文章信息
- 唐荣宏, 范莉莉, 王冬雪, 李亚杰, 郁宏伟, 杨保收, 鲍恩东
- TANG Ronghong, FAN Lili, WANG Dongxue, LI Yajie, YU Hongwei, YANG Baoshou, BAO Endong
- 禽流感DNA疫苗(H5亚型Re-8株)对黄羽肉鸡免疫程序的优化
- The immune program optimization of avian influenza DNA vaccine(H5 subtype Re-8 strain) on Yellow broiler
- 南京农业大学学报, 2021, 44(3): 521-525
- Journal of Nanjing Agricultural University, 2021, 44(3): 521-525.
- http://dx.doi.org/10.7685/jnau.202003008
-
文章历史
- 收稿日期: 2020-03-04
禽流感(avian influenza, AI)是A类禽类烈性传染病之一, 由正黏病毒科的A型流感病毒所引起, 该病毒为单股、负链RNA病毒, 禽类、鸟类、人类及低等哺乳动物为其易感动物[1]。A型流感病毒分为多种亚型, 其中H5、H7亚型属于高致病性强毒株, 由其引发的高致病性禽流感具有发病急、传播速度快、死亡率高等特点。2003年以来, H5亚型高致病性禽流感(highly pathogenic avian influenza, HPAI)在我国多数地区呈现不同程度的爆发, 严重危害养禽业的健康发展[2]。不仅如此, 我国已有HPAI(H5亚型)感染人并致死的报道[3-4]。H5亚型HPAI作为一种人兽共患病, 对人类安全及动物健康具有严重危害, 因此对其防控具有重要的经济及公共卫生意义。
目前我国主要采用免疫接种H5亚型禽流感灭活疫苗的方式来预防禽流感[5-6]。然而, 禽流感灭活疫苗具有干扰临床检疫(检测)及流行病学调查等缺点。作为第3代疫苗的DNA疫苗不仅具有灭活苗的安全性, 不会干扰临床检疫及流行病学调查, 而且还具有以下优点: 1)可诱导机体产生全面的免疫反应——体液免疫和细胞免疫; 2)能不断刺激机体产生抗体, 免疫效力持续时间长[7]; 3)有利于多价苗及多联苗的研制, 疫苗生产简便、易于运输且成本低廉。因此, 越来越多的学者致力于研究禽流感DNA疫苗, 包括其免疫原性、免疫保护效力、与其他类型疫苗联合免疫等方面。与用同一种疫苗免疫相比, 联合免疫更能刺激机体产生更强的体液免疫反应和更高的抗体效价[8-12]。本试验的主要目的在于探索禽流感(H5亚型Re-8株)DNA疫苗用于黄羽肉鸡的合理免疫程序, 及其与H5亚型禽流感灭活苗联合免疫的免疫效果, 为防控H5亚型禽流感提供新方法。
1 材料与方法 1.1 试验动物与受试疫苗210羽1日龄黄羽肉鸡购自温氏公司沧州孵化场。受试疫苗H5禽流感D7+rD8二价灭活疫苗购自广州市华南农大生物药品有限公司(批号1709001);H5亚型禽流感DNA疫苗(pDNA Re-8)由天津瑞普生物技术股份有限公司生物制品研究分院提供(1.0 mg·mL-1, 批号20160722)。
1.2 试验方法 1.2.1 试验分组将1日龄未免疫禽流感疫苗的210羽健康黄羽肉鸡随机均分为A、B、C、D、E、F、G共7组, 每组30羽。具体分组及处理见表 1。
| 组号Group No. | 免疫接种程序Immunization program | |
| 首免First immunization | 二免Secondary immunization | |
| A | 1日龄肌肉注射30 μg pDNA Re-8 1 day old intramuscular injection of 30 μg pDNA Re-8 |
无 None |
| B | 10日龄肌肉注射30 μg pDNA Re-8 10 days old intramuscular injection of 30 μg pDNA Re-8 |
无 None |
| C | 14日龄肌肉注射30 μg pDNA Re-8 14 days old intramuscular injection of 30 μg pDNA Re-8 |
无 None |
| D | 14日龄肌肉注射30 μg pDNA Re-8 14 days old intramuscular injection of 30 μg pDNA Re-8 |
35日龄肌肉注射30 μg pDNA Re-8 35 days old intramuscular injection of 30 μg pDNA Re-8 |
| E | 10日龄肌肉注射30 μg pDNA Re-8 10 days old intramuscular injection of 30 μg pDNA Re-8 |
31日龄颈背部皮下注射0.3 mL H5禽流感D7+rD8二价灭活疫苗31 days old subcutaneous immunization of the cervix and back with 0.3 mL H5 avian influenza D7+rD8 inactivated bivalent vaccine |
| F | 10日龄颈背部皮下注射0.3 mL H5禽流感D7+rD8二价灭活疫苗10 days old subcutaneous immunization of the cervix and back with 0.3 mL H5 avian influenza D7+rD8 inactivated bivalent vaccine | 31日龄颈背部皮下注射0.3 mL H5禽流感D7+rD8二价灭活疫苗31 days old subcutaneous immunization of the cervix and back with 0.3 mL H5 avian influenza D7+rD8 inactivated bivalent vaccine |
| G | 无 | 无 |
| 注: pDNA Re-8为禽流感DNA疫苗(H5亚型, Re-8株)。 Note: pDNA Re-8 is avian influenza DNA vaccine(H5 subtype, RE-8 strain). | ||
A、B和C组受试鸡分别于免疫前及免疫后1、2及3周采集血样; D、E、F组受试鸡于首免后1、2、3周以及加强免疫后1、2、4、6、8、10、12周采集血样; G组(空白对照组)受试鸡于1、7、14、21、28、35、42、49、63、77、91日龄时采集血样。各组受试鸡在各采样时间点均采取翅下静脉方式采集血液, 每羽0.5 mL。分离血清作为待检样品。
1.2.3 抗体检测采用血凝(HA)及血凝抑制(HI)方法检测抗体滴液(HI效价log2)。具体操作按照《中国兽药典》(2005版)所述进行, 即HA试验测出H5亚型禽流感Re-8株血凝价, 根据测定的血凝价配制HI试验所需要的4单位抗原。检测结束后, 对所得结果进行判断: 阳性(HI效价≥4)、阴性(HI效价≤3)、可疑(3 < HI效价 < 4)。对可疑样品应进行重检, 重检HI效价≥4判为阳性, HI效价≤3判为阴性。禽流感抗体HI效价≥4判为免疫合格。
1.3 数据处理抗体滴度(HI效价)采用算数平均数表示; 抗体合格率=合格抗体数/检测数×100%。用GraphPad及SPSS 16.0对试验数据进行统计分析。
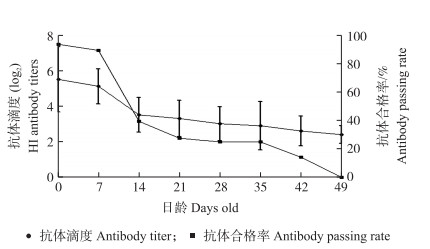
2 结果与分析 2.1 黄羽肉鸡禽流感母源抗体消长规律空白对照G组受试鸡血清样品的抗体滴度(HI效价)效价检测结果如图 1所示。黄羽肉鸡母源抗体HI效价在7~14 d时下降最快(由5.1降至3.5), 抗体合格率也由88.9%降至39.3%。14 d后其母源抗体的保护作用显著降低, 不足以产生对鸡群的免疫保护。
|
图 1 黄羽肉鸡禽流感(H5亚型Re-8株)母源抗体消长规律及母源抗体合格率 Fig. 1 Fluctuation and growth of maternal antibody to avian influenza(H5 subtype RE-8 strain)in Yellow broiler and qualified rate of maternal antibody |
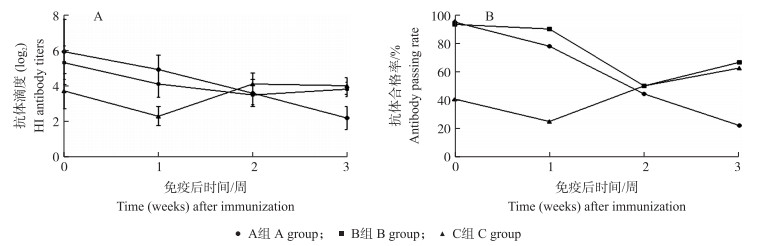
不同日龄(1、10、14日龄)受试鸡单次免疫禽流感DNA疫苗(pDNA Re-8株)后其血清样品抗体滴度检测结果(图 2)所示: C组(14日龄免疫)受试鸡血清抗体的HI效价及合格率在免疫DNA疫苗1周后即出现上升(由2.3升至4.1);B组(10日龄免疫)受试鸡血清抗体HI效价在免疫后2周出现上升(由3.5升至3.8);A组(1日龄组)受试鸡血清抗体HI效价免疫后3周内一直呈现下降趋势。统计学分析结果显示, A、B、C 3组受试鸡血清抗体HI效价及合格率差异均不显著(P>0.05), C组受试鸡呈现出的免疫效果优于A组和B组。
|
图 2 禽流感DNA疫苗(H5亚型Re-8株)单次免疫效果(A)和抗体合格率(B)比较 Fig. 2 Comparison of single immune effect(A)and passing rate of antibody(B)of avian influenza DNA vaccine(H5 subtype RE-8 strain) |
用pDNA Re-8疫苗对黄羽肉鸡进行不同日龄首免后, 再次追加免疫DNA疫苗或二价灭活疫苗, 受试鸡血清禽流感病毒抗体滴度检测结果如图 3所示。由图 3可以看出: F组(10和31日龄2次经颈背部皮下注射H5禽流感D7+rD8二价灭活疫苗)受试鸡血清抗体HI效价最高; 其次是E组受试鸡(10日龄, 肌肉注射pDNA Re-8疫苗, 31日龄时再次经过颈背部皮下注射H5禽流感D7+rD8二价灭活疫苗); 而D组(14和35日龄2次经肌肉注射pDNA Re-8疫苗)受试鸡血清抗体HI效价普遍低于F组和E组。然而, 3个试验组受试鸡血清中相应抗体的持续期均较长(抗体HI效价>5)。F组受试鸡抗体合格率在免疫期一直处于较高水平, D、E组受试鸡血清抗体合格率在免疫后持续增高, 免疫后2周均达到50%以上, 为鸡群提供较好的免疫保护。统计学分析结果显示, D、E 2组受试鸡的抗体HI效价及抗体合格率差异均不显著(P>0.05), 而D、F 2组受试鸡血清抗体HI效价及抗体合格率差异显著(P < 0.05), E、F 2组抗体HI效价及抗体合格率差异均不显著(P>0.05)。因此, F组的免疫效果最佳, 其次是E组, 然后是D组。D、E和F这3组受试鸡在免疫后抗体均能较快升至5.0, 可为鸡群提供长期的免疫保护。

|
图 3 加强免疫对H5禽流感DNA疫苗免疫鸡血清抗体滴度(HI效价, A)及抗体合格率(B)比较 Fig. 3 Comparison of HI titer(A)and passing rate(B)of serum antibody against H5 avian influenza DNA vaccine immunized chicken by enhanced immunization |
近年来, AI新毒株在中国广泛传播并导致人感染[1, 13], 其中至少包含6个亚型(H5N1、H6N1、H7N9、H9N2、H10N8和H5N6)的AIV[14]。以常用的H5N1亚型禽流感灭活疫苗应对新出现的AIV或许已不能满足AI防控的要求了。目前关于DNA疫苗的研究较多, 已显示出其在传染病预防方面的重要性[15-16]。因此, 本文选取禽流感(H5亚型, Re-8株)DNA疫苗与在市面上销售的H5禽流感D7+rD8二价灭活疫苗进行免疫效果的评价与分析, 为DNA疫苗的实际应用提供试验依据。
大部分灭活疫苗的免疫效果会受母源抗体的影响, 而DNA疫苗的免疫效果不受母源抗体的影响[17]。为更加贴近生产实际, 本研究将单次免疫禽流感疫苗的受试鸡日龄设为1、10和14日龄, 试验结果显示黄羽肉鸡免疫禽流感DNA疫苗的最佳日龄为14日龄, 其次为10日龄, 这一结果与文献[18]的结果不同。究其原因, 可能是随着雏鸡不断成长, 其免疫系统发育愈发成熟, 肌肉组织处于快速发育阶段, 进入肌纤维的DNA质粒及表达的抗原越多, 免疫系统对抗原的递呈越有效、应答越快[19-24], 当低日龄进行DNA疫苗免疫时, 雏鸡母源抗体过高会中和DNA疫苗抗原。
为探究较优的免疫程序以实现对黄羽肉鸡提供较好保护力, 本研究将DNA疫苗与市售禽流感二价疫苗进行单独及联合免疫, 结果显示, E组受试鸡血清抗体HI效价和抗体合格率在免疫期的前期低于F组, 但其抗体水平足以达到保护鸡群免于AIV感染, 且2组间的差异不显著, 说明禽流感DNA疫苗与目前市售灭活苗联合免疫可以给鸡群提供良好免疫保护。本试验中, D组受试鸡免疫效果整体比E组及F组差, 但其抗体HI效价大于4, 免疫合格率大于50%, 也可实现对鸡群的免疫保护。
通过对黄羽肉鸡禽流感DNA疫苗(H5亚型Re-8株)免疫效果和免疫程序研究, 我们初步认为以下2种免疫程序可以获得对禽流感较佳的免疫保护效果: 第1种为14日龄首免禽流感(H5亚型Re-8株)DNA疫苗, 每羽30 μg, 21 d后同等剂量加强免疫, 其所产生的HI抗体足够保护鸡群免于AIV的感染; 第2种为10日龄首免禽流感DNA疫苗, 每羽30 μg, 21 d后免疫禽流感H5亚型灭活苗0.3 mL, 受试鸡产生的抗体高, 持续期长。
| [1] |
Freidl G S, Meijer A, de Bruin E, et al. Influenza at the animal-human interface: a review of the literature for virological evidence of human infection with swine or avian influenza viruses other than A(H5N1)[J]. European Communicable Disease Bulletin, 2014, 19(18): 20793. |
| [2] |
Chen H L. H5N1 avian influenza in China[J]. Science in China Series C: Life Sciences, 2009, 52(5): 419-427. DOI:10.1007/s11427-009-0068-6 |
| [3] |
Shu Y L, Yu H J, Li D X. Lethal avian influenza A(H5N1) infection in a pregnant woman in Anhui Province, China[J]. The New England Journal of Medicine, 2006, 354(13): 1421-1422. DOI:10.1056/NEJMc053524 |
| [4] |
Korteweg C, Gu J. Pathology, molecular biology, and pathogenesis of avian influenza A(H5N1) infection in humans[J]. The American Journal of Pathology, 2008, 172(5): 1155-1170. DOI:10.2353/ajpath.2008.070791 |
| [5] |
Chen H. Avian influenza vaccination: the experience in China[J]. Revue Scientifique et Technique, 2009, 28(1): 267-274. DOI:10.20506/rst.28.1.1860 |
| [6] |
Tian G B, Zhang S H, Li Y B, et al. Protective efficacy in chickens, geese and ducks of an H5N1-inactivated vaccine developed by reverse genetics[J]. Virology, 2005, 341(1): 153-162. DOI:10.1016/j.virol.2005.07.011 |
| [7] |
Robinson H L, Boyle C A, Feltquate D M, et al. DNA immunization for influenza virus: studies using hemagglutinin- and nucleoprotein-expressing DNAs[J]. The Journal of Infectious Diseases, 1997, 176(Suppl 1): S50-S55. |
| [8] |
Oveissi S, Omar A R, Yusoff K, et al. DNA vaccine encoding avian influenza virus H5 and Esat-6 of Mycobacterium tuberculosis improved antibody responses against AIV in chickens[J]. Comparative Immunology, Microbiology and Infectious Diseases, 2010, 33(6): 491-503. DOI:10.1016/j.cimid.2009.08.004 |
| [9] |
Jiang Y P, Yu K Z, Zhang H B, et al. Enhanced protective efficacy of H5 subtype avian influenza DNA vaccine with Codon optimized HA gene in a pCAGGS plasmid vector[J]. Antiviral Research, 2007, 75(3): 234-241. DOI:10.1016/j.antiviral.2007.03.009 |
| [10] |
Li K, Gao H L, Gao L, et al. Enhancement of humoral and cellular immunity in chickens against reticuloendotheliosis virus by DNA prime-protein boost vaccination[J]. Vaccine, 2013, 31(15): 1944-1949. DOI:10.1016/j.vaccine.2013.02.010 |
| [11] |
Gao H L, Li K, Gao L, et al. DNA prime-protein boost vaccination enhances protective immunity against infectious bursal disease virus in chickens[J]. Veterinary Microbiology, 2013, 164(1/2): 9-17. |
| [12] |
Khurana S, Wu J, Dimitrova M, et al. DNA priming prior to inactivated influenza A(H5N1) vaccination expands the antibody epitope repertoire and increases affinity maturation in a boost-interval-dependent manner in adults[J]. The Journal of Infectious Diseases, 2013, 208(3): 413-417. DOI:10.1093/infdis/jit178 |
| [13] |
郝珊珊, 郑阳, 余远楠, 等. H9N2亚型禽流感病毒NA和M2基因的克隆与表达[J]. 畜牧与兽医, 2019, 51(5): 121-127. Hao S S, Zheng Y, Yu Y N, et al. Cloning and prokaryotic expression of NA and M2 genes of H9N2 subtype avian influenza virus[J]. Animal Husbandry & Veterinary Medicine, 2019, 51(5): 121-127 (in Chinese with English abstract). |
| [14] |
Bi Y H, Xie Q, Zhang S, et al. Assessment of the internal genes of influenza A(H7N9) virus contributing to high pathogenicity in mice[J]. Journal of Virology, 2015, 89(1): 2-13. DOI:10.1128/JVI.02390-14 |
| [15] |
Liu M. DNA vaccines: a review[J]. Journal of Internal Medicine, 2003, 253(4): 402-410. DOI:10.1046/j.1365-2796.2003.01140.x |
| [16] |
Ghanem A, Healey R, Adly F G. Current trends in separation of plasmid DNA vaccines: a review[J]. Analytica Chimica Acta, 2013, 760: 1-15. DOI:10.1016/j.aca.2012.11.006 |
| [17] |
胡北侠, 任素芳, 黄艳艳, 等. 禽流感疫苗免疫鸡群后HI抗体消长规律及免疫保护临界值的测定[J]. 山东农业科学, 2003, 35(5): 41-42. Hu B X, Ren S F, Huang Y Y, et al. Changes of HI antibody titer of chicken vaccinated with avian influenza vaccine and measurement of lowest protecting antibody titer[J]. Shandong Agricultural Sciences, 2003, 35(5): 41-42 (in Chinese). DOI:10.3969/j.issn.1001-4942.2003.05.016 |
| [18] |
李俊平. H5亚型禽流感DNA疫苗对鸭和鹌鹑的免疫保护效力研究[D]. 北京: 中国农业科学院, 2011. Li J P. Protective efficacy of H5 subtype avian influenza DNA vaccine in ducks and quails[D]. Beijing: Chinese Academy of Agricultural Sciences, 2011(in Chinese with English abstract). |
| [19] |
郭艳娜, 刘志远, 孙彤彤, 等. 低致病性A/Anhui/1/2013(H7N9)流感病毒感染小鼠肺组织的miRNA表达谱分析[J]. 南京农业大学学报, 2016, 39(3): 482-490. Guo Y N, Liu Z Y, Sun T T, et al. Analysis of miRNA expression profile in lung of mice infected with low pathogenic A/Anhui/1/2013(H7N9) influenza virus[J]. Journal of Nanjing Agricultural University, 2016, 39(5): 814-818 (in Chinese with English abstract). DOI:10.7685/jnau.201812037 |
| [20] |
Nobiron I, Thompson I, Brownlie J, et al. Cytokine adjuvancy of BVDV DNA vaccine enhances both humoral and cellular immune responses in mice[J]. Vaccine, 2001, 19(30): 4226-4235. DOI:10.1016/S0264-410X(01)00157-8 |
| [21] |
Rasoli M, Omar A R, Aini I, et al. Fusion of HSP70 gene of Mycobacterium tuberculosis to hemagglutinin(H5) gene of avian influenza virus in DNA vaccine enhances its potency[J]. Acta Virologica, 2010, 54(1): 33-39. DOI:10.4149/av_2010_01_33 |
| [22] |
Yao Q X, FischerK P, Li L N, et al. Immunogenicity and protective efficacy of a DNA vaccine encoding a chimeric protein of avian influenza hemagglutinin subtype H5 fused to CD154(CD40L) in Pekin ducks[J]. Vaccine, 2010, 28(51): 8147-8156. DOI:10.1016/j.vaccine.2010.09.081 |
| [23] |
Zhao K, Zhang Y, Zhang X Y, et al. Chitosan-coated poly(lactic-co-glycolic) acid nanoparticles as an efficient delivery system for Newcastle disease virus DNA vaccine[J]. International Journal of Nanomedicine, 2014, 9: 4609-4619. |




