文章信息
- 鲍春彤, 张晓光, 雷连成
- BAO Chuntong, ZHANG Xiaoguang, LEI Liancheng
- 坏死性凋亡及其相关疾病的研究进展
- Research progress of necroptosis and related diseases
- 南京农业大学学报, 2021, 44(1): 8-17
- Journal of Nanjing Agricultural University, 2021, 44(1): 8-17.
- http://dx.doi.org/10.7685/jnau.202006001
-
文章历史
- 收稿日期: 2020-06-02
2. 长江大学动物科学学院, 湖北 荆州 434000
2. College of Animal Science, Yangtze University, Jingzhou 434000, China
坏死性凋亡(necroptosis), 也被称作"程序性坏死", 是一种类似于坏死的细胞死亡方式, 依赖受体相互作用蛋白激酶1(receptor interacting serine/threonine protein kinase 1, RIPK1)、受体相互作用蛋白激酶3(receptor interacting serine/threonine protein kinase 3, RIPK3)和底物混合谱系激酶样蛋白(mixed lineage kinase domain like pseudokinase, MLKL), 不依赖半胱氨酸天冬氨酸蛋白酶(cysteine-aspartic proteases, Caspase)[1]。
凋亡(apoptosis)与坏死(necrosis)是两类主要的细胞死亡方式, 坏死曾经被认为是被动和非程序性的。随着研究的深入, 发现细胞坏死是一种主动、且可被调控的过程[2]。缺失或抑制凋亡信号通路中关键蛋白Caspase-8或FAS相关死亡结构域(Fas-associating protein with a novel death domain, FADD), 细胞在肿瘤坏死因子α(tumor necrosis factor α, TNF-α)诱导下仍会死亡, 且细胞死亡形态与坏死的细胞相似[3-4], 逐渐揭示了一种类似于坏死的不依赖Caspase的细胞死亡方式。2003年, Chan等[5]发现RIP依赖性的"程序性坏死"。Degterev等[6]在2005年阐述了小分子Nec-1对细胞坏死的调控作用, 首次将不受调控的坏死的概念更新为可以被Nec-1调控的细胞程序性坏死(necroptosis)。2008年Degterev等[7]证明RIPK1即为Nec-1的靶标, 并用siRNA筛选的方法, 证实基因组中很多基因对细胞程序性坏死都有调控作用, 找到了程序性坏死与细胞凋亡通路的交叉分子[8]。2018年细胞死亡命名委员会(Nomenclature Committee on Cell Death, NCCD)正式将坏死性凋亡定义为程序性坏死[1]。
发生坏死性凋亡的细胞胞膜破裂, 释放的胞内物质可以激发多种细胞(如巨噬细胞、成纤维细胞和内皮细胞等)参与固有免疫反应, 通过释放炎性细胞因子加剧炎症应答, 从而导致坏死性凋亡在不同生理或病理过程中扮演着双重角色。
坏死性凋亡的研究最初主要集中在急性脑损伤、癌症、神经退行性疾病等[6, 9-10]。自2013年以来, 相关研究成果激增, 发现坏死性凋亡不仅在多种器官和组织的非感染性病症如肺脏[11]和肾脏[12]疾病中扮演着重要角色, 同时在感染性疾病中也起到重要作用[13-14]。以信号通路中关键蛋白为靶点, 已经筛选出多种可用于治疗坏死性凋亡相关疾病的潜在药物及小分子化合物, 因此笔者对细胞坏死性凋亡的发生机制、与炎性因子释放的关系、相关常见病症及其治疗药物等方面进行综述。
1 细胞坏死性凋亡的发生机制细胞坏死性凋亡的发生需要RIPK1、RIPK3及MLKL的参与, MLKL作为执行分子导致胞膜裂解。肿瘤坏死因子受体1(tumor necrosis factor receptor 1, TNFR1)、干扰素受体(interferon receptor, IFNR)、Toll样受体3/4/9(Toll-like receptor 3/4/9, TLR3/4/9)以及胞浆感受器IFN调节因子的DNA依赖激活剂(DNA-dependent activator of IFN regulatory factors, DAI)活化后, 刺激信号激活胞内的RIPK家族激酶, 启动坏死性凋亡[15-19]。
细胞坏死性凋亡的调控过程精密复杂, 目前较为清晰的主要通路分为两类:
第1类为死亡受体参与且和细胞凋亡通路密不可分的信号通路。一方面, 当TNF-α刺激胞膜表面的TNFR1, TNFR1通过其C-端的死亡结构域(death domain, DD)募集TNFR1相关死亡结构域蛋白(TNF receptor 1-associated death domain protein, TRADD)、RIPK1和肿瘤坏死因子受体相关因子2(Tumor necrosis factor receptor related factor 2, TRAF2)等接头蛋白, 随后, TRAF2与胞内凋亡抑制蛋白(cellular inhibitors of apoptosis 1 and 2, cIAP1/2)识别并结合, 进一步募集线性泛素链组装复合体(linear ubiquitin Chain assem-bly complex, LUBAC)。形成的蛋白复合物支架, 与转化生长因子激酶1/转化生长因子结合蛋白(transforming growth factor kinase 1/transforming growth factor binding protein, TAK1/TAB)复合物和IκB激酶γ(IκB kinase, IKKγ)结合形成TNFR1复合物Ⅰ[20]。该复合物激活c-Jun氨基末端激酶(c-Jun N-terminal kinase, JNK)、p38丝裂原激活蛋白激酶(p38 mitogen activated protein kinase, p38)、细胞外调节蛋白激酶(extracellular regulated protein kinase, ERK)以及核因子κB(nuclear factor κB, NF-κB), 促进炎性基因表达[21-22]。另一方面, TNFR1复合物Ⅰ经肿瘤抑制因子头帕肿瘤综合征蛋白(cylindromatosis, CYLD)去泛素化, 募集TRADD、FADD及Caspase-8蛋白并结合后形成TNFR1复合物Ⅱa, 导致细胞凋亡, 也称为RIPK1非依赖凋亡(RIPK1 independent apoptosis, RIA)。当RIPK3和MLKL表达充分时, TNFR1复合物Ⅱa则会与RIPK3结合形成由FADD、Caspase-8、RIPK1和RIPK3蛋白分子组成的TNFR1复合物Ⅱb, 同样导致细胞凋亡, 这种凋亡则称为RIPK1依赖性凋亡(RIPK1 dependent apoptosis, RDA)。如果Caspase-8的催化活性被抑制(zVAD-FMK), 解除Caspase-8对RIPK1的抑制作用, 募集RIPK3并发生磷酸化, 活化的RIPK3募集MLKL形成TNFR1复合物Ⅱ(或称坏死小体, necrosome), 代谢物磷酸肌醇(IP)激酶调控MLKL[23], 使其发生磷酸化和寡聚化后, 从胞质部位转移至胞膜部位, 与相应的膜蛋白识别结合, 细胞发生坏死性凋亡[24]。
不同于TNF-α刺激下TNFR1介导的细胞坏死性凋亡信号通路, TNF家族死亡受体肿瘤坏死因子相关的凋亡诱导配体受体1(TNF related apoptosis inducing ligand receptor 1, TRAILR1)和受体2(TRAILR2)、死亡受体3(death receptor 3, DR3)和受体6(DR6)以及FAS(CD95)通过与细胞内的DD domain和接头蛋白FADD识别并结合, 形成死亡诱导信号复合物(death inducing signaling complex, DISC), 进而募集并活化Caspase-8, 最终致细胞凋亡[25]。但在特定条件下, 如Caspase-8被抑制剂(zVAD-FMK)抑制等, 则会形成坏死小体, 发生细胞坏死性凋亡。
第2类为无死亡受体参与的坏死性凋亡。受体相互作用蛋白(receptor-interacting protein, RIP)同型相互作用基序域(RIP homotypic interaction motifs, RHIM)在程序性坏死信号传导中起核心作用[26]。目前发现有4种含RHIM的蛋白质:RIPK1、RIPK3、β干扰素TIR结构域衔接蛋白(TIR-domain containing adaptor inducing interferon-β, TRIF)和DAI。TLR或病毒均需要包含RHIM的接头分子才能激活坏死性凋亡。胞外的poly IC、dsRNA、病毒RNA和脂多糖(lipopolysaccharide, LPS)分别激活胞膜表面的TLR3和TLR4受体, 募集含有RHIM域的接头分子TRIF, 并在泛Caspase-8抑制剂存在或Caspase-8(zVAD-FMK)被抑制的情况下, 形成坏死小体, 细胞发生坏死性凋亡[17-18, 27-28]。
除此之外, 干扰素家族也可诱导坏死性凋亡的发生。在巨噬细胞中, Ⅰ型干扰素(IFN-α和IFN-β)通过同源受体IFNARI, 激活小鼠骨髓巨噬细胞JAK1, 并在zVAD-FMK存在下, ISGF3复合物IRF9-STAT1-STAT2形成并持续激活最终导致坏死性凋亡发生[29]。Ⅱ型干扰素(IFN-γ)可协同Smac模拟物(birinapant, BV6)在干扰素调节因子1(interferon regulatory factor 1, IRF1)帮助下诱导癌细胞发生坏死性凋亡[30]。
MLKL磷酸化并不是坏死信号通路的终点, MLKL磷酸化后, Ca2+内流, 磷脂酰丝氨酸(phosphatidylserine, PS)从细胞膜的内侧翻转到细胞膜的表面造成膜损伤可以激活内涵体分选复合物Ⅲ(endosomal sorting complex required for transport-Ⅲ, ESCRT-Ⅲ), ESCRT-Ⅲ可控制质膜完整性的持续时间, 保证细胞的存活[31-32]。在感染、损伤或应激条件下, 多种模式识别受体(pattern recognition receptor, PRR)如TLR、NOD样受体(NOD-like receptor, NLR)、RIG-Ⅰ样受体(RIG-Ⅰ-like receptor, RLR)和RNA活化蛋白激酶(protein kinase R, PKR)等, 也参与细胞坏死性凋亡[17, 33-36]。细胞坏死性凋亡发生机制如图 1所示。
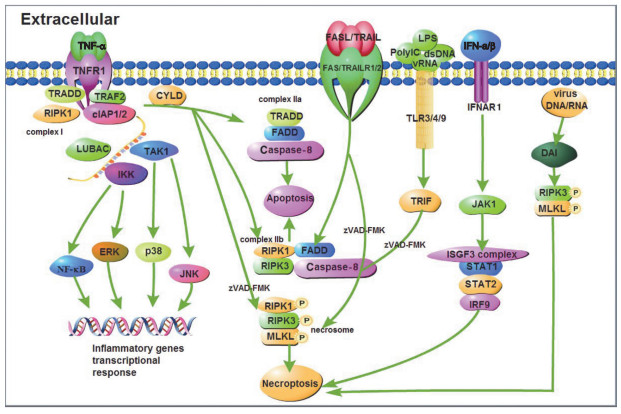
|
图 1 细胞坏死性凋亡发生机制示意图 Fig. 1 Schematic diagram of the mechanism of necroptosis TNF-α:肿瘤坏死因子Tumor necrosis factor α; TNFR1:肿瘤坏死因子受体1 Tumor necrosis factor receptor 1;TRADD:肿瘤坏死因子受体1相关死亡结构域蛋白Tumor necrosis factor receptor type 1-associated DEATH domain; TRAF2:肿瘤坏死因子受体相关因子2 TNF receptor-associated factor 2;RIPK1:受体相互作用蛋白激酶1 Receptor-interacting serine/threonine-protein kinase 1;cIAP1/2:细胞凋亡抑制蛋白1/2 Cellular inhibitor of apoptosis protein 1/2;LUBAC:线性泛素链组装复合体Linear ubiquitin chain assembly complex; TAK1:转化生长因子激酶1 TGF beta-activated kinase 1;IKK:IκB激酶IκB kinase; NF-κB:核因子活化B细胞κ轻链增强子Nuclear factor kappa-light-chain-enhancer of activated B cells; ERK:细胞外调节蛋白激酶Extracellular regulated protein kinases; p38:p38丝裂原激活蛋白激酶p38 mitogen-activated protein kinases; JNK:c-Jun氨基末端激酶c-Jun N-terminal kinase; CYLD:头帕肿瘤综合征蛋白Cylindromatosis; FADD:FAS相关死亡结构域Fas-associating protein with a novel death domain; Caspase-8:含半胱氨酸的天冬氨酸蛋白水解酶8 Cysteinyl aspartate specific proteinase 8;RIPK3:受体相互作用蛋白激酶3 Receptor-interacting serine/threonine-protein kinase 3;MLKL:混合谱系激酶结构域样假激酶Mixed lineage kinase domain like pseudokinase; FASL:FAS配体Fas ligand; TRAIL:肿瘤坏死因子相关凋亡诱导配体TNF-related apoptosis-inducing ligand; TRAILR1/2:肿瘤坏死因子相关凋亡诱导配体受体1/2 TNF-related apoptosis-inducing ligand receptor 1/2;LPS:脂多糖Lipopolysaccharide; PolyIC:聚肌胞苷酸(聚肌苷酸-聚胞苷酸)Polyinosinic(polycytidylic acid); dsDNA:双链DNA Double-stranded DNA; vRNA:病毒RNA Viral RNA; TLR3/4/9:Toll样受体3/4/9 Toll-like receptor 3/4/9;TRIF:β干扰素TIR结构域衔接蛋白TIR-domain-containing adapter-inducing interferon-β; IFN-α/β:干扰素α/β Interferon α/β; IFNAR1:干扰素α/β受体1 Interferon alpha/beta receptor 1;JAK1:酪氨酸受体激酶1 Janus kinase 1;ISGF3 complex:干扰素刺激基因因子3复合物IFN-stimulated gene factor 3 complex; STAT1/2:信号传导及转录激活蛋白1/2 Signal transducer and activator of transcription 1/2;IRF9:干扰素调节因子9 Interferon regulatory factor 9;DAI:IFN调节因子的DNA依赖激活剂DNA-dependent activator of IFN-regulatory factors. |
细胞坏死性凋亡的特点是细胞膜破裂、细胞器肿胀和细胞内成分外溢。当细胞发生坏死性凋亡时, 会因胞内成分"损伤相关分子模式(damage associated molecular pattern, DAMP)"的释放而促进炎症应答发生, 形成过度炎症。DAMP是在细胞遭受死亡、损伤、应激时, 释放或暴露的分子和细胞成分, 警告免疫系统机体处于危险状态。DAMP主要包括死亡细胞释放的细胞因子和警报蛋白, 例如IL-1家族细胞因子IL-1α、IL-1β、IL-18、IL-33、IL-36和IL-37, 以及S100蛋白S100A8、S100A9和S100A12[37]。随后DAMP由PRR识别, 通过PRR诱导细胞因子和趋化因子的表达来激活免疫应答。
细胞坏死性凋亡最初被认为是宿主为了抵抗具有抗凋亡作用的病毒而进化产生的一种防御方式, 但是后期发现伴随着坏死性凋亡的发生会导致炎性因子失衡, 引起急性或慢性炎症性疾病。例如, 发生肠炎时, RIPK3诱导的坏死性凋亡促进了MLKL的激活, 增加细胞因子IL-8、IL-1β、IL-33、HMGB1的表达, 导致上皮通透性增加, 加重肠道炎症[38]。在急性胰腺炎的小鼠模型中, RIPK3和MLKL介导的坏死性凋亡可加剧组织损伤和炎症[39-40], 在Gaucher病的小鼠模型中, RIPK3缺乏症具有保护作用[41]。此外, RIPK3缺乏症可改善缺血再灌注引起的损伤和炎症, 延长了肾脏和心脏同种异体移植的存活时间[42-44], 这表明细胞的坏死性凋亡可能在移植引发的炎症中起到关键作用。
但是, 也有研究发现, 坏死性凋亡的关键节点蛋白RIPK1、RIPK3及MLKL在多种疾病的炎症应答中发挥保护作用。RIPK1的Ser25磷酸化缺陷可导致多器官炎症; RIPK1的Ser25磷酸化可以抑制RIPK1依赖的坏死性凋亡导致的小鼠血清中IL-6和MCP-1的高表达, 显示RIPK1的磷酸化对于抵抗慢性增生性皮炎有重要作用[45]。在损伤性结肠炎模型中, 葡聚糖硫酸钠(dextran sodium sulfate, DSS)诱导Ripk3-/-小鼠结肠炎, 结肠损伤反而增加, 不同于RIPK3依赖的坏死会促进病理损伤的观点[38]。这是由于DSS主要以RIPK3非依赖的方式诱导Caspase非依赖的损伤, RIPK3在造血细胞的表达对于限制DSS诱导的结肠炎的损伤程度以及组织修复发挥着关键作用。因此, RIPK3以一种非依赖坏死性凋亡的方式, 通过表达IL-23和IL-1β促进IL-22介导组织修复[46]。表明细胞坏死性凋亡及关键蛋白的作用存在组织、细胞差异, 在维持炎性因子平衡和炎症损伤方面发挥着双重作用, 是调控炎症的重要靶点之一。
3 坏死性凋亡与疾病 3.1 感染性疾病感染是引发细胞坏死性凋亡的重要原因之一。某些病原体的感染诱导TNF-α和Ⅰ型干扰素大量表达, 导致细胞发生坏死性凋亡。细胞坏死性凋亡既可以通过增强炎症应答帮助清除病原, 可能造成过度炎症损伤组织, 或因导致细胞死亡而增加胞内病原的释放, 从而增强病原微生物的致病性。
利用斑马鱼模型研究发现, TNF-α在控制结核分枝杆菌(Mycobacterium tuberculosis, Mtb)中发挥重要作用, 但过量的TNF-α可导致组织损伤和器官功能障碍。斑马鱼感染Mtb后, TNF-α的过度释放引起RIPK1/RIPK3/MLKL依赖性坏死信号转导途径的激活, 一方面产生大量的ROS以消除和控制巨噬细胞内细菌的生长和繁殖; 另一方面, 诱导细胞发生坏死性凋亡, 导致细胞中未死亡细菌的释放[47]。结核菌分泌的坏死毒素(tuberculosis necrotizing toxin, TNT)能够水解烟酰胺腺嘌呤二核苷酸(nicotinamide adenine dinucleotide, NAD+), TNT对NAD+的消耗可激活RIPK3和MLKL, 引起坏死性凋亡以杀死受感染的巨噬细胞, 释放胞内细菌[48]。Ⅰ型干扰素在细菌诱导细胞坏死性凋亡中具有重要作用。2012年, Robinson等[49]发现在感染鼠伤寒沙门氏菌的过程中Ⅰ型干扰素诱导RIP激酶导致巨噬细胞发生坏死性凋亡。2017年, Hos等[50]进一步证明, Ⅰ型干扰素通过削弱Nrf2依赖的抗氧化应激反应而使感染了鼠伤寒沙门氏菌的巨噬细胞坏死性凋亡。
产生成孔毒素(pore-forming toxin, PFT)的多种细菌病原体感染也会造成多种细胞坏死性凋亡。例如肺炎链球菌、金黄色葡萄球菌、肺炎克雷伯菌、沙雷氏菌和单增李斯特菌都可以产生PFT, 促进巨噬细胞坏死性凋亡, 丧失免疫调节功能[51-52]。肺炎克雷伯菌(Klebsiella pneumoniae, KB)感染小鼠肺脏时, 通过RIPK1/RIPK3/MLKL依赖的坏死性凋亡途径促进中性粒细胞死亡, 阻止巨噬细胞对肺中性粒细胞的"胞吞作用", 影响机体对凋亡细胞的清除, 破坏体内炎性细胞因子的动态平衡[53]。黏质沙雷氏菌产生的PFT通过引起离子失衡, 以TNFR/TLR4受体非依赖的方式导致肺上皮细胞发生坏死性凋亡[54]。
在一些病理情况下, 如疱疹病毒和人痘病毒可编码一种结构和功能均与cFLIP相似的vFLIP蛋白, 可以抑制Caspase-8, 这虽然阻止了细胞凋亡但是却诱导细胞坏死性凋亡的发生[5, 55-56]。
有少数病毒, 例如单纯疱疹病毒(Herpes simplex virus, HSV)和巨细胞病毒(Cytomegalovirus, CMV), 能够抑制坏死, 从而逃避宿主抗病毒防御。小鼠巨细胞病毒(Mouse cytomegalovirus, MCMV)的M 45 基因编码的RIP激活病毒抑制剂(viral inhibitor of RIP activation, vIRA)是一种RHIM信号抑制因子[57-58], 它可以阻止RIPK3的激活和随后MLKL的激活[59]。人疱疹病毒基因HSV1和HSV2编码R1蛋白ICP6和ICP10, 抑制Caspase-8激活和RIPK1与RIPK3之间的RHIM信号传导[60-61]。已知的所有依赖RIP3激酶的坏死性死亡途径都需要RHIM介导的信号转导, 使MLKL磷酸化[62-63], 但均可被vIRA阻断[64]。然而, 爱泼斯坦-巴尔病毒(Epstein-Barr virus, EBV)抑制细胞坏死的机制不同于MCMV和HSV。EBV编码的潜伏膜蛋白1(latent membrane protein 1, LMP1)缺少RHIM域, LMP1通过其C端激活区直接与RIPK1和RIPK3相互作用, 调节蛋白的翻译后修饰。LMP1促进K63多聚泛素化RIPK1, 抑制RIPK1蛋白表达和抑制K63多聚泛素化RIPK3, 使细胞从坏死性凋亡转为存活[65]。
寄生虫感染也会引起细胞坏死性凋亡, 广州管圆线虫感染引起宿主脑组织实质和海马区小胶质细胞和星形胶质细胞的凋亡和坏死, 感染后Caspase-3、Caspase-4、Caspase-6和RIP3的mRNA水平升高, Caspase-4、裂解的Caspase-3、裂解的Caspase-6、RIP3和pRIP3蛋白水平明显升高。但是, FADD、Beclin-1或LC3B的mRNA或蛋白质水平都没有明显变化, 表明在感染广州管圆线虫的小鼠脑组织同时发生了凋亡和坏死性凋亡[66]。
3.2 非感染性疾病坏死性凋亡在非感染性疾病中也发挥着重要作用。如在神经系统疾病中, 如缺血性脑损伤[67]、阿尔茨海默症(Alzheimer ’ s disease, AD)[68]、帕金森病(Parkinson ’ s disease, PD)[69]。在脑缺血/再灌注(cerebral ischemia/reperfusion, I/R)损伤后, 血管周围M1型小胶质细胞产生的TNF-α介导内皮细胞发生坏死性凋亡, 加重缺血性卒中后对血脑屏障的破坏[67]。阿尔茨海默症的发病特征为严重的神经元缺失, Caccamo等[68]发现减弱细胞坏死性凋亡的激活可减少AD小鼠模型中小鼠神经元的缺失; RIPK1/RIPK3/MLKL介导的坏死性凋亡导致的轴突细胞变性严重危害帕金森病患者的神经系统[69]。在不同的肝脏疾病中, 细胞坏死性凋亡途径的关键蛋白RIPK1和RIPK3分别起到不同的作用。RIPK1参与的炎症反应影响慢性炎症和肝癌的发病过程[70]。非酒精性脂肪肝(non-alcoholic fatty liver, NAFLD)和非酒精性脂肪性肝炎(non-alcoholic steatohepatitis, NASH)的慢性肝细胞死亡相关疾病与坏死性凋亡也有关系。肝细胞损伤、死亡、炎症和氧化应激是NAFLD的主要病理特征。NAFLD和NASH患者外周血TNF-α以及肝脏中RIPK3、MLKL表达增加, 且RIPK3缺失可减轻肝脏的损伤、脂肪变性、炎症、坏死和氧化应激的影响, 因此, RIPK3被认为是NAFLD的治疗靶点[71]。此外, 酒精性肝硬化晚期患者不良预后与RIPK3的表达量相关[72]。
3.3 癌症诱导癌细胞发生坏死性凋亡是治疗癌症的一种方法, 但是坏死性凋亡也参与肿瘤细胞的致病过程。细胞坏死性凋亡可以促进肿瘤细胞的外渗和迁移, 例如肿瘤细胞以表达的淀粉样蛋白前体为介质, 通过其在内皮细胞上的DR6, 诱导内皮细胞发生坏死性凋亡而导致肿瘤细胞外渗和转移[73]。当RIPK1/RIPK3介导发生胰腺肿瘤(pancreatic tumor, PDA)坏死性凋亡时, 释放的可溶性因子CXCL1, 通过抑制性巨噬细胞诱导的免疫抑制性肿瘤微环境, 促进PDA的生长和侵袭[74]。RIPK3或TNFR1的基因失活可减轻结肠炎和癌症的临床症状, 提示RIPK3介导的坏死性凋亡可促进慢性炎症和结肠直肠癌的发生[75-76]。
4 抑制坏死性凋亡的药物研究 4.1 抑制坏死性凋亡的中药小分子化合物中药是中国文化的瑰宝, 很多中药小分子在抑制细胞坏死性凋亡中起重要作用。它们主要靶向细胞坏死性凋亡信号通路中关键效应蛋白RIPK1、RIPK3及MLKL。在神经系统疾病、实质性脏器疾病及炎症性疾病, 药物主要通过抑制细胞坏死性凋亡发挥治疗作用。在癌症治疗中, 大多数癌细胞由于细胞凋亡机制失调而具有耐药性, 通过诱导癌细胞发生细胞坏死性凋亡起抗癌作用。试验证实具有抗坏死性凋亡作用的药物如下。
神经系统疾病药物:姜黄素通过减弱坏死作用, 保护原皮层神经元免受铁引起的神经毒性[77]。槲皮素通过抑制大鼠脊髓损伤后巨噬细胞/小胶质细胞向M1表型的极化来防止少突胶质细胞坏死[78]。川芎素通过靶向RIPK1/RIPK3/MLKL途径减少缺血性脑卒中后大鼠脑坏死[79]。在脑缺血模型中, 茴香霉素通过上调与Hsc70相互作用的蛋白羧基末端从而导致RIPK1和RIPK3蛋白的水平降低, 减轻细胞坏死性凋亡, 从而起到治疗作用[80]。近期研究证实, 银杏叶提取物761(Gingko biloba extract 761, EGB761)通过减轻RIP1介导的线粒体功能障碍和产生ROS来改善细胞坏死性凋亡[81]。
肝脏疾病药物:酒精性肝病(alcoholic liver disease, ALD)是以肝细胞过度死亡和炎症为特征的一种常见疾病, 肝细胞坏死是引起肝脏炎症的主要原因。没食子酸(gallic acid, GA)通过Nrf2依赖机制使坏死信号蛋白RIPK1和RIPK3的表达减少, 限制了乙醇刺激下的肝细胞坏死[82]。
癌症相关疾病药物:紫草素通过RIPK1/RIPK3/MLKL信号通路诱导"坏死性凋亡"杀伤鼻咽癌[83]。黄连素联合顺铂可诱导卵巢癌细胞的坏死和凋亡, 显著增加卵巢癌细胞的死亡, 即黄连素可提高化疗药物的抗癌作用[84]。
炎症性疾病药物:没食子酸可减轻脂多糖诱导的神经炎症——蛋白聚集和坏死[85]。GA是一种酚酸, 几乎存在于植物的所有部位。GA阻止了LPS诱导的Caspase-3激活(程序性细胞死亡的生物标志物)和LPS诱导的RIPK1和RIPK3水平的升高(坏死的生物标志物), 表明GA抑制了LPS诱导的大鼠脑部黑质纹状体多巴胺能系统的凋亡和坏死。
4.2 小分子抑制剂类药物坏死性凋亡是在一系列因子激活和高表达的精准调控下进行的细胞程序性死亡。现有的针对坏死性凋亡途径中关键蛋白的小分子抑制剂也体现出了较好的治疗作用, 可以考虑作为潜在的治疗药物。
4.2.1 RIPK1抑制剂RIP1(RIPK也称RIP)抑制剂Necrostatin-1(Nec-1), 最早是通过筛选坏死病抑制剂而被发现的, 并已在各种与细胞坏死性凋亡相关的动物模型中得到了广泛验证[86-88]。另一种有效的Nec-1衍生物Nec-1s(7-Cl-O-Nec-1)对RIP1具有更高的特异性[89]。RIP1抑制剂还有GSK ’ 963、GSK ’ 481、RIPA-56等[90]。
4.2.2 RIPK3抑制剂与RIP1不同, RIP3对细胞凋亡没有作用, 而靶向抑制RIP3似乎在控制坏死病方面更具特异性。有趣的是, 沉默RIP3可以保护细胞和组织免于坏死性坏死[91], 诸如GSK’ 840、GSK’ 843和GSK’ 872的小分子可抑制RIP3依赖性坏死性肾病[92-94]。
4.2.3 MLKL抑制剂MLKL作为坏死性凋亡的执行者, 也是重要的药物靶标, 已有研究发现了丰富的抑制剂。通过在MLKL中对半胱氨酸进行共价修饰, MLKL抑制剂保护细胞免受TNF诱导的坏死[95]。化合物TC13172通过诱导MLKL的Cys-86处的共价结合对其进行抑制[96]。此外, GW806742X靶向抑制活化的MLKL的假激酶结构域, 保护其免于坏死性凋亡[51]。此外, 索拉非尼是一种多靶点激酶抑制剂, 该药主要用于治疗肾癌、肝癌、甲状腺癌以及部分白血病患者。索拉非尼除了靶向Braf/Mek/Erk和VEGFR途径外, 还靶向坏死性途径, 并且缓解急性炎症中RIPK1/3介导的病理作用[97]。
5 问题与展望坏死性凋亡是一种重要的、自主调控的宿主细胞防御机制, 甚至是一种可以抑制病原体的宿主防御机制。现有研究揭示了坏死性凋亡对机体生理作用的影响、坏死性凋亡通路与节点蛋白、坏死性炎症启动的炎症应答机制等。细胞坏死性凋亡是免疫系统不同参与者相互协调的结果, 细胞坏死性凋亡在时间和空间上多角度影响免疫系统的不同细胞群, 因此有必要揭示细胞坏死过程调控免疫细胞表型的分子机制, 阐明细胞坏死性凋亡与炎症应答的内在关系。细胞坏死性凋亡和凋亡之间的交叉调节增加了相关通路的复杂性, 如Caspase-8、FADD蛋白与RIPK1在细胞凋亡和坏死性凋亡通路中复杂的关系。其他的凋亡检查点如Caspase-9、Bal2、Bax是否对程序性坏死起调节作用仍有待深入研究。另外, 坏死性凋亡存在组织差异性, 与多种病症的发生密切相关, 这可能对精准治疗有一定指导作用。研究坏死性凋亡的诱因对凋亡细胞类型、机制以及凋亡在疾病中的作用十分必要。针对细胞坏死性凋亡通路蛋白作为某些疾病的治疗靶点, 几种坏死途径抑制剂已被开发用于试验治疗, 中药提纯物及化学小分子坏死激活剂或抑制剂可能在某些疾病特异性治疗中发挥作用, 但其作用靶点及机制有待进一步研究。
| [1] |
Galluzzi L, Vitale I, Aaronson S A, et al. Molecular mechanisms of cell death:recommendations of the Nomenclature Committee on Cell Death 2018[J]. Cell Death and Differentiation, 2018, 25(3): 486-541. DOI:10.1038/s41418-017-0012-4 |
| [2] |
Laster S M, Wood J G, Gooding L R. Tumor necrosis factor can induce both apoptic and necrotic forms of cell Lysis[J]. Journal of Immunology, 1988, 141(8): 2629-2634. |
| [3] |
Kawahara A, Ohsawa Y, Matsumura H, et al. Caspase-independent cell killing by fas-associated protein with death domain[J]. Journal of Cell Biology, 1998, 143(5): 1353-1360. DOI:10.1083/jcb.143.5.1353 |
| [4] |
Wallach D, Varfolomeev E E, Malinin N L, et al. Tumor necrosis factor receptor and Fas signaling mechanisms[J]. Annual Review of Immunology, 1999, 17: 331-367. DOI:10.1146/annurev.immunol.17.1.331 |
| [5] |
Chan F K M, Shisler J, Bixby J G, et al. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses[J]. The Journal of Biological Chemistry, 2003, 278(51): 51613-51621. DOI:10.1074/jbc.M305633200 |
| [6] |
Degterev A, Huang Z H, Boyce M, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury[J]. Nature Chemical Biology, 2005, 1(2): 112-119. DOI:10.1038/nchembio711 |
| [7] |
Degterev A, Hitomi J, Germscheid M, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins[J]. Nature Chemical Biology, 2008, 4(5): 313-321. DOI:10.1038/nchembio.83 |
| [8] |
Hitomi J, Christofferson D E, Ng A, et al. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway[J]. Cell, 2008, 135(7): 1311-1323. DOI:10.1016/j.cell.2008.10.044 |
| [9] |
You Z R, Savitz S I, Yang J S, et al. Necrostatin-1 reduces histopathology and improves functional outcome after controlled cortical impact in mice[J]. Journal of Cerebral Blood Flow & Metabolism, 2008, 28(9): 1564-1573. |
| [10] |
Wu W, Liu P, Li J Y. Necroptosis:an emerging form of programmed cell death[J]. Critical Reviews in Oncology/Hematology, 2012, 82(3): 249-258. DOI:10.1016/j.critrevonc.2011.08.004 |
| [11] |
Sauler M, Bazan I S, Lee P J. Cell death in the lung:the apoptosis-necroptosis axis[J]. Annual Review of Physiology, 2019, 81: 375-402. DOI:10.1146/annurev-physiol-020518-114320 |
| [12] |
Belavgeni A, Meyer C, Stumpf J, et al. Ferroptosis and necroptosis in the kidney[J]. Cell Chemical Biology, 2020, 27(4): 448-462. DOI:10.1016/j.chembiol.2020.03.016 |
| [13] |
Paudel S, Ghimire L, Jin L L, et al. NLRC4 suppresses IL-17A-mediated neutrophil-dependent host defense through upregulation of IL-18 and induction of necroptosis during Gram-positive pneumonia[J]. Mucosal Immunology, 2019, 12(1): 247-257. DOI:10.1038/s41385-018-0088-2 |
| [14] |
Orzalli M H, Kagan J C. Apoptosis and necroptosis as host defense strategies to prevent viral infection[J]. Trends in Cell Biology, 2017, 27(11): 800-809. DOI:10.1016/j.tcb.2017.05.007 |
| [15] |
Lee S Y, Kim H, Li C M, et al. Casein kinase-1γ1 and 3 stimulate tumor necrosis factor-induced necroptosis through RIPK3[J]. Cell Death and Disease, 2019, 10(12): 923. DOI:10.1038/s41419-019-2146-4 |
| [16] |
Brault M, Olsen T M, Martinez J, et al. Intracellular nucleic acid sensing triggers necroptosis through synergistic typeⅠIFN and TNF signaling[J]. Journal of Immunology, 2018, 200(8): 2748-2756. DOI:10.4049/jimmunol.1701492 |
| [17] |
Takemura R, Takaki H, Okada S, et al. PolyI:C-induced, TLR3/RIP3-dependent necroptosis backs up immune effector-mediated tumor elimination in vivo[J]. Cancer Immunology Research, 2015, 3(8): 902-914. DOI:10.1158/2326-6066.CIR-14-0219 |
| [18] |
Huang Z J, Zhou T, Sun X W, et al. Necroptosis in microglia contributes to neuroinflammation and retinal degeneration through TLR4 activation[J]. Cell Death and Differentiation, 2018, 25(1): 180-189. DOI:10.1038/cdd.2017.141 |
| [19] |
Kesarwani P, Chakraborty P, Gudi R, et al. Blocking TCR restimulation induced necroptosis in adoptively transferred T cells improves tumor control[J]. Oncotarget, 2016, 7(43): 69371-69383. DOI:10.18632/oncotarget.12674 |
| [20] |
Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation[J]. Nature, 2015, 517(7534): 311-320. DOI:10.1038/nature14191 |
| [21] |
Zhang J Z, Clark K, Lawrence T, et al. An unexpected twist to the activation of IKKβ:TAK1 primes IKKβ for activation by autophosphorylation[J]. The Biochemical Journal, 2014, 461(3): 531-537. DOI:10.1042/BJ20140444 |
| [22] |
Newton K, Dixit V M. Signaling in innate immunity and inflammation[J]. Cold Spring Harbor Perspectives in Biology, 2012, 4(3): a006049. |
| [23] |
Dovey C M, Diep J, Clarke B P, et al. MLKL requires the inositol phosphate code to execute necroptosis[J]. Molecular Cell, 2018, 70(5): 936-948. DOI:10.1016/j.molcel.2018.05.010 |
| [24] |
Wang H Y, Sun L M, Su L J, et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3[J]. Molecular Cell, 2014, 54(1): 133-146. DOI:10.1016/j.molcel.2014.03.003 |
| [25] |
Wilson N S, Dixit V, Ashkenazi A. Death receptor signal transducers:nodes of coordination in immune signaling networks[J]. Nature Immunology, 2009, 10(4): 348-355. DOI:10.1038/ni.1714 |
| [26] |
Baker M O D G, Shanmugam N, Pham C L L, et al. RHIM-based protein:protein interactions in microbial defence against programmed cell death by necroptosis[J]. Seminars in Cell & Developmental Biology, 2020, 99: 86-95. |
| [27] |
Berger A K, Hiller B E, Thete D, et al. Viral RNA at two stages of reovirus infection is required for the induction of necroptosis[J]. Journal of Virology, 2017, 91(6): e02404-16. |
| [28] |
Tsukamoto H, Takeuchi S, Kubota K, et al. Lipopolysaccharide(LPS)-binding protein stimulates CD14-dependent Toll-like receptor 4 internalization and LPS-induced TBK1-IKKε-IRF3 axis activation[J]. Journal of Biological Chemistry, 2018, 293(26): 10186-10201. DOI:10.1074/jbc.M117.796631 |
| [29] |
McComb S, Cessford E, Alturki N A, et al. Type-I interferon signaling through ISGF3 complex is required for sustained Rip3 activation and necroptosis in macrophages[J]. Proc Natl Acad Sci USA, 2014, 111(31): E3206-E3213. DOI:10.1073/pnas.1407068111 |
| [30] |
Cekay M J, Roesler S, Frank T, et al. Smac mimetics and type Ⅱ interferon synergistically induce necroptosis in various cancer cell lines[J]. Cancer Letters, 2017, 410: 228-237. DOI:10.1016/j.canlet.2017.09.002 |
| [31] |
Gong Y N, Guy C, Crawford J C, et al. Biological events and molecular signaling following MLKL activation during necroptosis[J]. Cell Cycle(Greorgetown, Tex.), 2017, 16(19): 1748-1760. DOI:10.1080/15384101.2017.1371889 |
| [32] |
Gong Y N, Guy C, Olauson H, et al. ESCRT-Ⅲ Acts downstream of MLKL to regulate necroptotic cell death and its consequences[J]. Cell, 2017, 169(2): 286-300. DOI:10.1016/j.cell.2017.03.020 |
| [33] |
Teng X, Chen W W, Liu Z H, et al. NLRP3 inflammasome is involved in Q-VD-OPH induced necroptosis following cerebral ischemia-reperfusion injury[J]. Neurochemical Research, 2018, 43(6): 1200-1209. DOI:10.1007/s11064-018-2537-4 |
| [34] |
Schock S N, Chandra N V, Sun Y F, et al. Induction of necroptotic cell death by viral activation of the RIG-I or STING pathway[J]. Cell Death and Differentiation, 2017, 24(4): 615-625. DOI:10.1038/cdd.2016.153 |
| [35] |
Wang W, Wang W H, Azadzoi K M, et al. Activation of innate antiviral immune response via double-stranded RNA-dependent RLR receptor-mediated necroptosis[J]. Scientific Reports, 2016, 6: 22550. DOI:10.1038/srep22550 |
| [36] |
Downey J, Pernet E, Coulombe F, et al. RIPK3 interacts with MAVS to regulate type I IFN-mediated immunity to Influenza A virus infection[J]. PLoS Pathogens, 2017, 13(4): e1006326. DOI:10.1371/journal.ppat.1006326 |
| [37] |
Krysko D V, Garg A D, Kaczmarek A, et al. Immunogenic cell death and DAMPs in cancer therapy[J]. Nature Reviews Cancer, 2012, 12(12): 860-875. DOI:10.1038/nrc3380 |
| [38] |
Negroni A, Colantoni E, Pierdomenico M, et al. RIP3 AND pMLKL promote necroptosis-induced inflammation and alter membrane permeability in intestinal epithelial cells[J]. Digestive and Liver Disease, 2017, 49(11): 1201-1210. DOI:10.1016/j.dld.2017.08.017 |
| [39] |
He S D, Wang L, Miao L, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-A[J]. Cell, 2009, 137(6): 1100-1111. DOI:10.1016/j.cell.2009.05.021 |
| [40] |
Wu J F, Huang Z, Ren J M, et al. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis[J]. Cell Research, 2013, 23(8): 994-1006. DOI:10.1038/cr.2013.91 |
| [41] |
Vitner E B, Salomon R, Farfel-Becker T, et al. RIPK3 as a potential therapeutic target for Gaucher's disease[J]. Nature Medicine, 2014, 20(2): 204-208. DOI:10.1038/nm.3449 |
| [42] |
Linkermann A, Brasen J H, Darding M, et al. Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury[J]. Proc Natl Acad Sci USA, 2013, 110(29): 12024-12029. DOI:10.1073/pnas.1305538110 |
| [43] |
Lau A, Wang S, Jiang J, et al. RIPK3-mediated necroptosis promotes donor kidney inflammatory injury and reduces allograft survival[J]. American Journal of Transplantation, 2013, 13(11): 2805-2818. DOI:10.1111/ajt.12447 |
| [44] |
Pavlosky A, Lau A, Su Y, et al. RIPK3-mediated necroptosis regulates cardiac allograft rejection[J]. American Journal of Transplantation, 2014, 14(8): 1778-1790. DOI:10.1111/ajt.12779 |
| [45] |
Dondelinger Y, Delanghe T, Priem D, et al. Serine 25 phosphorylation inhibits RIPK1 kinase-dependent cell death in models of infection and inflammation[J]. Nature Communications, 2019, 10(1): 1729. DOI:10.1038/s41467-019-09690-0 |
| [46] |
Moriwaki K, Balaji S, McQuade T, et al. The necroptosis adaptor RIPK3 promotes injury-induced cytokine expression and tissue repair[J]. Immunity, 2014, 41(4): 567-578. DOI:10.1016/j.immuni.2014.09.016 |
| [47] |
Roca F J, Ramakrishnan L. TNF dually mediates resistance and susceptibility to Mycobacteria via mitochondrial reactive oxygen species[J]. Cell, 2013, 153(3): 521-534. DOI:10.1016/j.cell.2013.03.022 |
| [48] |
Pajuelo D, Gonzalez-Juarbe N, Tak U, et al. NAD+ depletion triggers macrophage necroptosis, a cell death pathway exploited by Mycobacterium tuberculosis[J]. Cell Reports, 2018, 24(2): 429-440. DOI:10.1016/j.celrep.2018.06.042 |
| [49] |
Robinson N, McComb S, Mulligan R, et al. Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar typhimurium[J]. Nature Immunology, 2012, 13(10): 954-962. DOI:10.1038/ni.2397 |
| [50] |
Hos N J, Ganesan R, Gutiérrez S, et al. Type I interferon enhances necroptosis of Salmonella typhimurium-infected macrophages by impairing antioxidative stress responses[J]. The Journal of Cell Biology, 2017, 216(12): 4107-4121. DOI:10.1083/jcb.201701107 |
| [51] |
González-Juarbe N, Gilley R P, Hinojosa C A, et al. Pore-forming toxins induce macrophage necroptosis during acute bacterial pneumonia[J]. PLoS Pathogens, 2015, 11(12): e1005337. DOI:10.1371/journal.ppat.1005337 |
| [52] |
Ahn D, Prince A. Participation of necroptosis in the host response to acute bacterial pneumonia[J]. Journal of Innate Immunity, 2017, 9(3): 262-270. DOI:10.1159/000455100 |
| [53] |
Jondle C N, Gupta K, Mishra B B, et al. Klebsiella pneumoniae infection of murine neutrophils impairs their efferocytic clearance by modulating cell death machinery[J]. PLoS Pathogens, 2018, 14(10): e1007338. DOI:10.1371/journal.ppat.1007338 |
| [54] |
González-Juarbe N, Bradley K M, Shenoy A T, et al. Pore-forming toxin-mediated ion dysregulation leads to death receptor-independent necroptosis of lung epithelial cells during bacterial pneumonia[J]. Cell Death and Differentiation, 2017, 24(5): 917-928. DOI:10.1038/cdd.2017.49 |
| [55] |
Bertin J, Armstrong R C, Ottilie S, et al. Death effector domain-containing herpesvirus and poxvirus proteins inhibit both Fas- and TNFR1-induced apoptosis[J]. Proc Natl Acad Sci USA, 1997, 94(4): 1172-1176. DOI:10.1073/pnas.94.4.1172 |
| [56] |
Guo H Y, Kaiser W J, Mocarski E S. Manipulation of apoptosis and necroptosis signaling by herpesviruses[J]. Medical Microbiology and Immunology, 2015, 204(3): 439-448. DOI:10.1007/s00430-015-0410-5 |
| [57] |
Upton J W, Kaiser W J, Mocarski E S. Cytomegalovirus M45 cell death suppression requires receptor-interacting protein(RIP)homotypic interaction motif(RHIM)-dependent interaction with RIP1[J]. The Journal of Biological Chemistry, 2008, 283(25): 16966-16970. DOI:10.1074/jbc.C800051200 |
| [58] |
Upton J W, Kaiser W J, Mocarski E S. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine Cytomegalovirus vIRA[J]. Cell Host & Microbe, 2019, 26(4): 564. |
| [59] |
Kaiser W J, Sridharan H, Huang C Z, et al. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL[J]. The Journal of Biological Chemistry, 2013, 288(43): 31268-31279. DOI:10.1074/jbc.M113.462341 |
| [60] |
Guo H Y, Omoto S, Harris P A, et al. Herpes simplex virus suppresses necroptosis in human cells[J]. Cell Host & Microbe, 2015, 17(2): 243-251. |
| [61] |
He S D, Han J H. Manipulation of host cell death pathways by Herpes simplex virus[M]//Current Topics in Microbiology and Immunology. Berlin:Springer, 2020.
|
| [62] |
Murphy J M, Czabotar P E, Hildebrand J M, et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism[J]. Immunity, 2013, 39(3): 443-453. DOI:10.1016/j.immuni.2013.06.018 |
| [63] |
Xie T, Peng W, Yan C Y, et al. Structural insights into RIP3-mediated necroptotic signaling[J]. Cell Reports, 2013, 5(1): 70-78. DOI:10.1016/j.celrep.2013.08.044 |
| [64] |
Upton J W, Kaiser W J, Mocarski E S. Virus inhibition of RIP3-dependent necrosis[J]. Cell Host & Microbe, 2010, 7(4): 302-313. |
| [65] |
Liu X L, Li Y S, Peng S L, et al. Epstein-Barr virus encoded latent membrane protein 1 suppresses necroptosis through targeting RIPK1/3 ubiquitination[J]. Cell Death & Disease, 2018, 9(2): 53. |
| [66] |
Zhang M Y, Yiyue X, Tong P, et al. Apoptosis and necroptosis of mouse hippocampal and parenchymal astrocytes, microglia and neurons caused by Angiostrongylus cantonensis infection[J]. Parasites & Vectors, 2017, 10(1): 1-19. |
| [67] |
Chen A Q, Fang Z, Chen X L, et al. Microglia-derived TNF-α mediates endothelial necroptosis aggravating blood brain-barrier disruption after ischemic stroke[J]. Cell Death & Disease, 2019, 10(7): 487. |
| [68] |
Caccamo A, Branca C, Piras I S, et al. Necroptosis activation in Alzheimer's disease[J]. Nature Neuroscience, 2017, 20(9): 1236-1246. DOI:10.1038/nn.4608 |
| [69] |
Oñate M, Catenaccio A, Salvadores N, et al. The necroptosis machinery mediates axonal degeneration in a model of Parkinson disease[J]. Cell Death and Differentiation, 2020, 27(4): 1169-1185. DOI:10.1038/s41418-019-0408-4 |
| [70] |
Kondylis V, Pasparakis M. RIP kinases in liver cell death, inflammation and cancer[J]. Trends in Molecular Medicine, 2019, 25(1): 47-63. DOI:10.1016/j.molmed.2018.10.007 |
| [71] |
Afonso M B, Rodrigues P M, Carvalho T, et al. Necroptosis is a key pathogenic event in human and experimental murine models of non-alcoholic steatohepatitis[J]. Clinical Science, 2015, 129(8): 721-739. DOI:10.1042/CS20140732 |
| [72] |
Zhang Z Z, Xie G M, Liang L, et al. RIPK3-mediated necroptosis and neutrophil infiltration are associated with poor prognosis in patients with alcoholic cirrhosis[J]. Journal of Immunology Research, 2018, 2018: 1509851. |
| [73] |
Strilic B, Yang L D, Albarrán-Juárez J, et al. Tumour-cell-induced endothelial cell necroptosis via death receptor 6 promotes metastasis[J]. Nature, 2016, 536(7615): 215-218. DOI:10.1038/nature19076 |
| [74] |
Seifert L, Werba G, Tiwari S, et al. The necrosome promotes pancreatic oncogenesis via CXCL1 and Mincle-induced immune suppression[J]. Nature, 2016, 532(7598): 245-249. DOI:10.1038/nature17403 |
| [75] |
Shi J, Li J, Su W, et al. Loss of periodontal ligament fibroblasts by RIPK3-MLKL-mediated necroptosis in the progress of chronic periodontitis[J]. Scientific Reports, 2019, 9(1): 2902. DOI:10.1038/s41598-019-39721-1 |
| [76] |
Tortola L, Nitsch R, Bertrand M J M, et al. The tumor suppressor Hace1 is a critical regulator of TNFR1-mediated cell fate[J]. Cell Reports, 2016, 16(12): 3414. |
| [77] |
Dai M C, Zhong Z H, Sun Y H, et al. Curcumin protects against iron induced neurotoxicity in primary cortical neurons by attenuating necroptosis[J]. Neuroscience Letters, 2013, 536: 41-46. DOI:10.1016/j.neulet.2013.01.007 |
| [78] |
Fan H, Tang H B, Shan L Q, et al. Quercetin prevents necroptosis of oligodendrocytes by inhibiting macrophages/microglia polarization to M1 phenotype after spinal cord injury in rats[J]. Journal of Neuroinflammation, 2019, 16(1): 206. DOI:10.1186/s12974-019-1613-2 |
| [79] |
Chtourou Y, Slima A B, Makni M, et al. Naringenin protects cardiac hypercholesterolemia-induced oxidative stress and subsequent necroptosis in rats[J]. Pharmacological Reports, 2015, 67(6): 1090-1097. DOI:10.1016/j.pharep.2015.04.002 |
| [80] |
Tang M B, Li Y S, Li S H, et al. Anisomycin prevents OGD-induced necroptosis by regulating the E3 ligase CHIP[J]. Scientific Reports, 2018, 8: 6379. DOI:10.1038/s41598-018-24414-y |
| [81] |
Tu J L, Chen W P, Cheng Z J, et al. EGb761 ameliorates cell necroptosis by attenuating RIP1-mediated mitochondrial dysfunction and ROS production in both in vivo and in vitro models of Alzheimer's disease[J]. Brain Research, 2020, 1736: 146730. DOI:10.1016/j.brainres.2020.146730 |
| [82] |
Zhou Y, Jin H H, Wu Y, et al. Gallic acid protects against ethanol-induced hepatocyte necroptosis via an NRF2-dependent mechanism[J]. Toxicology in Vitro, 2019, 57: 226-232. DOI:10.1016/j.tiv.2019.03.008 |
| [83] |
Liu T C, Sun X, Cao Z W. Shikonin-induced necroptosis in nasopharyngeal carcinoma cells via ROS overproduction and upregulation of RIPK1/RIPK3/MLKL expression[J]. Onco Targets and Therapy, 2019, 12: 2605-2614. DOI:10.2147/OTT.S200740 |
| [84] |
Liu L, Fan J Y, Ai G H, et al. Berberine in combination with cisplatin induces necroptosis and apoptosis in ovarian cancer cells[J]. Biological Research, 2019, 52(1): 37. DOI:10.1186/s40659-019-0243-6 |
| [85] |
Liu Y L, Hsu C C, Huang H J, et al. Gallic acid attenuated LPS-induced neuroinflammation:protein aggregation and necroptosis[J]. Molecular Neurobiology, 2020, 57(1): 96-104. DOI:10.1007/s12035-019-01759-7 |
| [86] |
Deng X X, Li S S, Sun F Y. Necrostatin-1 prevents necroptosis in brains after ischemic stroke via inhibition of RIPK1-mediated RIPK3/MLKL signaling[J]. Aging and Disease, 2019, 10(4): 807-817. DOI:10.14336/AD.2018.0728 |
| [87] |
Zhang L P, Feng Q M, Wang T. Necrostatin-1 protects against paraquat-induced cardiac contractile dysfunction via RIP1-RIP3-MLKL-dependent necroptosis pathway[J]. Cardiovascular Toxicology, 2018, 18(4): 346-355. DOI:10.1007/s12012-017-9441-z |
| [88] |
Cui H W, Zhu Y J, Yang Q M, et al. Necrostatin-1 treatment inhibits osteocyte necroptosis and trabecular deterioration in ovariectomized rats[J]. Scientific Reports, 2016, 6: 33803. DOI:10.1038/srep33803 |
| [89] |
Wang Q W, Zhou T, Liu Z J, et al. Inhibition of receptor-interacting protein kinase 1 with necrostatin-1s ameliorates disease progression in elastase-induced mouse abdominal aortic aneurysm model[J]. Scientific Reports, 2017, 7: 42159. DOI:10.1038/srep42159 |
| [90] |
Beal A M, Bertin J, Reilly M A. Use of RIP1 kinase small-molecule inhibitors in studying necroptosis[J]. Methods in Molecular Biology, 2018, 1857: 109-124. |
| [91] |
Onizawa M, Oshima S, Schulze-Topphoff U, et al. The ubiquitin-modifying enzyme A20 restricts ubiquitination of the kinase RIPK3 and protects cells from necroptosis[J]. Nature Immunology, 2015, 16(6): 618-627. DOI:10.1038/ni.3172 |
| [92] |
Geserick P, Wang J, Schilling R, et al. Absence of RIPK3 predicts necroptosis resistance in malignant melanoma[J]. Cell Death & Disease, 2015, 6(9): e1884. |
| [93] |
Zhou T, Wang Q W, Phan N, et al. Identification of a novel class of RIP1/RIP3 dual inhibitors that impede cell death and inflammation in mouse abdominal aortic aneurysm models[J]. Cell Death & Disease, 2019, 10(3): 226. |
| [94] |
Gautheron J, Vucur M, Schneider A T, et al. The necroptosis-inducing kinase RIPK3 dampens adipose tissue inflammation and glucose intolerance[J]. Nature Communications, 2016, 7: 11869. DOI:10.1038/ncomms11869 |
| [95] |
Sun L M, Wang H Y, Wang Z G, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase[J]. Cell, 2012, 148(1/2): 213-227. |
| [96] |
Yan B, Liu L, Huang S Q, et al. Discovery of a new class of highly potent necroptosis inhibitors targeting the mixed lineage kinase domain-like protein[J]. Chemical Communications, 2017, 53(26): 3637-3640. DOI:10.1039/C7CC00667E |
| [97] |
Martens S, Jeong M, Tonnus W, et al. Sorafenib tosylate inhibits directly necrosome complex formation and protects in mouse models of inflammation and tissue injury[J]. Cell Death & Disease, 2017, 8(6): e2904. |




