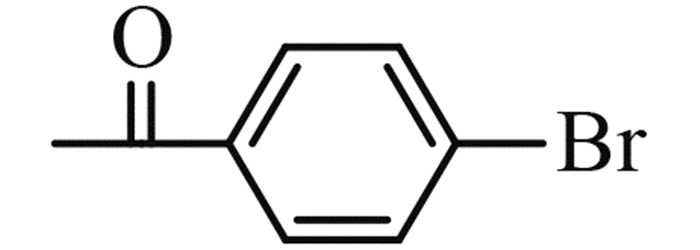
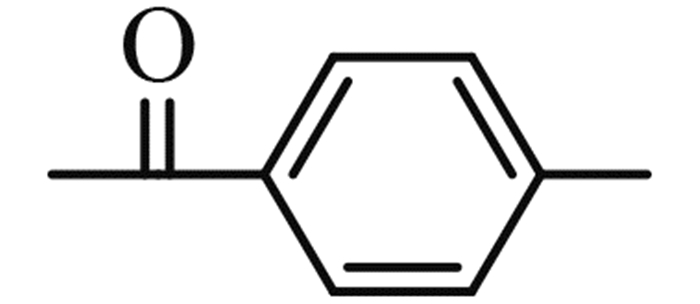
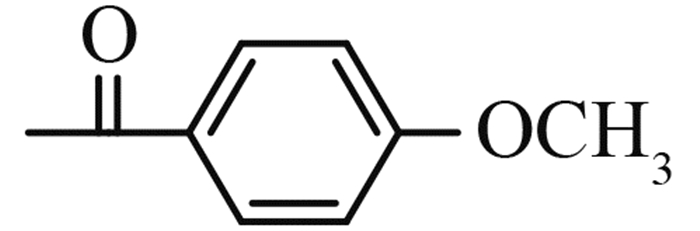
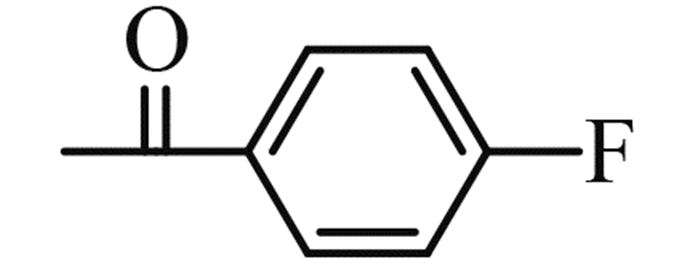
2. 天津化学化工协同创新中心, 天津 300071
2. Collaborative Innovatiaon Center of Chemical Science and Engineering (Tianjin), Tianjin 300071, China
In recent years,due to the increasing resistance of fungi to fungicides,it is necessary to develop new environmentally friendly fungicide. Moxifloxacin is the fourth-generation fluoroquinolone drug with broad-spectrum antifungal activity,good pharmaco- kinetics property,low drug resistance,and safe to use[1-2]. It also has very broad application prospect,and huge market potential[3]. As the branched-chain,(S,S)-2,8-diazabicyclo [4.3.0] nonane is the key intermediate for the synthesis of moxifloxacin. Its special diazabicyclo structure strengthens moxifloxacin’s activity against gram-positive bacteria,and also reduces adverse reaction while keeps the original activity [4].
Heterocyclic compounds with benzimidazole moiety usually showed high biological activity[5-6],including antiparasitic,antiviral,antifungal and anticancer activity. Aromatic heterocyclic ring containing two nitrogen atoms,can form hydrogen bonds with receptors[6-7]. The synthesis of 1-substituted and 2-substituted benzimidazole derivatives has become a hot research topic recently[7-13].
Since (S,S)-2,8- diazabicyclo [4.3.0] nonane and benzimidazole have special nitrogen heterocyclic structures,we designed and synthesized a series of novel hetereocycles with 2,8-diazabicyclo [4.3.0] nonane and benzimidazole moiety by using active substructure splicing method (Scheme 1). The antifungal activity and insecticidal activity of these compounds were also evaluated.
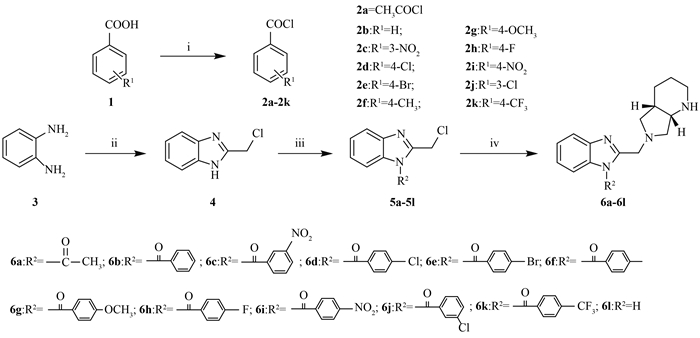
|
Reagents and conditions:(i)(COCl)2, DMF, CH2Cl2, rt;(ii)ClCH2COOH, HCl, H2O, re- flux, NH3·H2O;(iii)compounds 2a-2k, Et3N, CH2Cl2, 0 ℃; reflux;(iv)(S, S)-2, 8-diazabicyclo [4.3.0] nonane, K2CO3, CH3CN, rt. Scheme1 Synthesis of compounds 6a-6l |
1 Materials and methods 1.1 Instruments
All chemicals and reagents were of analytical grade. Reactions were monitored with analytical thin-layer chromatography (TLC). The melting points were determined on an X-4 binocular microscope melting point apparatus (Beijing Tech Instruments Co.,Beijing,China) and were uncorrected. 1H NMR spectra were recorded at 400 MHz on a Bruker AV 400 spectrometer in a CDCl3 solution with tetrame- thylsilane as the internal standard. High resolution mass spectrometry (HRMS) data were obtained on a Varian QFT-ESI instrument.
1.2 Synthetic procedures 1.2.1 General procedure for synthesis of compounds 2b-2kTo a solution of acid 1 (10 mmol) and oxalyl chloride (20 mmol) in dry CH2Cl2 (20 mL),0.1 mL DMF was added. The mixture was stirred at room temperature for 4 h. The reaction mixture was concentrated under reduced pressure. The crude acid chloride 2 was used in the subsequent step without further purification [16-17].
1.2.2 General synthetic procedure for compound 4Compounds 4 were prepared according to the literature procedures [14-16].
1.2.3 General procedure for synthesis of compounds 5a-5kTo a solution of 2-(chloromethyl)-1H-benzo [d] imidazole 4 (10 mmol) and triethylamine (15 mmol) in CH2Cl2 (20 mL),acyl chlorides 2 (10 mmol) was added dropwise at 0 ℃. The reaction mixture was allowed to warm to room temperature,and was then refluxed for 2 h. After the reaction completed,the mixture was diluted with CH2Cl2,washed with 1 mol/L HCl (50 mL×3),followed by brine (10 mL×3). The combined organic layers were dried over MgSO4,concentrated in vacuo,and purified by column chromatography on silica gel to afford 5a-5k[18].
(2-chloromethyl-benzoimidazol-1-yl)-ethanone (5a): yield 80%,a yellow solid,m. p. 80-81 ℃; 1H NMR,δ: 7.82 (dd,J = 6.4,2.6 Hz,1H,Ar-H),7.71 (dd,J = 6.7,2.4 Hz,1H,Ar-H),7.45-7.39 (m,2H,Ar-H),5.11 (s,2H,CH2),2.89 (s,3H,CH3).
(2-chloromethyl-benzoimidazol-1-yl)-phenyl-methanone (5b): yield 83%,a yellow solid,m. p. 80-81 ℃; 1H NMR,δ: 8.16 (d,J = 7.3 Hz,2H,Ar-H),7.79-7.51 (m,3H,Ar-H),7.50 (t,J = 7.7 Hz,2H,Ar-H),7.34-7.31 (m,2H,Ar-H),4.93 (s,2H,CH2).
(2-chloromethyl-benzoimidazol-1-yl)-(3-nitro-phenyl)-methanone (5c): yield 85%,a yellow solid,m. p. 128-129 ℃; 1H NMR,δ: 8.70 (s,1H,Ar-H),8.60 (d,J = 8.2 Hz,1H,Ar-H),8.11 (d,J = 7.7 Hz,1H,Ar-H),7.84-7.77 (m,2H,Ar-H),7.36 (t,J = 7.7 Hz,1H,Ar-H),7.17 (t,J = 7.8 Hz,1H,Ar-H),6.60 (d,J = 8.3 Hz,1H,Ar-H),5.10 (s,2H,CH2).
(2-chloromethyl-benzoimidazol-1-yl)-(4-chloro-phenyl)-methanone (5d): yield 80%,a yellow solid,m. p. 110-111 ℃; 1H NMR,δ: 7.79 (t,J = 8.8 Hz,3H,Ar-H),7.54 (d,J = 8.4 Hz,2H,Ar-H),7.33 (t,J = 7.7 Hz,1H,Ar-H),7.1 (t,J = 7.8 Hz,1H,Ar-H),6.71 (d,J = 8.3 Hz,1H,Ar-H),5.08 (s,2H,CH2).
(2-chloromethyl-benzoimidazol-1-yl)-(4-bromo-phenyl)-methanone (5e): yield 80%,a yellow solid,m. p. 108-109 ℃; 1H NMR,δ: 7.80 (d,J = 8.1 Hz,1H,Ar-H),7.75-7.61 (m,4H,Ar-H),7.33 (t,J = 7.5 Hz,1H,Ar-H),7.18 (t,J = 7.7 Hz,1H,Ar-H),6.71 (d,J = 8.3 Hz,1H,Ar-H),5.07 (s,2H,CH2).
(2-chloromethyl-benzoimidazol-1-yl)-p-tolyl-methanone (5f): yield 87%,a yellow solid,m. p. 73-74 ℃; 1H NMR,δ: 7.79 (d,J = 8.1 Hz,1H,Ar-H),7.72 (d,J = 7.8 Hz,2H,Ar-H),7.37-7.28 (m,3H,Ar-H),7.16 (t,J = 7.8 Hz,1H,Ar-H),6.76 (d,J = 8.3 Hz,1H,Ar-H),5.07 (s,2H,CH2),2.50 (s,3H,CH3).
(2-chloromethyl-benzoimidazol-1-yl)-(4-methoxy-phenyl)-methanone (5g): yield 83%,a yellow solid,m. p. 77-78 ℃; 1H NMR,δ: 7.80 (t,J = 8.7 Hz,3H,Ar-H),7.31 (t,J = 7.7 Hz,1H,Ar-H),7.17 (t,J = 7.8 Hz,1H,Ar-H),7.02 (d,J = 8.1 Hz,2H,Ar-H),6.83 (d,J = 8.2 Hz,1H,Ar-H),5.07 (s,2H,CH2),3.93 (s,3H,CH3).
(2-chloromethyl-benzoimidazol-1-yl)-(4-fluoro-phenyl)-methanone (5h): yield 80%,a yellow solid,m. p. 77-78 ℃; 1H NMR,δ: 7.98-7.77 (m,3H,Ar-H),7.37-7.18 (m,4H,Ar-H),6.73 (d,J = 8.3 Hz,1H,Ar-H),5.11 (s,2H,CH2).
(2-chloromethyl-benzoimidazol-1-yl)-(4-nitro-phenyl)-methanone (5i): yield 85%,a yellow solid,m. p. 80-81 ℃; 1H NMR,δ: 8.44 (d,J = 7.1 Hz,2H,Ar-H),8.02 (d,J = 7.1 Hz,2H,Ar-H),7.84 (d,J = 8.1 Hz,1H,Ar-H),7.38 (t,J = 7.7 Hz,1H,Ar-H),7.20 (t,J = 7.8 Hz,1H,Ar-H),6.59 (d,J = 8.3 Hz,1H,Ar-H),5.12 (d,J = 1.5 Hz,2H,CH2).
(2-chloromethyl-benzoimidazol-1-yl)-(3-chloro-phenyl)-methanone (5j): yield 80%,a yellow solid,m. p. 87-88 ℃; 1H NMR,δ: 8.14 (t,J = 1.8 Hz,1H,Ar-H),8.08-8.00 (m,1H,Ar-H),7.65 (dt,J = 6.6,3.3 Hz,2H,Ar-H),7.60-7.57 (m,1H,Ar-H),7.44 (t,J = 7.9 Hz,1H,Ar-H),7.37-7.30 (m,2H,Ar-H),4.94 (s,2H,CH2).
(2-chloromethyl-benzoimidazol-1-yl)-(4-trifluoromethyl-phenyl)-methanone (5k): yield 86%,a yellow solid,m. p. 78-79 ℃; 1H NMR,δ: 7.97 (d,J = 8.1 Hz,2H,Ar-H),7.85 (dd,J = 10.3,8.3 Hz,3H,Ar-H),7.42-7.31 (m,1H,Ar-H),7.25-7.16 (m,1H,Ar-H),6.63 (d,J = 8.3 Hz,1H,Ar-H),5.12 (s,2H,CH2).
1.2.4 General procedure for the synthesis of compounds 6a-6lTo a mixture of (S,S)-2,8-diazabicyclo [4.3.0] nonane (10 mmol) and anhydrous K2CO3 (15 mmol) in CH3CN (20 mL),compound 5 (5 mmol) was added. After the reaction completed,the mixture was filtrated. The solvent was removed and the residue was dissolved by CH2Cl2. The mixture was washed with saturated NaHCO3 solution and water. The combined organic layers were dried over MgSO4,concentrated in vacuo,and purified by column chromatography on silica gel to afford title compounds 6a-6l[19-20].
1.3 Bioassays 1.3.1 Antifungal activityThe fungicidal activity of title compounds was tested in vitro against Physalospora piricala,Fusarium graminearum,Phytophthora infestans,Rhizoctonia cerealis,Sclerotinia sclerotiorum,Cochliobolus heterostrophus and Fusarium moniliforme,their relative inhibitory rates (%) were determined by the mycelium growth rate method[21]. Carbendazim,chlorothalonil and thiram were used as controls. After the mycelia grew completely,the diameters of the mycelia were measured and the inhibition rate was calculated according to the formula:
I/% = [(D1 - D2)/D1] × 100
In the formula,I is the inhibition rate,D1 is the average diameter of mycelia in the blank test,and D2 is the average diameter of mycelia in the presence of one of those compounds.
1.3.2 Insecticidal activityInsecticidal activity against Mythimna separata Walker was tested in a greenhouse [22]. The bioassay was operated at (25±1) ℃ by statistical requirements. The compounds were dissolved with acetone at various concentrations,0.306 μL of the tested solution was applied to the thoracic tergite of fourth-instar larva with a platinum loop. After the treatment,the insects were observed under standard rearing conditions. Mortalities were calculated after 72 h. Each treatment was performed in triplicate.
Insecticidal activity against Culex pipiens pallens[22] was also evaluated. compounds (1 mL) was added to water (99 mL) to get the test solutions of different concentrations. 20 fourth-instar mosquito larva were transferred into 10 mL of the test solution and raised for 2 d,and the results were expressed by the lethality rate.
2 Results and discussion 2.1 Synthesis and analytical spectral dataThe physical and chemical data of the title compounds 6a-6l were shown in Table 1. The 1H NMR data of the title compounds 6a-6l were shown in Table 2. The 13C NMR data of the title compounds 6d,6e,6g and 6i were shown in Table 3.
|
|
Table 1 Physical and chemical data of the title compounds 6a-6l |
|
|
Table 2 1H NMR data of the title compounds 6a-6l |
|
|
Table 3 13C NMR data of the title compounds 6 |
The synthetic route of the intermediates and title compounds are illustrated in Scheme 1. Compound 4 was prepared from o-phenylenediamine and chloroacetic acid via a cyclization reaction in the acidic environment,followed by the ammonia neutralization. It can be conveniently obtained with high yields. Compounds 5a-5l can be obtained through the acylation reaction in CH2Cl2 with Et3N as base with high yields.
It was noteworthy that the target compounds 6a-6l can’t be obtained in acetone with anhydrous K2CO3 and KI,while they were formed in the N-alkylation reaction in CH3CN. It might indicated the poor activity of the reactants in acetone.
2.2 Antifungal activityThe antifungal activities of the target compounds against seven pathogenic fungis were listed in Table 4. Most of them showed moderate activity at the concentration of 50 μg/mL. Inhibition rate against P. infestans ranged from 50.0% to 62.5% for compounds 6d-6g. Compounds 6d-6h exhibited activity toward S. sclerotiorum,however,all of them were less effective than chlorothaloni. Inhibition rates against P. piricala of compounds 6h-6i were as good as carbendazim. Compounds 6d-6l exhibited inhibition rates of 55.0%-67.6% against R. cerealis. Compounds 6d-6g were less effective than carbendazim against C. heterostrophus and F. moniliforme. However,the fungicidal activities of other title compounds are not notable.
|
|
Table 4 Anti-fungal activity (inhibition rate,%) of title compounds at 50 μg/mL |
|
|
Table 5 Insecticidal activity(lethality rates,%)of title compounds |
2.3 Insecticidal activity
The insecticidal activity was evaluated against M. separata and C.pipiens pallens. Chloratraniliprole was used as control. We found that the insecticidal activities of most new compounds against M. separata were over 50% at the concentration of 200 mg/L. Among them,compounds 6e and 6k showed higher activities. Most of the target compounds also exhibited insecticidal activity against C. pipiens pallens at the concentration of 2 mg/L. Compounds 6h,6i and 6k showed higher lethality rates against C. pipiens pallens.
In conclusion,a series of 2,8-diazabicyclo [4.3.0] nonane containing benzimidazole moiety has been synthesized and characterized. All 12 compounds showed moderate antifungal activity against seven pathogenic fungi and moderate insecticidal activity against M. separata and C. pipiens pallens. 2,8-Diazabicyclo [4.3.0] nonane and benzimidazole are developed and applied widely in the field of fungicide. Interestingly,to a certain extent,these novel structures exhibited insecticidal activity,which means they are worth for our further modification.
| [1] | LAI S H. Pharmacological property and recent progress in application of moxifloxacin [J]. Evaluat Anal Drug Use Hospit China, 2010, 10 (8) :682–683 . |
| [2] | LU D Q, WANG W B, LING X Q, et al. Progress in synthesis and applications of moxifloxacin hydrochloride [J]. Mod Chem Ind, 2014, 34 (2) :33–37 . |
| [3] | GAO J H. Study on synthesis of key intermediate of moxifloxacin[D]. Zhejiang: Zhejiang University of Technology, 2012. |
| [4] | XU L. Synthetic study of intermediate of moxifloxaein drug[D]. Zhejiang: Zhejiang University, 2005. |
| [5] | MENG J P, GENG R X, ZHOU C H, et al. Advances in the research of benzimidazole drugs [J]. Chin J New Drugs, 2009, 18 (16) :1505–1514 . |
| [6] | ZHOU S F. Design, synthesis, antibacterial of new benzimidazole derivatives[D]. Taian: Shandong Agricultural University, 2012. |
| [7] | MWNG J P, LU Y H, IBRAYIM H, et al. Advances in the research of benzimidazole compounds as enzyme inhibitors [J]. Chin J Biochem Pharmaceut, 2008, 29 (6) :418–421 . |
| [8] | KHALAFI-NEZHAD A, SOLTANI RAD M N, MOHABATKAR H, et al. Design, synthesis, antibacterial and QSAR studies of benzimidazole and imidazole chloroaryloxyalkyl derivatives [J]. Bioorg Med Chem, 2005, 13 (6) :1931–1938 . |
| [9] | GVEN , ERDOĞAN T, GKER H, et al. Synthesis and antimicrobial activity of some novel phenyl and benzimidazole substituted benzyl ethers [J]. Bioorg Med Chem Lett, 2007, 17 (8) :2233–2236 . |
| [10] | ZHANG Y, YANG S, SONG B A, et al. Recent advances in the antimicrobial activity of benzimidazole compounds [J]. Agrochemicals, 2008, 47 (3) :164–170 . |
| [11] | GKER H, ZDEN S, YILDIZ S, et al. Synthesis and potent antibacterial activity against MRSA of some novel 1, 2-disubstituted-1 H-benzimidazole-N-alkylated-5-carboxamidines [J]. Eur J Med Chem, 2005, 40 (10) :1062–1069 . |
| [12] | LI Y, MA H Q, WANG Y L. Progress in the synthesis and application of benzimidazoles and their derivatives [J]. Chin J Org Chem, 2008, 28 (2) :210–217 . |
| [13] | OHEMENG K A, NGUYEN V N. 2-substituted phenyl-benzimidazole antibacterial agents: US 5942532 A[P]. 1999-08-24. |
| [14] | SONG L, XIE Y Y, WANG H Z. Synthesis and antibacterial activity of benzimidazole schiff base [J]. Chin J Med Chem, 2000, 10 (2) :92–94 . |
| [15] | JIANG H Y, YIN Y B, ZHANG H B, et al. The synthesis and characteristics of 2-chloromethyl benzimidazole [J]. New Chem Mater, 2011, 39 (5) :74–81 . |
| [16] | JIA J H, CUI Y H, HAN L, et al. Syntheses, third-order optical nonlinearity and DFT studies on benzoylferrocene derivatives [J]. Dyes Pigm, 2014, 104 :137–145 . |
| [17] | LI X H, CUI Z N, CHEN X Y, et al. Synthesis of 2-acyloxycyclohexylsulfonamides and evaluation on their fungicidal activity [J]. Int J Mol Sci, 2013, 14 (11) :22544–22557 . |
| [18] | BAI Y B, ZHANG A L, TANG J J, et al. Synthesis and antifungal activity of 2-chloromethyl-1H benzimidazole derivatives against phytopathogenic fungi in vitro [J]. J Agric Food Chem, 2013, 61 (11) :2789–2795 . |
| [19] | STABILE P, LAMONICA A, RIBECAI A, et al. Mild, convenient and versatile Cu-mediated synthesis of N-aryl-2-imidazolidinones [J]. Tetrahedron Lett, 2010, 51 (24) :3232–3235 . |
| [20] | BOLLIGER J L, FRECH C M. Transition metal-free amination of aryl halides—A simple and reliable method for the efficient and high-yielding synthesis of N-arylated amines [J]. Tetrahedron, 2009, 65 (6) :1180–1187 . |
| [21] | CHEN N C. Bioassay technology for pestcides[M]. Beijing: Beijing Agricultural University Press, 1991 : 161 . |
| [22] | WANG B L, MA Y, XIONG L X, et al. Synthesis and insecticidal activity of novel N-pyridylpyrazole carbonyl thioureas [J]. Chin J Chem, 2012, 30 (4) :815–821 . |
 2016, Vol. 18
2016, Vol. 18