文章信息
- 季书立, 贠阳, 贾沛璐, 甘振丁, 张昊, 张莉莉, 王恬
- JI Shuli, YUN Yang, JIA Peilu, GAN Zhending, ZHANG Hao, ZHANG Lili, WANG Tian
- 竹叶提取物对哺乳仔猪回肠形态和抗氧化功能的影响
- Effects of bamboo leaf extract on the morphological structure and antioxidant function in ileum of suckling piglets
- 南京农业大学学报, 2021, 44(4): 733-739
- Journal of Nanjing Agricultural University, 2021, 44(4): 733-739.
- http://dx.doi.org/10.7685/jnau.202009019
-
文章历史
- 收稿日期: 2020-09-21
氧化应激是内、外环境因素诱导机体氧化系统和抗氧化系统失衡的状态[1]。母猪在分娩时, 仔猪从低氧的宫内环境进入高氧的宫外环境, 引起血液氧分压骤变、细胞内活性氧(reactive oxygen species, ROS)爆发式增多、氧化还原失衡, 造成大脑、肝脏和肠道等代谢旺盛组织的氧化损伤, 导致新生仔猪发病率和死亡率升高[2-3]。
肠道组织既是营养物质消化、吸收和代谢的主要器官, 也是最大的免疫器官, 对机体抵御病原体及有害物质的侵袭起着重要作用[4]。在新生阶段, 仔猪肠道形态结构和生理功能尚不完善, 对各种应激反应高度敏感, 易导致肠道损伤和多种疾病的发生, 严重影响仔猪的生长发育[5]。添加适宜的抗氧化活性物质是缓解仔猪肠道损伤、促进肠道发育的一种有效方法[6-8]。
近年的研究表明, 黄酮类物质是一种高效的抗氧化剂, 能够有效缓解由氧化应激引起的肠道损伤, 改善肠道完整性, 提高其抗氧化能力[9-10]。竹叶提取物(bamboo leaf extract, BLE)是一种源于竹叶、富含黄酮和多酚的天然活性物质, 具有抗氧化和抑菌等多种生物学功效, 被广泛应用于食品和化妆品行业[11-12]。研究表明, 竹叶黄酮提取物可通过上调核转录因子E2相关因子2(nuclear factor erythroid 2-related factor 2, Nrf2)和血红素氧合酶1(heme oxygenase-1, HO-1)的表达缓解油酸诱导的HepG2细胞的氧化应激[13]。BLE还可以提高小鼠血清和肝脏的抗氧化酶活性, 降低丙二醛(malondialdehyde, MDA)含量, 减少氧化应激引起的急性胃黏膜损伤和肝损伤[14-15]。近年来, BLE更是以其丰富的原料来源、明确的功能因子、良好的食用安全性, 在饲料添加剂领域崭露头角。Shen等[16]研究发现, 日粮中添加BLE能有效提高肉鸡肌肉清除自由基的能力和抗氧化酶的活性。粟明月等[17]报道, 奶牛日粮中添加BLE能显著降低血浆中的MDA含量。但BLE对哺乳仔猪肠道健康影响的研究尚未见报道。在前期研究中我们发现, 灌喂适宜水平的BLE能在一定程度上促进哺乳仔猪的生长, 但其潜在机制尚不明确。鉴于初生仔猪易受环境影响发生肠道氧化应激, 阻碍仔猪健康发育, 故本试验旨在研究BLE对哺乳仔猪回肠形态和抗氧化性能的影响, 以期为BLE作为抗氧化剂在生猪养殖中的应用提供参考。
1 材料与方法 1.1 材料BLE由浙江新篁生物科技有限公司提供, 其有效成分包括总黄酮70 mg·g-1、总多酚50.42 mg·g-1。
1.2 试验动物与饲养管理本试验在苏州安佑太仓种猪场进行。试验选取体重相近、胎次相同的3日龄雄性仔猪64头, 随机分为4组, 每组8个重复, 每个重复2头仔猪。对照组(CON组)仔猪每天灌喂羧甲基纤维素钠溶液10 mL, 试验组分别灌喂含100、200和300 mg·kg-1 BLE的羧甲基纤维素钠溶液10 mL, 试验期21 d。试验期间仔猪自由采食母乳和饮水, 并按照猪场的正常免疫措施等进行饲养管理。
1.3 样品采集试验结束时, 从每个重复中随机选取一头仔猪, 前腔静脉采集血液, 4 ℃、3 000 r·min-1离心10 min, 分离血清, 保存于-80 ℃冰箱备用。腹腔注射戊巴比妥钠溶液, 放血致死后剖开腹腔, 分离回肠。从回肠中段取2 cm肠段纵向切开后, 先用预冷的生理盐水冲洗内容物, 再置于4%的多聚甲醛中固定, 用于形态学观察。用无菌载玻片刮取回肠黏膜, 保存于-80 ℃冰箱备用。
1.4 测定指标及方法 1.4.1 肠道组织形态学观察将保存于4%多聚甲醛固定液中的回肠组织用不同梯度的乙醇溶液干燥处理。经二甲苯洗净, 石蜡包埋, 制备成5 μm的切片后, 用苏木精和伊红染色, 置于光学双目显微镜下观察。每张切片选取20个完整的绒毛和隐窝, 使用Image-Pro Plus软件(Media Cybernetics, USA)测量其绒毛高度(villi height, VH)和隐窝深度(crypt depth, CD), 计算其比值(VH/CD)。
1.4.2 氧化损伤以及抗氧化相关指标测定取适量回肠黏膜按照1∶4(m/v, 质量体积比)的比例加入预冷的生理盐水, 冰水浴中破碎、匀浆后, 3 500 r·min-1离心10 min, 取上清液保存于-20 ℃备用。回肠黏膜匀浆和血清中的总蛋白含量采用二喹啉甲酸(BCA)法测量。MDA、谷胱甘肽(glutathione, GSH)含量和总抗氧化能力(total antioxidant capacity, T-AOC)以及总超氧化物歧化酶(superoxide dismutase, SOD)、过氧化氢酶(catalase, CAT)、谷胱甘肽过氧化物酶(glutathione peroxidase, GSH-Px)活性均采用试剂盒(南京建成生物科技有限公司)进行测量。
1.4.3 回肠黏膜RNA的提取和mRNA表达量的测定称取0.1 g回肠黏膜样品, 加入1 mL Trizol后按照说明书提取回肠黏膜总RNA。经NanoDrop1000(Thermo Scientific, UK)进行纯度和浓度的检测后, 再用15 g·L-1琼脂糖凝胶电泳检测RNA的完整性。用反转录试剂盒将RNA反转录为cDNA, 于-20 ℃保存。使用SYBR Premix Ex Taq试剂盒(TaKaRa, 大连)进行实时荧光定量PCR(qPCR)反应。qPCR程序: 95 ℃ 30 s, 95 ℃ 5 s, 60 ℃ 30 s, 40个循环。目的基因的相对表达含量以β-actin为内参, 采用2-ΔΔCT法计算。引物序列见表 1。
| 基因Gene | 引物对序列Primers pairs sequence(5′→3′) | 登录号Accession No. |
| β-actin | TCATGGACTCTGGGGATGGG/GCAGCTCGTAGCTCTTCTCC | DQ_845171.1 |
| Nrf2 | GATGTTGCAGCAGGAAACAGG/GAAAGCCCAAATGGTCCCAG | NM_001185152.1 |
| SOD2 | GGACAAATCTGAGCCCTAACG/CCTTGTTGAAACCGAGCC | NM_214127 |
| GSH-Px | CAAGTCCTTCTACGACCTCA/GAAGCCAAGAACCACCAG | NM_001115136 |
| HO-1 | TACCGCTCCCGAATGAACAC/TGGTCCTTAGTGTCCTGGGT | NM_001004027.1 |
| GR | GTGAGCCGACTGAACACCAT/CAGGATGTGAGGAGCTGTGT | AY368271 |
| CAT | CTGTAAGGCTAGTCGGACACC/ATATCAGGTTTCTGCGCGGC | NM_214301.2 |
| 注: Nrf2: 核转录因子E2相关因子2基因Nuclear factor erythroid 2-related factor 2 gene; SOD2: 超氧化物歧化酶基因2Superoxide dismutase 2 gene; GSH-Px: 谷胱甘肽过氧化物酶基因Glutathione peroxidase gene; HO-1: 血红素氧合酶1基因Heme oxygenase 1 gene; GR: 谷胱甘肽还原酶基因Glutathione reductase gene; CAT: 过氧化氢酶基因Catalase gene. | ||
将冷冻的回肠黏膜切成小块, 用蛋白裂解液RIPA和苯甲磺酰氟研磨, 提取冷冻肠黏膜中的蛋白质, 并用BCA法测定蛋白浓度。将蛋白样品平衡至统一浓度后, 加入等体积的上样缓冲液进行变性处理, 样品-80 ℃保存。
用十二烷基硫酸钠-聚丙烯酰胺凝胶电泳(SDS-PAGE)对蛋白样品进行电泳, 在300 mA条件下将蛋白湿转至聚偏二氟乙烯膜上。在室温条件下用50 g·L-1脱脂奶粉封闭膜2 h后, 用含有0.1%吐温20的三羟甲基氨基甲烷-盐酸缓冲溶液(TBST)清洗干净, 再依次经4 ℃一抗孵育过夜, 室温二抗孵育, TBST清洗, 显影液浸泡, 最后用天能4600SF全自动化学发光图像分析系统(上海天能科技有限公司)扫描, 扫描图像用Image-Pro Plus软件进行量化分析。
1.5 数据统计与分析数据经Excel 2010初步处理后, 采用SPSS 20.0软件进行单因素方差分析, 用Tukey法进行差异显著性比较。试验结果数据用平均值±标准误(x±SE)表示。
2 结果与分析 2.1 竹叶提取物对哺乳仔猪回肠黏膜形态的影响由图 1和表 2可知: 灌喂BLE改善了哺乳仔猪回肠绒毛形态。与CON组相比, 灌喂BLE可提高仔猪回肠绒毛高度(VH), 其剂量为300 mg·kg-1时效果显著(P < 0.05), 但对隐窝深度(CD)无显著影响(P>0.05)。另外, 300 mg·kg-1 BLE组仔猪VH/CD值相对于CON组显著提高(P < 0.05)。说明BLE能促进仔猪回肠发育。
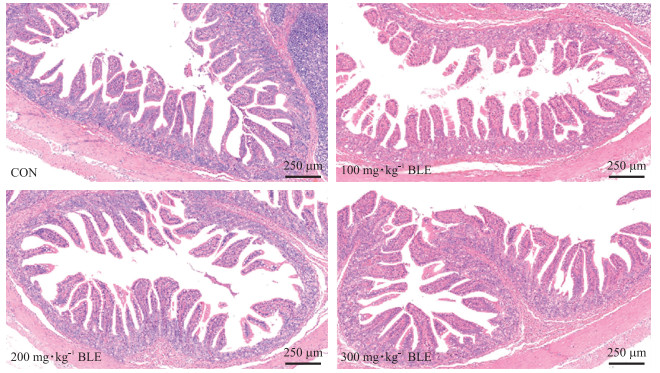
|
图 1 竹叶提取物(BLE)对哺乳仔猪回肠绒毛形态的影响 Fig. 1 Effects of dietary supplementation with bamboo leaf extrat(BLE)on histologic morphology in the ileum of suckling piglets |
| 指标Items | 灌喂BLE剂量/(mg·kg-1)Addition levels of BLE | |||
| 0(CON) | 100 | 200 | 300 | |
| 绒毛高度/μm Villi height(VH) | 315.05±10.11 | 342.60±23.54 | 372.75±13.30 | 394.00±19.59* |
| 隐窝深度/μm Crypt depth(CD) | 131.22±6.90 | 128.26±12.36 | 124.12±7.40 | 148.19±9.71 |
| VH/CD | 2.39±0.15 | 2.75±0.23 | 3.04±0.14* | 2.75±0.09 |
| 注: *表示与对照组相比差异显著(P < 0.05)。下同。 Note: *means significant difference at 0.05 level when compared with CON group. The same as follows. | ||||
由表 3可知: 与CON组相比, 200和300 mg·kg-1 BLE组仔猪血清中MDA含量均显著降低(P < 0.05)。此外, 200和300 mg·kg-1 BLE组仔猪血清中T-AOC和GSH-Px活性较CON组均显著提高(P < 0.05)。
| 指标Items | 灌喂BLE水平/(mg·kg-1)Addition levels of BLE | |||
| 0(CON) | 100 | 200 | 300 | |
| MDA含量/(nmol·mL-1)Content of MDA | 3.82±0.43 | 2.76±0.29 | 2.22±0.13* | 2.41±0.29* |
| SOD活性/(U·mL-1)Activity of SOD | 141.05±2.59 | 148.60±3.39 | 150.00±1.57 | 146.23±1.65 |
| GSH含量/(mg·L-1)Content of GSH | 0.46±0.09 | 0.50±0.07 | 0.57±0.07 | 0.52±0.08 |
| GSH-Px活性/(U·mL-1)Activity of GSH-Px | 194.44±7.00 | 198.71±7.33 | 230.05±8.52* | 236.66±8.99* |
| T-AOC活性/(U·mL-1)Activity of T-AOC | 10.82±1.62 | 15.15±1.68 | 20.20±1.14* | 16.49±1.21* |
| 注: MDA: 丙二醛Malondialdehyde; SOD: 超氧化物歧化酶Superoxide dismutase; GSH: 谷胱甘肽Glutathione; GSH-Px: 谷胱甘肽过氧化物酶Glutathione peroxidase; T-AOC: 总抗氧化能力Total antioxidant capacity. 下同。The same as follows. | ||||
由表 4可知: 与CON组相比, 灌喂BLE能显著降低哺乳仔猪回肠黏膜MDA含量(P < 0.05)。300 mg·kg-1 BLE组仔猪回肠黏膜SOD和GSH-Px活性相对于CON组显著提高(P < 0.05), 200 mg·kg-1 BLE组仔猪回肠黏膜中GSH-Px活性相对于CON组显著提高(P < 0.05), 但对CAT和T-AOC活性影响不显著(P < 0.05)。
| 指标Items | 灌喂BLE水平/(mg·kg-1)Addition levels of BLE | |||
| 0(CON) | 100 | 200 | 300 | |
| MDA含量/(nmol·mg-1)Content of MDA | 1.00±0.12 | 0.62±0.11* | 0.57±0.11* | 0.48±0.09* |
| SOD活性/(U·mg-1)Activity of SOD | 251.42±6.49 | 263.14±4.23 | 271.09±7.24 | 280.64±5.23* |
| GSH含量/(mg·g-1)Content of GSH | 7.96±0.82 | 11.51±1.45 | 9.69±1.29 | 8.06±0.85 |
| GSH-Px活性/(U·mg-1)Activity of GSH-Px | 60.61±6.24 | 73.64±10.89 | 87.19±5.16* | 91.41±4.85* |
| CAT活性/(U·mg-1)Activity of CAT | 1.96±0.27 | 1.84±0.20 | 2.26±0.15 | 1.86±0.18 |
| T-AOC活性/(U·mg-1)Activity of T-AOC | 0.59±0.02 | 0.69±0.08 | 0.65±0.06 | 0.58±0.01 |
由图 2可以看出: 与CON组相比, 200和300 mg·kg-1 BLE组仔猪回肠黏膜中Nrf2和GSH-Px mRNA表达水平均显著提高(P < 0.05)。同时, 与CON组相比, 300 mg·kg-1 BLE组仔猪回肠黏膜中SOD2和HO-1 mRNA表达量也均显著提高(P < 0.05), 而灌喂BLE对哺乳仔猪回肠黏膜中CAT和GR mRNA表达没有显著影响(P>0.05)。
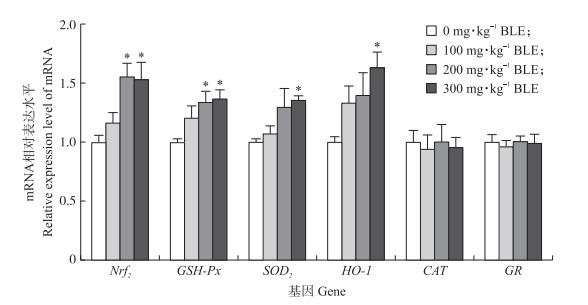
|
图 2 BLE对哺乳仔猪回肠黏膜抗氧化基因mRNA表达量的影响 Fig. 2 Effects of dietary supplementation with BLE on antioxidant gene expression level in the ileum mucosa of suckling piglets |
由图 3可以看出: 与CON组相比, 200和300 mg·kg-1 BLE组仔猪回肠黏膜Nrf2和SOD2蛋白表达水平均显著提高(P < 0.05), 表明BLE可以激活Nrf2蛋白并上调其下游SOD2蛋白的表达。

|
图 3 BLE对哺乳仔猪回肠黏膜Nrf2和SOD2蛋白表达量的影响 Fig. 3 Effects of dietary supplementation with BLE on the protein expression levels of Nrf2 and SOD2 in the ileum mucosa of suckling piglets |
小肠作为机体营养物质消化吸收代谢的主要器官, 对动物体的生长发育起着重要作用。尤其在哺乳阶段仔猪尚不成熟的肠道形态结构和生理功能将迅速发育, 其健康状况决定了仔猪的存活率及后期生长速率[18]。氧化应激会引起肠上皮细胞的凋亡, 损伤肠道绒毛的完整性, 影响肠道功能[19-20]。诸多研究表明, 改善动物肠道氧化还原平衡对促进绒毛形态和组织发育具有积极意义[21-22]。本试验结果显示, 灌喂BLE能提高哺乳仔猪回肠VH和VH/CD值。肠道绒毛的增长与上皮细胞的发育和成熟密切相关, 在生长阶段, 大量细胞由隐窝迁移至绒毛顶端, 促使绒毛增长。这说明BLE可能通过提高仔猪回肠肠道抗氧化能力, 促进仔猪肠道黏膜上皮细胞的发育, 从而促进肠道绒毛的生长[23]。另一方面, 小肠VH和VH/CD值越大, 说明肠上皮表面积越大, 肠道的消化吸收能力就会越强[24]。这意味着BLE具有促进回肠绒毛发育, 提高仔猪消化能力的作用, 这与前人的研究结果一致[25]。
对于需氧生物来说, 一定浓度的氧化剂可以促进其生长、细胞分化、凋亡和免疫等各种生命活动[26]。但在新生阶段, 动物机体发育不完善, 抗氧化机能不健全, 很容易受到外界环境影响导致应激反应的发生。由此产生的大量ROS堆积在体内得不到清除, 导致机体氧化还原系统的平衡被打破, 并造成氧化损伤[27]。
MDA作为脂质氧化的主要产物之一, 其水平的高低常被用来衡量机体氧化损伤的程度[28]。本试验结果显示, 灌喂200或300 mg·kg-1 BLE均能有效降低仔猪血清和回肠黏膜中的MDA含量, 这可能与黄酮类和多酚类植物提取物可以通过清除自由基和螯合过渡金属离子来降低脂质氧化的能力有关[29]。此外, 灌喂300 mg·kg-1 BLE能够显著提高仔猪血清中的T-AOC、SOD和GSH-Px活性以及回肠黏膜中的SOD和GSH-Px活性, 从而提高机体的抗氧化水平。
还有研究表明, 许多黄酮类、多酚类植物衍生物可以作为Nrf2通路的潜在诱导剂[30-31]。Nrf2是氧化还原稳态的关键调节因子, 负责调节细胞防御机制对抗氧化应激。正常生理状态下, Nrf2被其负调控因子Kelch样环氧氯丙烷相关蛋白1锚定在细胞质中。当机体发生氧化应激时, Nrf2被激活并移位至细胞核中, 与小Maf蛋白聚合形成二聚体后再与抗氧化反应元件结合, 调控其下游Ⅱ相解毒酶和抗氧化酶基因的表达[32]。本试验结果显示, 灌喂200和300 mg·kg-1的BLE均能显著上调哺乳仔猪回肠黏膜Nrf2 mRNA和蛋白的表达, 并促进其下游HO-1、GSH-Px和SOD2等抗氧化基因的表达。HO-1能够催化铁与血红素基团结合为胆绿素, 降低血红素基团促氧能力。胆绿素的生成能够有效清除过氧化物和超氧化物自由基, 降低氧化损伤的发生[33]。这表明BLE能够激活Nrf2并调节其相关抗氧化酶的表达, 提高机体的抗氧化能力。Shen等[34]发现日粮中添加BLE可以上调肉鸡肝脏中SOD和GSH-Px mRNA的表达, 提高肝脏抗氧化能力。本试验结果与上述研究结果相一致。
综上所述, 灌喂BLE促进了哺乳仔猪肠道绒毛形态结构的发育, 这可能是因为BLE通过激活Nrf2信号通路, 提高哺乳仔猪回肠抗氧化能力, 从而缓解出生应激带来的肠道损伤, 有利于仔猪肠道及机体的健康发育。但有关BLE对仔猪回肠炎症水平的影响以及仔猪回肠抗炎能力和肠道发育之间是否存在相关性仍不明确, 需进一步研究。
| [1] |
Pizzino G, Irrera N, Cucinotta M, et al. Oxidative stress: harms and benefits for human health[J]. Oxidative Medicine and Cellular Longevity, 2017, 2017: 1-13. |
| [2] |
Muller D P. Free radical problems of the newborn[J]. The Proceedings of the Nutrition Society, 1987, 46(1): 69-75. DOI:10.1079/PNS19870009 |
| [3] |
Gill R S, Lee T F, Liu J Q, et al. Cyclosporine treatment reduces oxygen free radical generation and oxidative stress in the brain of hypoxia-reoxygenated newborn piglets[J]. PLoS One, 2012, 7(7): e40471. DOI:10.1371/journal.pone.0040471 |
| [4] |
Sangild P T, Thymann T, Schmidt M, et al. Invited review: the preterm pig as a model in pediatric gastroenterology[J]. Journal of Animal Science, 2013, 91(10): 4713-4729. DOI:10.2527/jas.2013-6359 |
| [5] |
Smith W J. The neonatal pig: development and survival[J]. Preventive Veterinary Medicine, 1997, 31(1/2): 153-154. |
| [6] |
王彬, 刘俊泽, 陈志杰, 等. 甘蔗提取物对敌草快诱导断奶仔猪氧化损伤的防护效果及肠道微生物参与的机制[J]. 南京农业大学学报, 2020, 43(3): 514-522. Wang B, Liu J Z, Chen Z J, et al. Protective effects of sugar cane extract against oxidative damage induced by diquat and mechanism mediated by intestinal microbiota in weaned piglets[J]. Journal of Nanjing Agricultural University, 2020, 43(3): 514-522 (in Chinese with English abstract). DOI:10.7685/jnau.201906025 |
| [7] |
Gan Z D, Wei W Y, Wu J M, et al. Resveratrol and curcumin improve intestinal mucosal integrity and decrease m6A RNA methylation in the intestine of weaning piglets[J]. ACS Omega, 2019, 4(17): 17438-17446. DOI:10.1021/acsomega.9b02236 |
| [8] |
Zhang H, Li Y, Chen Y P, et al. N-acetylcysteine protects against intrauterine growth retardation-induced intestinal injury via restoring redox status and mitochondrial function in neonatal piglets[J]. European Journal of Nutrition, 2019, 58(8): 3335-3347. DOI:10.1007/s00394-018-1878-8 |
| [9] |
Xiao D F, Yuan D X, Tan B H, et al. The role of Nrf2 signaling pathway in Eucommia ulmoides flavones regulating oxidative stress in the intestine of piglets[J]. Oxidative Medicine and Cellular Longevity, 2019, 2019: 9719618. |
| [10] |
Niu Y, Zhang J F, Wan X L, et al. Effect of fermented Ginkgo biloba leaves on nutrient utilisation, intestinal digestive function and antioxidant capacity in broilers[J]. British Poultry Science, 2019, 60(1): 47-55. DOI:10.1080/00071668.2018.1535166 |
| [11] |
姜文进, 李栋, 黄骆镰, 等. 竹叶抗氧化物作为大黄鱼冷藏保鲜剂的生物学效应研究[J]. 食品工业科技, 2013, 34(5): 325-329. Jiang W J, Li D, Huang L L, et al. Study on biological antioxidant effect of bamboo leaves as a novel refrigeration preservative on large yellow croaker[J]. Science and Technology of Food Industry, 2013, 34(5): 325-329 (in Chinese with English abstract). |
| [12] |
倪向梅. 从竹叶中提取化妆品用防腐剂的研究[D]. 无锡: 江南大学, 2011. Ni X M. The research on extracting cosmetics preservative from bamboo leaves[D]. Wuxi: Jiangnan University, 2011(in Chinese with English abstract). |
| [13] |
Yu Y, Li Z M, Cao G T, et al. Bamboo leaf flavonoids extracts alleviate oxidative stress in HepG2 cells via naturally modulating reactive oxygen species production and Nrf2-mediated antioxidant defense responses[J]. Journal of Food Science, 2019, 84(6): 1609-1620. DOI:10.1111/1750-3841.14609 |
| [14] |
Zhang S, Chen J, Sun A D, et al. Protective effects and antioxidant mechanism of bamboo leaf flavonoids on hepatocytes injured by CCl4[J]. Food and Agricultural Immunology, 2014, 25(3): 386-396. DOI:10.1080/09540105.2013.810709 |
| [15] |
黄进波, 萧钦, 周佳佳, 等. 竹叶黄酮对乙醇诱导小鼠急性胃黏膜损伤的保护作用[J]. 现代药物与临床, 2015, 30(7): 779-783. Huang J B, Xiao Q, Zhou J J, et al. Protection of bamboo leaf flavonoids on ethanol-induced acute gastric mucosal injury in mice[J]. Drugs & Clinic, 2015, 30(7): 779-783 (in Chinese with English abstract). |
| [16] |
Shen M M, Zhang L L, Chen Y N, et al. Effects of bamboo leaf extract on growth performance, meat quality, and meat oxidative stability in broiler chickens[J]. Poultry Science, 2019, 98(12): 6787-6796. DOI:10.3382/ps/pez404 |
| [17] |
栗明月, 方洛云, 苏汉书, 等. 竹叶提取物对奶牛泌乳性能、血液常规指标、免疫和抗氧化性能的影响[J]. 动物营养学报, 2019, 31(7): 3302-3309. Li M Y, Fang L Y, Su H S, et al. Effects of bamboo leaf extracts on lactation performance, blood routine indexes and immune and antioxidant ability of dairy cows[J]. Chinese Journal of Animal Nutrition, 2019, 31(7): 3302-3309 (in Chinese with English abstract). DOI:10.3969/j.issn.1006-267x.2019.07.043 |
| [18] |
Barszcz M, Skomiał J. The development of the small intestine of piglets-chosen aspects[J]. Journal of Animal and Feed Sciences, 2011, 20(1): 3-15. DOI:10.22358/jafs/66152/2011 |
| [19] |
Yuan D X, Hussain T, Tan B, et al. The evaluation of antioxidant and anti-inflammatory effects of Eucommia ulmoides flavones using diquat-challenged piglet models[J]. Oxidative Medicine and Cellular Longevity, 2017, 2017: 8140962. |
| [20] |
Yin J, Liu M F, Ren W K, et al. Effects of dietary supplementation with glutamate and aspartate on diquat-induced oxidative stress in piglets[J]. PLoS One, 2015, 10(4): e0122893. |
| [21] |
王斐, 何进田, 沈明明, 等. 日粮添加姜黄素对宫内发育迟缓断奶仔猪生长性能和肠道组织形态的影响[J]. 畜牧与兽医, 2019, 51(6): 23-30. Wang F, He J T, Shen M M, et al. Effects of curcumin supplementation on growth performance and intestinal histomorphology of weaned piglets with intrauterine growth retardation[J]. Animal Husbandry and Veterinary Medicine, 2019, 51(6): 23-30 (in Chinese with English abstract). |
| [22] |
Zheng Y W, Zhang J Y, Zhou H B, et al. Effects of dietary pyrroloquinoline quinone disodium supplementation on inflammatory responses, oxidative stress, and intestinal morphology in broiler chickens challenged with lipopolysaccharide[J]. Poultry Science, 2020, 99(11): 5389-5398. DOI:10.1016/j.psj.2020.08.007 |
| [23] |
Dunsford B R, Knabe D A, Haensly W E. Effect of dietary soybean meal on the microscopic anatomy of the small intestine in the early-weaned pig[J]. Journal of Animal Science, 1989, 67(7): 1855-1863. |
| [24] |
Pluske J R, Hampson D J, Williams I H. Factors influencing the structure and function of the small intestine in the weaned pig: a review[J]. Livestock Production Science, 1997, 51(1/2/3): 215-236. |
| [25] |
王咏梅, 陈冰, 曹俊明, 等. 桑叶黄酮对凡纳滨对虾肠道黏膜形态和肠道菌群的影响[J]. 动物营养学报, 2020, 32(4): 1817-1825. Wang Y M, Chen B, Cao J M, et al. Effects of mulberry leaf flavonoids on intestinal mucosal morphology and intestinal flora of Litopenaeus vannamei[J]. Chinese Journal of Animal Nutrition, 2020, 32(4): 1817-1825 (in Chinese with English abstract). DOI:10.3969/j.issn.1006-267x.2020.04.040 |
| [26] |
Cano-Europa E, Blas-Valdivia V, Franco-Colin M. Regulation of the redox environment[J]. Plant Cell, 2015, 21(10): 3198-3211. |
| [27] |
Robles R, Palomino N, Robles A. Oxidative stress in the neonate[J]. Early Human Development, 2001, 65: S75-S81. DOI:10.1016/S0378-3782(01)00209-2 |
| [28] |
Pirinccioglu A G, Gökalp D, Pirinccioglu M, et al. Malondialdehyde(MDA) and protein carbonyl(PCO) levels as biomarkers of oxidative stress in subjects with familial hypercholesterolemia[J]. Clinical Biochemistry, 2010, 43(15): 1220-1224. |
| [29] |
Mira L, Tereza Fernandez M, Santos M, et al. Interactions of flavonoids with iron and copper ions: a mechanism for their antioxidant activity[J]. Free Radical Research, 2002, 36(11): 1199-1208. |
| [30] |
Stefanson A L, Bakovic M. Dietary regulation of Keap1/Nrf2/ARE pathway: focus on plant-derived compounds and trace minerals[J]. Nutrients, 2014, 6(9): 3777-3801. |
| [31] |
Zhai X H, Lin M S, Zhang F, et al. Dietary flavonoid genistein induces Nrf2 and phase Ⅱ detoxification gene expression via ERKs and PKC pathways and protects against oxidative stress in Caco-2 cells[J]. Molecular Nutrition & Food Research, 2013, 57(2): 249-259. |
| [32] |
Tu W, Wang H, Li S, et al. The anti-inflammatory and anti-oxidant mechanisms of the Keap1/Nrf2/ARE signaling pathway in chronic diseases[J]. Aging and Disease, 2019, 10(3): 637-651. |
| [33] |
González-Burgos E, Carretero M E, Gómez-Serranillos M P. Diterpenoids isolated from Sideritis species protect astrocytes against oxidative stress via Nrf2[J]. Journal of Natural Products, 2012, 75(10): 1750-1758. |
| [34] |
Shen M M, Xie Z C, Jia M H, et al. Effect of bamboo leaf extract on antioxidant status and cholesterol metabolism in broiler chickens[J]. Animals, 2019, 9(9): 699. |




