文章信息
- 贾兵, 余桃, 郭国凌, 王友煜, 许波, 刘普, 衡伟, 朱立武
- JIA Bing, YU Tao, GUO Guoling, WANG Youyu, XU Bo, LIU Pu, HENG Wei, ZHU Liwu
- ‘砀山酥梨’宿萼果与脱萼果内源激素含量比较及其信号传导基因的表达差异
- Comparision of endogenous hormones content and expression difference of signal transduction genes between the calyx-persistence fruit and calyx-shedding fruit in 'Dangshansuli'pear
- 南京农业大学学报, 2021, 44(3): 428-436
- Journal of Nanjing Agricultural University, 2021, 44(3): 428-436.
- http://dx.doi.org/10.7685/jnau.202008002
-
文章历史
- 收稿日期: 2020-08-03
中国是世界上梨(Pyrus spp.)栽培面积最大的国家, 2017年, 梨栽培面积约96万hm2, 总产量约1 653万t, 分别占世界总面积和总产量的69.1%和68.4%[1]。‘砀山酥梨’果实有宿萼果与脱萼果之分, 宿萼果果实大, 但果形不端正, 风味比脱萼果差, 外源生长调节剂如PBO、PP333、Flusilazole可调控果实萼片的脱落, 提高脱萼果率, 在生产上得到了应用[2]。
梨属植物可划分成宿萼组和脱萼组, 其中西洋梨属宿萼组, 因此, 未见有欧美学者开展梨果萼片脱落与宿存等方面的研究。白梨、新疆梨中的‘砀山酥梨’‘库尔勒香梨’等品种属脱萼组[3]。国内主要围绕植物生长调节剂、授粉、光照等对梨萼片发育的影响, 脱萼果与宿萼果中内源激素含量的比较等方面开展了相关研究。
梨果实萼片宿存与脱落过程基因表达谱分析表明, 萼片宿存与脱落涉及激素合成、应答、信号传导基因的表达[4]。IAA通过膜转运蛋白(auxin influx transporter, AUX)进入膜内, 与受体蛋白1(transport inhibitor response protein 1, TIR1)结合, 并与CUL1(cullin 1)蛋白相互作用, 形成泛素连接酶复合体, 介导转录抑制子蛋白(auxin/indole-3-acetic acid, Aux/IAA)的降解, 将生长素响应因子ARF(auxin response factor, ARF)从Aux/IAA-ARF二聚体中释放出来, 转录激活生长素早期响应基因Aux/IAA、GH3(Gretchen Hagen 3)和SAUR(small auxin up RNA)3大家族基因表达, 调控植物生长以及细胞增大等生理过程[5]; CTK通过与细胞膜类组氨酸激酶受体蛋白1(cytokinin receptor 1 protein, CRE1)结合, 被转运至细胞质[6], 由组氨酸转运蛋白(AHP)转运传至细胞核内, 促使B型反应调节子(B-ARR)活化, 最终激活A型反应调节子(A-ARR)或其他靶基因的转录, 调控细胞分裂和幼嫩组织分化[7]; GA进入膜内与受体蛋白(GA insensitive dwarf 1, GID1)特异性结合形成GID1-GA二联复合体, 并与DELLA蛋白结合形成GID1-GA-DELLA三联复合体, 诱导DELLA蛋白的26S泛素化降解, 解除对GA抑制作用, 调控植物的发育[8]; ABA进入细胞内与受体蛋白(PYR/PYL/RCAR)结合形成二联复合物, 并与A组2C类蛋白磷酸酶(PP2C)结合, 抑制其活性, 而造成蔗糖非发酵-1-相关蛋白激酶2(SnRK2)活性的增强, 调控ABA应答元件结合蛋白(ABF)活性而激活ABA生理效应[9]。目前, 植物内源激素信号传导途径已经很清楚, 但有关宿萼果与脱萼果内源激素如何影响下游传导基因的表达还未见报道。
本试验在‘砀山酥梨’形成宿萼果与脱萼果的关键时期, 比较不同外源生长调节剂处理的宿萼果与脱萼果内源IAA、ZR、GA3和ABA的含量, 并从NCBI梨基因组数据库中查询、克隆相关信号传导基因, 分析其在宿萼果与脱萼果的表达差异, 旨在揭示‘砀山酥梨’幼果中内源激素含量及其信号传导基因的表达与萼片宿存与脱落的关系, 为人工施用外源生长调节剂提高‘砀山酥梨’脱萼果率的生产实践奠定理论基础。
1 材料与方法 1.1 试验材料以‘砀山酥梨’为试材, 于盛花期喷施400 mg·L-1赤霉素(GA3)作为宿萼处理, 喷施200 mg·L-1多效唑(PP333)作为脱萼处理, 并以喷施清水为对照。每个处理3棵树, 3次重复。于盛花后10 d, 幼果能分辨出宿萼果与脱萼果时, 分别取叶片和幼果备用。
1.2 试验方法 1.2.1 内源激素测定采用酶联免疫法(enzyme-linked immunosorbent assay, ELISA)测定叶片中GA3、IAA、ZR和ABA含量。
1.2.2 石细胞染色测定石细胞染色采用间苯三酚染色法。
1.2.3 总RNA提取及cDNA合成RNA提取使用StarSpin Plant RNA Mini Kit(Genstar公司), 合成cDNA采用M-MLV RTase cDNA Synthesis Kit(大连宝生物工程有限公司)。
1.2.4 实时荧光定量PCR从NCBI梨基因组数据库(https://www.ncbi.nlm.nih.gov/genome/12793)查询、克隆获得了IAA、GA、CTK和ABA信号传导相关基因的全长序列, 并根据获得的序列使用Primer Premier 5.0软件设计实时荧光定量PCR(RT-qPCR)引物(表 1), 内参采用梨GAPDH基因。
| 基因编号Gene ID | 引物名称Primer name | 引物序列Primer sequences(5′→3′) |
| Pbr009498.1 | pbAUX1-F/R | CTTGGGATGACCACCTACACTG/GTCCGCCGAAAGTGTAGAGAAT |
| Pbr022779.1 | pbTIR1-F/R | GCTTGGGGTCTGAGTTGAGTTT/CTTGAGTTCCTTGCATCCTGAG |
| Pbr029871.1 | pbAUX/IAA1-F/R | TGAGAGTGAGAAGCACCGATTT/GCTCTCCACAATCCGCTACTCT |
| Pbr008768.1 | pbAUX/IAA2-F/R | ATTTGGTCTACGACAGGGGTTG/AAAACAACACACAACACCACCC |
| Pbr008404.1 | pbARF1-F/R | ATGGCTTTCGAGACTGAGGACT/CTTGGTGATGAGAAGGGTGACA |
| Pbr035088.1 | pbARF2-F/R | CCAAGGATCTTCATGGTTACGA/GAGATCACAGATGATGGCATGC |
| Pbr021158.1 | pbGH3.6-F/R | TCTTCGGAGTGCTACTTTGGTG/TTCTTGCTGCTCCTTCTCATTG |
| Pbr040072.1 | pbSAUR32-F/R | TTTCCTTTTCTTGCATGTGCTC/GTTTGTGCGAAACATGGAAATC |
| Pbr023315.1 | pbCRE1-F/R | ACGGAACAGCAACCCTACCAGT/GCGAACATTGCCCACGTCACA |
| Pbr005256.1 | pbCRE2-F/R | GAGCAAGGGCAACTGGAAAGGG/TCTGGTGTGGAATCTGGAGGCA |
| Pbr015157.1 | pbCRE3-F/R | CATCAGCAAGATCCGCCACCAT/TCCACCCAACCACCCACGAAA |
| Pbr012151.3 | pbCRE4-F/R | CCAAAGTCTGCTCTGCGGGAAG/CGGATTCTGCGTGTTGCCTCAA |
| Pbr006568.1 | pbAHP1-F/R | AGCTCCAGCATTGGTGCTCAGA/AGCCGCCACAACCTGTCTCT |
| Pbr042238.1 | pbAHP6-F/R | ACAGCAGCAACGAGTCTTGGC/TGGTACAGTCGCAGTGGGTCAA |
| Pbr011993.1 | pbARR1-F/R | TGATAGCGGCAGAGGCATAGGA/GCGAGGCTGGACATTACTGGTG |
| Pbr020017.1 | pbARR11-F/R | CGGTCCACACAGCCATTTCCAA/AGGAAAGGAGTCTGCCGTGCTT |
| Pbr006571.1 | pbGID1-F/R | TGATGGATGGACAGCCCTGAGG/CGCTCCTGCCCACCAAACATAG |
| Pbr017104.1 | pbGID2-F/R | ACAGCCCTGAAGTGGGTGAAGT/CGCTCCTGCCCACCAAACATAG |
| Pbr029796.1 | pbGID4-F/R | CCGAATTGGTGGCGTTCTGTGA/ACAACAGCACAGCACTCCTCCT |
| Pbr040316.1 | pbDELLA2-F/R | ACCAGCTTCAGCGGCCTTCA/ACAGGAGAACGGCGTACCAGTT |
| Pbr040300.1 | pbDELLA5-F/R | GGTCGGCTCAGGAAGATGGGA/GGTGGTAGAGGTCGCCTGTGTTT |
| Pbr026078.1 | pbTF5-F/R | ACGGTGTGATGGCTGGCTCA/AGCATTGACTCGGGCAGACTGT |
| Pbr011993.1 | pbARR1-F/R | CGGTCCACACAGCCATTTCCAA/AGGAAAGGAGTCTGCCGTGCTT |
| Pbr042468.1 | pbPYR1-F/R | CGTGCATGGGTTTGACCGTGA/CGACGCCAGCTTCTGGAGATTC |
| Pbr013616.1 | pbPYL8-F/R | GGCACCACAAGCACGACCTTAA/GCACCACACACCTGCTGACAAA |
| Pbr009416.1 | pbPP2C-2-F/R | TTGCTGCGGTGTTGTTGGAGAA/GTTGCGTTAGGCTGCGACTCTT |
| Pbr019878.1 | pbPP2C-50-F/R | TCGTGTTTGTGGCGTTCTTGCT/AGCGGAAGTCCATCAGCACCAT |
| Pbr007881.1 | pbSnRK2-1-F/R | ATCGGCAGGCAATGACGGTTG/CGATGTACTTGACGGCGACGAG |
| Pbr040276.1 | pbSnRK2-2-F/R | GTAGTGGAGCGTGACCAGCCTA/ACAGGACCGCCTGCTTTGGAT |
| Pbr040390.1 | pbABF-5-F/R | CCACAACAGCAGCCACTCTTCC/GCCCAAACCAACCGTGCCAAT |
| Pbr007589.1 | pbABF-5-5-F/R | ATCGGTGGCGGTGTTCAGAGT/CATACTGCTGCTGCTGGTGAGG |
| 注: AUX: 生长素膜转运蛋白基因Auxin influx transporter gene; TIR: 生长素受体蛋白基因Transport inhibitor response gene; AUX/IAA: 生长素转录抑制子基因Auxin/indole-3-acetic acid in early auxin-response gene; ARF: 生长素响应因子基因Auxin response factor gene; GH3: 生长素早期响应基因Gretchen hagen 3 in early auxin-response gene; SAUR: 生长素早期响应基因Small auxin up RNA in early auxin-response gene; CRE: 细胞分裂素结合受体类组氨酸激酶受体蛋白基因Cytokinin receptor protein gene; AHP: 磷酸转运蛋白基因Arabidopsis histidine phosphotransfer protein gene; ARR: A型反应调节子A-type response regulator gene; GID1: GA受体蛋白基因GA insensitive dwarf 1 gene; GID2: SCF-E3复合体中F-box蛋白基因F-box subunit of the SCF-E3 complex named GA insensitive dwarf 2 gene; DELLA: 转录抑制因子基因Transcription repressors gene; TF: 转录因子基因Transcription factors gene; PYR: ABA受体蛋白基因Gene for soluble ABA receptors gene; PP2C: 蛋白磷酸酶2C基因Protein phosphatases 2C gene; SnRK: 蔗糖非发酵相关蛋白激酶基因Sucrose non-fermenting related protein kinase gene; ABF: ABA应答元件结合蛋白基因ABRE-blinding factor gene. | ||
采用Excel 2019、GraphPad Prism 8和SPSS 23统计软件分析相关数据。
2 结果与分析 2.1 ‘砀山酥梨’宿萼果与脱萼果的发育过程比较如图 1所示: 从‘砀山酥梨’盛花后10 d开始, 宿萼果与脱萼果即开始形成(图 1-A、B), 在外观形态上已有幼果在萼筒下部出现离层线, 即形成脱萼果, 而宿萼果则没有(图 1-C、D), 此期为宿萼果与脱萼果分化关键期。从盛花后12 d开始, 脱萼果在萼筒离层处萼片与幼果完全分离, 而宿萼果萼片与幼果正常生长(图 1-E、F)。盛花后15 d, 脱萼果在萼筒离层处萼片已经完全脱落, 而宿萼果萼片与幼果正常生长(图 1-G、H)。盛花后165 d, ‘砀山酥梨’宿萼果与脱萼果果实成熟, 宿萼果萼片宿存且肉质化, 脱萼果则没有(图 1-I、J)。成熟果实石细胞染色结果表明, 宿萼果中石细胞较脱萼果多(图 1-K、L)。

|
图 1 ‘砀山酥梨’宿萼果(CPF)与脱萼果(CSF)的发育过程 Fig. 1 Development process of the calyx-persistence fruit(CPF)and calyx-shedding fruit(CSF)of 'Dangshansuli'pear A. 盛花后10 d CPF; B. 盛花后10 d CSF; C. 盛花后10 d CPF萼片发育放大图; D. 盛花后10 d CSF萼片发育放大图; E. 盛花后12 d CPF; F. 盛花后12 d CSF; G. 盛花后15 d CPF; H. 盛花后15 d CSF; I. 盛花后165 d CPF; J. 盛花后165 d CSF; K. 成熟CPF石细胞染色图; L. 成熟CSF石细胞染色图。 A. The CPF at 10 d after full bloom; B. The CSF at 10 d after full bloom; C. The CPF enlarged view at 10 d after full bloom; D. The CSF enlarged view at 10 d after full bloom; E. The CPF at 12 d after full bloom; F. The CSF at 12 d after full bloom; G. The CPF at 15 d after full bloom; H. The CSF at 15 d after full bloom; I. The mature CPF at 165 d after full bloom; J. The mature CSF at 165 d after full bloom; K. The stone cells chromatogram of mature CPF; L. The stone cells chromatogram of mature CSF. |
‘砀山酥梨’对照的宿萼果率为56.67%, 400 mg·L-1 GA3处理宿萼果率为100%, 200 mg·L-1PP333处理宿萼果率为16.2%, 且三者之间差异显著(P < 0.05)。说明外源GA3处理能显著促进宿萼果的发育, PP333处理显著促进脱萼果的发育。
2.3 ‘砀山酥梨’幼果中内源激素含量的比较如图 2所示: ‘砀山酥梨’盛花后10 d, 对照和PP333处理宿萼果中内源IAA和GA3含量显著高于脱萼果, GA3处理宿萼果IAA和GA3含量均显著高于对照和PP333处理的脱萼果。不同处理宿萼果中ZR和ABA含量均与脱萼果无显著差异。表明: 在‘砀山酥梨’宿萼果与脱萼果形成的关键时期, 宿萼果中内源激素含量与脱萼果存在明显的差异, 且3个处理宿萼果中IAA、GA3含量显著高于脱萼果。

|
图 2 外源生长调节剂处理对‘砀山酥梨’幼果中内源激素含量的影响
Fig. 2 Effects of exogenous growth regulators on endogenous hormone content in young fruit of 'Dangshansuli'pear
CK. 清水对照Water control; PP333. 200 mg·L-1 PP333脱萼处理200 mg·L-1 PP333 calyx-shedding treatment; GA3. 400 mg·L-1 GA3宿萼处理400 mg·L-1 GA3 calyx-persistent treatment. 不同小写字母表示不同处理差异显著(P < 0.05)。Different lowercases letters indicate significant difference at 0.05 level. 下同。The same as follows. |
如图 3所示: ‘砀山酥梨’盛花后10 d, 对照和PP333处理宿萼果中IAA信号传导基因pbAUX1、pbTIR1、pbARF1、pbARF2、pbGH3.6、pbSAUR32相对表达量均显著高于脱萼果; GA3处理宿萼果pbTIR1、pbARF1、pbARF2、pbGH3.6、pbSAUR32基因相对表达量均显著高于对照和PP333处理的脱萼果。对照和PP333处理宿萼果中生长素信号传导基因pbAUX/IAA1、pbAUX/IAA2相对表达量低于脱萼果; GA3处理宿萼果pbAUX/IAA1基因相对表达量与对照脱萼果的无显著差异, 但显著高于PP333处理的脱萼果; GA3处理宿萼果pbAUX/IAA2基因相对表达量与对照和PP333处理的脱萼果无显著差异。表明: 宿萼果中高浓度的IAA促使膜转运蛋白基因pbAUX1和核内受体蛋白基因pbTIR1高表达, 生长素抑制基因pbAUX/IAA1、pbAUX/IAA2表达量显著下降, 生长素响应因子pbARF1、pbARF2表达量显著上升, 激活了原初反应基因pbGH3.6、pbSAUR32, 调控宿萼果的发育。
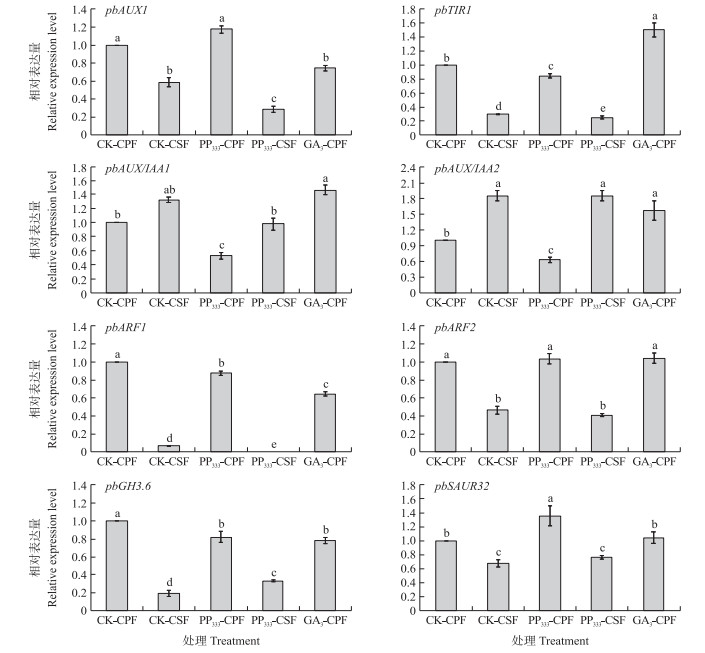
|
图 3 ‘砀山酥梨’宿萼果与脱萼果生长素(IAA)信号传导基因表达量的比较 Fig. 3 Comparision of the expression level on auxin signaling transduction genes between the CPF and CSF of 'Dangshansuli'pear |
如图 4所示: ‘砀山酥梨’盛花后10 d, 对照和PP333处理宿萼果中CTK信号传导基因pbCRE1、pbCRE2、pbCRE3、pbCRE4、pbARR1、pbARR11相对表达量均显著高于脱萼果; GA3处理的宿萼果6个基因相对表达量均显著高于对照和PP333处理的脱萼果。而对照和PP333处理宿萼果中CTK信号传导基因pbAHP1、pbAHP6相对表达量显著低于脱萼果; GA3处理的宿萼果pbAHP6基因相对表达量显著低于对照和PP333处理的脱萼果。表明宿萼果中CTK膜转运能力强, 但只发生在细胞质内, 其下游核内转运关键基因pbAHP1、pbAHP6并未响应, 核内CTK效应基因pbARR1、pbARR11的激活与ZR无关, 可能受其他激素参与调控。

|
图 4 ‘砀山酥梨’宿萼果与脱萼果细胞分裂素(CTK)信号传导基因表达量的比较 Fig. 4 Comparison of the expression level on cytokinin signaling transduction genes between the CPF and CSF of 'Dangshansuli'pear |
如图 5所示: ‘砀山酥梨’盛花后10 d, 对照宿萼果中GA信号传导基因pbGID1、pbGID2、pbGID4、pbDELLA2、pbDELLA5、pbTF5相对表达量均显著高于脱萼果; PP333处理宿萼果pbGID1、pbGID2、pbGID4、pbTF5基因相对表达量也显著高于脱萼果; GA3处理宿萼果pbGID1、pbGID2、pbGID4、pbTF5基因相对表达量均显著高于对照和PP333处理的脱萼果。表明: 宿萼果核内受体蛋白基因pbGID1表达量也显著高于脱萼果, 诱导泛素化酶体蛋白基因pbGID2和转录因子pbTF5的表达, 参与调控宿萼果的发育。

|
图 5 ‘砀山酥梨’宿萼果与脱萼果赤霉素(GA)信号传导基因表达量的比较 Fig. 5 Comparison of the expression level on gibberellin signaling transduction genes between the CPF and CSF of 'Dangshansuli'pear |
如图 6所示: ‘砀山酥梨’盛花后10 d, 对照宿萼果中ABA信号传导基因pbPP2C-2、pbPP2C-50、pbSnRK2-1、pbSnRK2-2、pbABF-5、pbABF-5-5相对表达量显著高于脱萼果; 而pbPYR1、pbPYL8表达量与脱萼果无显著差异。PP333处理的宿萼果8个基因相对表达量均显著高于脱萼果; GA3处理宿萼果pbPP2C-2、pbPP2C-50、pbSnRK2-2、pbABF-5-5基因相对表达量均显著高于对照和PP333处理的脱萼果。
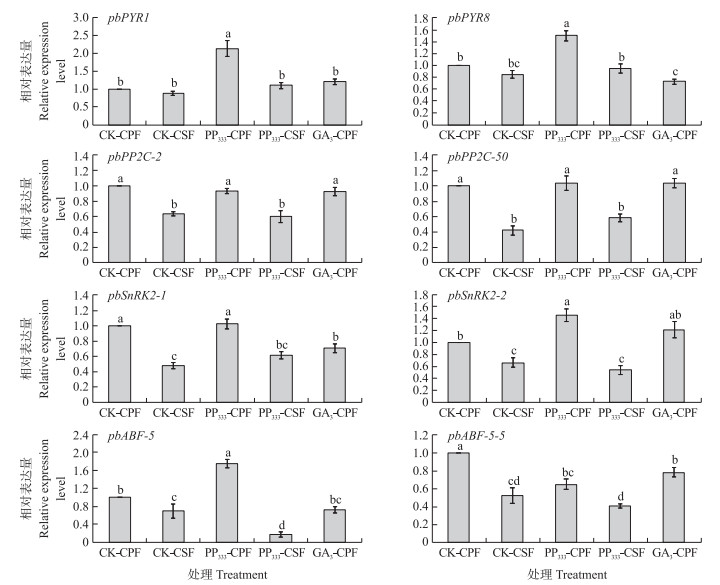
|
图 6 ‘砀山酥梨’宿萼果与脱萼果脱落酸(ABA)信号传导基因表达量的比较 Fig. 6 Comparison of the expression level on abscisic acid signaling transduction genes between the CPF and CSF of 'Dangshansuli'pear |
由于宿萼果与脱萼果中ABA含量并无明显差异, 且ABA核内受体蛋白基因pbPYR1、pbPYR8表达量也无显著差异, ABA抑制因子pbPP2C-2、pbPP2C-50表达量却较脱萼果显著增加, ABA生理效应被抑制, 说明ABA信号传导相关基因的表达可能受IAA、GA3等激素共同调控, ABA信号传导基因pbPP2C-2、pbPP2C-50、pbSnRK2-2、pbABF-5-5可能参与调控宿萼果的发育。
3 讨论研究表明, ‘砀山酥梨’果心IAA含量高有利于果实萼片宿存[10-11]; ‘库尔勒香梨’幼果中IAA含量高有利于果萼的宿存[12-13]; ‘南果梨’萼片和果心高浓度IAA有利于萼片宿存[14]。本研究中, 对照与外源生长调节剂处理的‘砀山酥梨’宿萼果内IAA含量均显著高于脱萼果, 表明梨幼果中高IAA水平有利于萼片的宿存, 这与齐笑笑[4]和贾晓辉等[15]的研究结果一致。AUX1作为IAA转运蛋白基因, 对于IAA膜、核间转运发挥着重要作用。仇志浪等[16]研究表明, 在即将脱落的甜樱桃幼果果柄中, AUX1显著下调, 说明高AUX1基因表达水平利于组织宿存。外源IAA处理可显著诱导其受体TIR1的表达[17], 高表达的TIR1与活性IAA结合, 介导IAA抑制因子AUX/IAA的降解, 造成ARF解离, 并调控下游基因GH3、SAUR的表达[5]。本研究中宿萼果内pbAUX1和pbTIR1表达量均显著高于脱萼果, 而宿萼果中pbAUX/IAA1、pbAUX/IAA2基因表达量显著下降, 而pbGH3.6、pbSAUR32表达量显著增加, 与前人研究一致。Damodharan等[18]通过沉默番茄AtARF17, 引起番茄花器官的脱落, 证实AtARF17调控花器官的宿存。本研究表明, 宿萼果内pbARF1、pbARF2表达量均显著高于脱萼果, 可能与AtARF17功能一致, 对萼片的宿存发挥着积极作用, 其具体功能有待于进一步研究。
梨果萼中高浓度GA3有利于萼片宿存[19], GA能够延缓离体桤木茎叶的黄变[20], 也可通过促进百合切花内源IAA的合成, 延缓叶片衰老[21]。GA作为重要的生长调节剂之一, 参与植物光形态建成、果实膨大等多种生理调控[22]。GA信号可以被GID1/DELLA感知, GID1表达量与赤霉素含量呈正相关[23], GID2是GA信号传导途径中一个重要的调节因子, 是介导DELLA蛋白降解的重要蛋白酶体编码基因, 其突变体gid2中SLRl(Slender rice 1)不能正常降解, 从而抑制GA信号向下游的传导[24]。本研究中, 宿萼果中GA3含量与pbGID1、pbTF5基因表达量均显著高于脱萼果, 说明GA3参与调控果萼的宿存。
CTK对叶片衰老有抑制作用, 而ABA对叶片衰老有促进作用[25]。拟南芥CTK信号途径的ARR蛋白和ABA信号途径的SnRK激酶互作, 参与调控植物衰老过程[26]。CTK响应因子CRF基因在AHK3被激活的条件下表达增加, 对叶片衰老具有负调控作用, 通过直接或间接调控下游的转录靶点, 包括响应因子基因ARR6、ARR9和ARR11的表达, 进而影响叶片衰老[27]。萼片为叶的变态, 具有叶片的功能。本研究中, 尽管‘砀山酥梨’宿萼果中ZR、ABA含量与脱萼果无显著差异, 但CTK膜转运蛋白基因pbCRE1/2/3/4和核内反应调节子pbARR1/11表达量均显著高于脱萼果; 宿萼果中ABA抑制因子pbPP2C-2、pbPP2C-50表达量却较脱萼果显著增加, ABA生理效应被抑制, 说明CTK和ABA等激素之间相互作用, 可能间接调控宿萼果与脱萼果的发育。
| [1] |
张绍铃, 谢智华. 我国梨产业发展现状、趋势、存在问题与对策建议[J]. 果树学报, 2019, 36(8): 1067-1072. Zhang S L, Xie Z H. Current status, trends, main problems and the suggestions on development of pear industry in China[J]. Journal of Fruit Science, 2019, 36(8): 1067-1072 (in Chinese with English abstract). |
| [2] |
衡伟, 陈捷, 叶振风, 等. '砀山酥梨'幼果花萼发育及其调控技术研究[J]. 安徽农业大学学报, 2010, 37(2): 238-243. Heng W, Chen J, Ye Z F, et al. Development of calyx and its controlling techniques of young fruit of 'Dangshansu' pear[J]. Journal of Anhui Agricultural University, 2010, 37(2): 238-243 (in Chinese with English abstract). |
| [3] |
金敏, 张倩, 陶书田, 等. IAA及其运输载体PIN对库尔勒香梨萼片脱落与宿存影响的研究[J]. 现代农业科技, 2020(8): 46-49. Jin M, Zhang Q, Tao S T, et al. Effect of IAA and its carrier PIN on sepal exfoliation and persistence of Korla fragrant pear[J]. Modern Agricultural Science and Technology, 2020(8): 46-49 (in Chinese with English abstract). DOI:10.3969/j.issn.1007-5739.2020.08.030 |
| [4] |
齐笑笑. 梨果实萼片宿存与脱落过程基因表达谱分析及PsIDA、PsJOINTLESS基因功能的初步研究[D]. 南京: 南京农业大学, 2014. Qi X X. Investigation of genes expression of calyx survival and shedding of pear by digital gene expression and functional analysis of PsIDA and PsJOINTLESS[D]. Nanjing: Nanjing Agricultural University, 2014(in Chinese with English abstract). |
| [5] |
冯寒骞, 李超. 生长素信号转导研究进展[J]. 生物技术通报, 2018, 34(7): 24-30. Feng H Q, Li C. Research advances of auxin signal transduction[J]. Biotechnology Bulletin, 2018, 34(7): 24-30 (in Chinese with English abstract). |
| [6] |
Anantharaman V, Aravind L. The CHASE domain: a predicted ligand-binding module in plant cytokinin receptors and other eukaryotic and bacterial receptors[J]. Trends in Biochemical Sciences, 2001, 26(10): 579-582. DOI:10.1016/S0968-0004(01)01968-5 |
| [7] |
齐莹, 施和平, 李玲. 细胞分裂素信号转导分子机制[J]. 生命科学研究, 2004, 8(4): 88-92. Qi Y, Shi H P, Li L. Studies on molecular mechanisms of cytokinin signaling[J]. Life Science Research, 2004, 8(4): 88-92 (in Chinese with English abstract). |
| [8] |
王文然, 樊秀彩, 张文颖, 等. 果树赤霉素代谢与信号途径研究进展[J]. 生物技术通报, 2017, 33(11): 1-7. Wang W R, Fan X C, Zhang W Y, et al. Study progress on gibberellin metabolism and signaling transduction pathway in fruits trees[J]. Biotechnology Bulletin, 2017, 33(11): 1-7 (in Chinese with English abstract). |
| [9] |
宋松泉, 刘军, 徐恒恒, 等. 脱落酸代谢与信号传递及其调控种子休眠与萌发的分子机制[J]. 中国农业科学, 2020, 53(5): 857-873. Song S Q, Liu J, Xu H H, et al. ABA metabolism and signaling and their molecular mechanism regulating seed dormancy and germination[J]. Scientia Agricultura Sinica, 2020, 53(5): 857-873 (in Chinese with English abstract). |
| [10] |
刘妮, 陶书田, 李雷廷, 等. '砀山酥梨'幼果萼片脱落期内源激素含量变化[J]. 南京农业大学学报, 2013, 36(6): 147-150. Liu N, Tao S T, Li L T, et al. Changes in endogenous hormones levels of young fruit of 'Dangshansuli'pear during calyx abscission processes[J]. Journal of Nanjing Agricultural University, 2013, 36(6): 147-150 (in Chinese with English abstract). DOI:10.7685/j.issn.1000-2030.2013.06.024 |
| [11] |
Su J, Jia B, Jia S, et al. Effect of plant growth regulators on calyx abscission, fruit quality, and auxin-repressed protein(ARP) gene expression in fruitlets of 'Dangshansuli' pear(Pyrus bretschneideri Rehd.)[J]. The Journal of Horticultural Science and Biotechnology, 2015, 90(2): 135-142. DOI:10.1080/14620316.2015.11513164 |
| [12] |
牛建新, 何子顺. 梨果萼脱落宿存过程中果萼幼果内源激素的变化动态[J]. 果树学报, 2009, 26(4): 431-434. Niu J X, He Z S. Dynamic changes of phytohormone content in pear calyx and young fruit during calyx growth and development[J]. Journal of Fruit Science, 2009, 26(4): 431-434 (in Chinese with English abstract). |
| [13] |
李长江, 李鹏, 井春芝, 等. '库尔勒香梨'幼果内源激素分布差异与果实萼片脱落的关系[J]. 西北农业学报, 2017, 26(11): 1631-1638. Li C J, Li P, Jing C Z, et al. The relationship between endogenous hormones distribution in fruitlets and calyx shedding of 'Korla fragrant pear'[J]. Acta Agriculturae Boreali-Occidentalis Sinica, 2017, 26(11): 1631-1638 (in Chinese with English abstract). DOI:10.7606/j.issn.1004-1389.2017.11.009 |
| [14] |
汪晓谦, 商叶, 刘维成, 等. 生长调节剂及钙、硼肥对南果梨萼片脱落、果实品质及木质素代谢的影响[J]. 沈阳农业大学学报, 2019, 50(4): 399-405. Wang X Q, Shang Y, Liu W C, et al. Effect of growth regulators, Ca and B fertilizers on calyx abscission, fruit quality, and lignin metabolism in Nanguo pear[J]. Journal of Shenyang Agricultural University, 2019, 50(4): 399-405 (in Chinese with English abstract). |
| [15] |
贾晓辉, 王文辉, 李世强, 等. '库尔勒香梨'花萼端不同形状果实的矿质元素和内源激素含量比较[J]. 园艺学报, 2015, 42(4): 751-758. Jia X H, Wang W H, Li S Q, et al. The comparison of the mineral elements, endogenous hormones in different shapes and positions of 'Korla xiangli' pear[J]. Acta Horticulturae Sinica, 2015, 42(4): 751-758 (in Chinese with English abstract). |
| [16] |
仇志浪, 文壮, 杨鵾, 等. 基于蛋白质组分析探究甜樱桃幼果脱落的分子机制[C]//中国园艺学会2019年学术年会暨成立90周年纪念大会论文摘要集, 2019: 30. Qiu Z L, Wen Z, Yang K, et al. The molecular mechanism of young fruit shedding of sweet cherry based on proteome analysis[C]//Abstracts of Papers from the Academic Annual Meeting of China Horticultural Society in 2019 and its 90th Anniversary, 2019: 30(in Chinese with English abstract). |
| [17] |
王波. 拟南芥和盐生植物灰绿藜液泡膜焦磷酸酶基因与TIR1基因表达相关性分析[D]. 乌鲁木齐: 新疆大学, 2007. Wang B. Analysis on gene expression correlation between vacuolar H+-pyrophophatase and TRANSPORT INHIBITOR RESPONSE1(TIR1) in Arabidopsis thaliana and halophyte Chenopodium glaucum[D]. Urumqi: Xinjiang University, 2007(in Chinese with English abstract). |
| [18] |
Damodharan S, Zhao D Z, Arazi T. A common miRNA160-based mechanism regulates ovary patterning, floral organ abscission and lamina outgrowth in tomato[J]. The Plant Journal, 2016, 86(6): 458-471. DOI:10.1111/tpj.13127 |
| [19] |
姜彦辰. 梨萼片脱落与宿存果实内源激素及品质的差异研究[D]. 南京: 南京农业大学, 2011. Jiang Y C. Research of differences of endogenous hormones and quality in calyx persistent or fall off fruits[D]. Nanjing: Nanjing Agricultural University, 2011(in Chinese with English abstract). |
| [20] |
Hicklenton P R. GA3and benzylaminopurine delay leaf yellowing in cut Alstroemeria stems[J]. HortScience, 1991, 26(9): 1198-1199. DOI:10.21273/HORTSCI.26.9.1198 |
| [21] |
蔡军伙, 连芳青, 魏绪英. PP333、GA3对麝香百合切花品质及叶绿素含量的影响初探[J]. 江西农业大学学报, 2002, 24(5): 623-626. Cai J H, Lian F Q, Wei X Y. A study on the influence of PP333 and GA3 on the quality and chlorophyll content of Lilium longiflorum Thunb[J]. Acta Agriculturae Universitatis Jiangxiensis, 2002, 24(5): 623-626 (in Chinese with English abstract). DOI:10.3969/j.issn.1000-2286.2002.05.014 |
| [22] |
商建秀, 张胜伟, 孙颖. 油菜素内酯、赤霉素与光共同调控拟南芥的细胞伸长和光形态建成[J]. 生物化学与生物物理进展, 2013, 40(3): 228-230. Shang J X, Zhang S W, Sun Y. BR, GA and light co-regulate cell elongation and photo-morphogenesis in Arabidopsis thaliana[J]. Progress in Biochemistry and Biophysics, 2013, 40(3): 228-230 (in Chinese with English abstract). |
| [23] |
丁伟, 周葱, 刘超, 等. 缺铁胁迫对梨叶片中GA信号转导相关基因的影响[J]. 西北植物学报, 2015, 35(2): 233-238. Ding W, Zhou C, Liu C, et al. Effect of iron-deficiency on the expression of GA signal transduction related genes in leaf of 'Dangshansuli' pear(Pyrus bretschneideri Rehd.)[J]. Acta Botanica Boreali-Occidentalia Sinica, 2015, 35(2): 233-238 (in Chinese with English abstract). |
| [24] |
Gomi K, Sasaki A, Itoh H, et al. GID2, an F-box subunit of the SCF E3 complex, specifically interacts with phosphorylated SLR1 protein and regulates the gibberellin-dependent degradation of SLR1 in rice[J]. The Plant Journal, 2004, 37(4): 626-634. DOI:10.1111/j.1365-313X.2003.01990.x |
| [25] |
彭凯轩, 章薇, 朱晓仙, 等. 细胞分裂素延缓叶片衰老的机制研究进展[J]. 植物生理学报, 2021, 57(1): 12-18. Peng K X, Zhang W, Zhu X X, et al. Research progress on the mechanisms of cytokinin-inhibited leaf senescence[J]. Plant Physiology Journal, 2021, 57(1): 12-18 (in Chinese with English abstract). |
| [26] |
Huang X, Hou L, Meng J, et al. The antagonistic action of abscisic acid and cytokinin signaling mediates drought stress response in Arabidopsis[J]. Molecular Plant, 2018, 11(7): 970-982. DOI:10.1016/j.molp.2018.05.001 |
| [27] |
Zwack P J, de Clercq I, Howton T C, et al. Cytokinin response factor 6 represses cytokinin-associated genes during oxidative stress[J]. Plant Physiology, 2016, 172(2): 1249-1258. |



