文章信息
- 姚宏, 郑玉才, 李志雄, 王云浩, 谈永萍, 徐亚欧, 王爱林, 董传豪, 冯卫东, 饶开晴
- YAO Hong, ZHENG Yucai, LI Zhixiong, WANG Yunhao, TAN Yongping, XU Ya'ou, WANG Ailin, DONG Chuanhao, FENG Weidong, RAO Kaiqing
- 低氧环境下蛋内注射CuSO4对藏鸡和肉鸡鸡胚发育的影响
- Effects of in ovo injection of CuSO4 on the development of Tibetan chicken and broiler chicken embryos hatched under hypoxia conditions
- 南京农业大学学报, 2020, 43(6): 1079-1086
- Journal of Nanjing Agricultural University, 2020, 43(6): 1079-1086.
- http://dx.doi.org/10.7685/jnau.201910038
-
文章历史
- 收稿日期: 2019-10-24
2. 甘孜州畜牧业科学研究所, 四川 康定 626000
2. Ganzi Prefectural Livestock Research Institute, Kangding 626000, China
藏鸡是我国肉蛋两用型的地方家禽品种, 主要生活在海拔2 200~4 000 m的西藏的高原地区, 四川西北地区亦分布广泛[1]。藏鸡长期生活在高原低氧环境中, 低氧环境不仅是制约藏鸡生长繁殖的重要因素, 也是致使低海拔鸡无法在高原地区饲养的主要原因。氧气对鸡胚血管生成和抗氧化性能的调控是影响鸡胚发育的主要因素[2-3]。Cu2+作为机体必需的微量元素之一, 它不仅是蛋内营养物质的重要组成部分[4], 而且Cu2+还是铜锌超氧化物歧化酶(Cu/Zn-SOD)的活性中心[5], 它能够提高机体的抗氧化能力; 同时Cu2+也是血管形成的重要辅助因子, 调控血管内皮生长因子(vascular endothelial growth factor, VEGF)基因的表达, 从而影响血管内皮细胞的出芽和迁移, 促进血管生成[6-8]。Cu2+的缺乏将导致新血管生成受阻[9]; Urso等[10]研究发现, 血管生成障碍所引发的局部贫血与血清中Cu2+水平相关; 饲粮中添加Cu2+可以提高小鼠心肌VEGF蛋白的表达, 促使心肌血管形成[11]; 种蛋内注射纳米铜可有效增加9胚龄鸡胚尿囊膜上的血管数量和长度, 上调20日龄鸡胚胸肌组织VEGF基因的表达[12], 以上研究均表明Cu2+在血管生成中的重要作用。目前在低氧环境下改变种蛋内Cu2+含量对鸡胚血管生成和抗氧化性能影响的研究尚未见报道。因此本试验通过向藏鸡和科宝肉鸡种蛋内注射CuSO4的方式, 探讨Cu2+在低氧条件下对鸡胚发育的影响及机制, 从而为提高藏鸡的生产力及低海拔家禽的高原环境适应力提供科学的参考依据。
1 材料与方法 1.1 试验设计选择产蛋中期质量相近的藏鸡种蛋(甘孜州海螺沟畜牧业有限责任公司)和科宝肉鸡种蛋(温江正大畜禽有限公司)各132枚, 随机平均分成3组:对照组(90 μg·mL-1 NaCl溶液)、低剂量组(Cu2+质量浓度为75 μg·mL-1的CuSO4溶液)、高剂量组(Cu2+质量浓度为300 μg·mL-1的CuSO4溶液)。
1.2 蛋内注射CuSO4及鸡胚的孵化种蛋小头端用体积分数为75%乙醇消毒后打孔, 对照组使用1 mL一次性灭菌注射器抽取高压灭菌后的生理盐水100 μL, 垂直进针约1 cm注入蛋清; 低剂量、高剂量组以相同的方法注入100 μL不同质量浓度的CuSO4注射液。在高原低氧环境(海拔约2 000 m的四川省甘孜州康定县)孵化, 孵化条件参照科裕孵化箱使用说明书中禽类种蛋的孵化条件。藏鸡、肉鸡种蛋孵化至12胚龄时, 从每组编号前10的种蛋各取活着的6枚鸡胚进行尿囊膜的固定和采集。1日龄时每组各取10只小鸡采集血清和肝脏, 血清于-20 ℃冰箱保存, 肝脏置于液氮速冻后移至-80 ℃冰箱保存。
1.3 12胚龄鸡胚尿囊膜固定及血管面积、血管数量测定在12胚龄的鸡胚气室端开孔, 将甲醇-丙酮(1:1)固定液2 mL从孔滴入, 固定1 h后, 将尿囊膜和壳膜一并剪下贴于载玻片, 干燥30 min, 于显微镜下观察拍照, 用Image-Pro Plus 6.0图像分析软件进行图像分析, 以血管面积/尿囊膜面积的比值作为最终结果。根据直径大小将血管分为3类进行统计, 分别为大血管(直径≥100 μm)、中血管(50 μm≤直径 < 100 μm)、小血管(直径 < 50 μm)。
1.4 出雏率和组织质量的测定出雏率等指标由每组编号为21~44的24枚种蛋提供, 出雏率=出雏数量/受精种蛋数量。各组织质量使用电子天平称量, 计算出雏质量/蛋质量和组织体比(组织质量/体质量)。
1.5 与抗氧化相关指标的测定用试剂盒检测1日龄小鸡血清中Cu/Zn-SOD和GSH-Px活性以及MDA含量。将0.1 g肝脏加入装有0.9 mL生理盐水的EP管中, 在冰上匀浆, 2 500 r·min-1离心后得到体积分数为10%肝脏匀浆液, 取上清液用生理盐水按1:9稀释成体积分数为1%的肝脏匀浆液。1%匀浆液用作蛋白含量的测定以及Cu/Zn-SOD和GSH-Px活性的检测, 10%匀浆液用于MDA含量的检测。具体方法参照试剂盒说明书。
1.6 肝脏中VEGF基因表达的测定使用RNAiso Reagent试剂盒(Invitrogen公司)提取1日龄小鸡肝脏总RNA, 再用反转录试剂盒(TaKaRa公司)将肝脏总RNA进行反转录(RT)获得cDNA。用Primer Premier 5.0和Oligo 6.0软件设计目的基因(VEGF)和内标基因(β-actin)引物。引物信息见表 1, 由苏州金唯智生物科技有限公司合成。用荧光定量PCR(qPCR)分析肝组织中VEGF基因和β-actin基因的表达, 按SYBR GREENⅠ荧光定量试剂盒(TaKaRa公司)建议的反应体系, 混合反应物, 总体积10 μL。反应程序:95 ℃预变性15 s, 95 ℃变性15 s, 60 ℃退火30 s, 共40个循环。
| 目的基因 Target genes |
GenBank登录号 GenBank accession No. |
PCR产物长度/bp PCR products size |
引物对序列 Primer pairs sequences (5′→3′) |
退火温度/℃ Annealing temperature |
| β-actin | NM205518 | 300 | TGCGTGACATCAAGGAGAAG/TGCCAGGGTACATTGTGGTA | 60 |
| VEGF | AY168004 | 261 | GAAAGGGGAAGGGTCAA/GAGAAATCAGGCTCCAGAA | 60 |
| 注:β-actin:β-肌动蛋白基因β-actin gene; VEGF:血管内皮生长因子基因Vascular endothelial growth factor gene. | ||||
出雏质量/蛋质量、组织质量/体质量及基因表达、酶活性等数据采用SPSS 19.0软件进行多重比较分析, 出雏率使用SPSS 19.0软件进行卡方检验。用2-ΔΔCT法对基因进行相对定量表达分析。所有数值均以平均值±标准误(x±SE)表示。
2 结果与分析 2.1 低氧环境下种蛋内注射CuSO4对藏鸡、肉鸡出雏率和出雏质量/蛋质量和出雏组织体比的影响如图 1所示:低氧孵化下肉鸡出雏率随蛋内注射CuSO4剂量的增加而上升, 且高剂量组显著高于对照组(P < 0.05);肉鸡低剂量组出雏质量/蛋质量和腿体比显著高于对照组(P < 0.05), 高剂量组肝体比显著高于对照组(P < 0.05);其他组织体比(脑、胸肌、心、肺)3组之间均无显著差异(P>0.05)(图未列出)。藏鸡蛋内注射CuSO4组与对照组相比出雏率差异不显著(P < 0.05);藏鸡蛋内注射CuSO4组与对照组相比出雏质量/蛋质量差异不显著(P>0.05);低剂量组肝体比显著高于对照组(P < 0.05);其他组织体比(脑、胸肌、腿肌、心、肺)各组之间均无显著差异(P>0.05)(图未列出)。
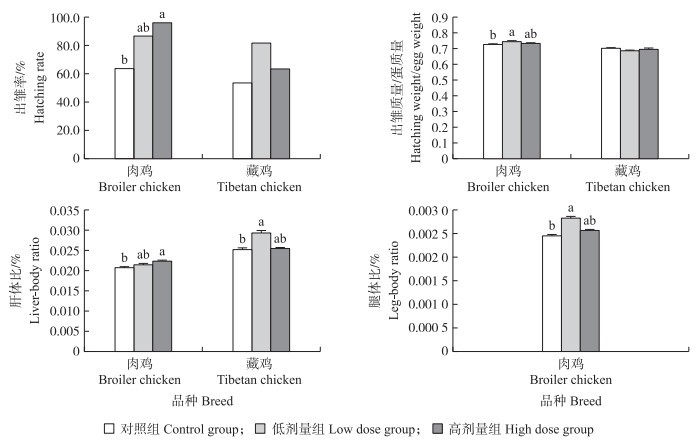
|
图 1 低氧环境下种蛋内注射CuSO4对肉鸡和藏鸡出雏率、出雏质量/蛋质量及1日龄组织体比的影响 Fig. 1 Effects of in ovo injection of CuSO4 on hatching rate, hatching weight/egg weight, tissue weight/body weight in one-day-old broiler chickens and Tibetan chickens under hypoxia condition 不同小写字母表示同一品种差异显著(P < 0.05)。图 2同此。 The data with different lowercase letters indicate significant difference on the same breed(P < 0.05). The same as in the Fig. 2. |
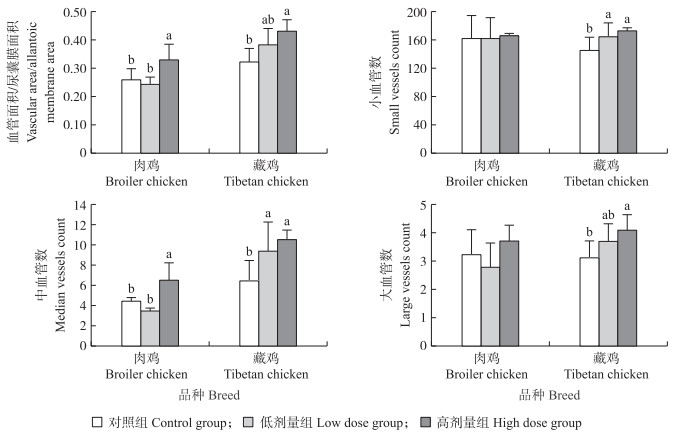
|
图 2 低氧环境下种蛋内注射CuSO4对12胚龄肉鸡、藏鸡尿囊膜血管面积及血管数的影响 Fig. 2 Effects of in ovo injection of CuSO4 on chorioallantoic membrane(CAM)vascular area and number in chickens and broiler Tibetan chickens of 12 embryonic age(E12)under hypoxia conditions |
蛋内注射CuSO4能有效促进低氧孵化下肉鸡的血管生成, 其中高剂量组尿囊膜血管面积以及中血管数都显著高于对照组(P < 0.05), 而对小血管数没有影响(P>0.05)(图 2); 蛋内注射CuSO4也能促进低氧孵化下藏鸡尿囊膜上血管的生成, 高剂量组血管面积和大、中、小血管数均高于对照组, 且差异显著(P < 0.05), 低剂量组中血管数和小血管数也显著高于对照组(P < 0.05)(图 2)。
2.3 低氧环境下种蛋内注射CuSO4对1日龄藏鸡、肉鸡血清和肝脏中Cu/Zn-SOD、GSH-Px活性及MDA含量的影响种蛋内注射低、高剂量的CuSO4均能提高低氧孵化1日龄肉鸡血清Cu/Zn-SOD的活性(P < 0.05), 而藏鸡仅在低剂量组与对照组间有显著差异(P < 0.05), 3组中肉鸡的Cu/Zn-SOD活性均高于藏鸡; 蛋内注射CuSO4对低氧孵化下藏鸡、肉鸡血清内GSH-Px活性均无显著影响, 但藏鸡低剂量组GSH-Px活性显著高于高剂量组(P < 0.05), 并极显著高于低剂量组的肉鸡(P < 0.01);高剂量CuSO4能显著降低低氧孵化下1日龄藏鸡和肉鸡血清中MDA含量(P < 0.05);肉鸡各组的MDA含量均极显著低于藏鸡(图 3)。Cu2+对于低氧孵化下肉鸡肝脏内Cu/Zn-SOD和GSH-Px活性均无显著影响(P>0.05), 但高剂量CuSO4可显著降低肉鸡肝脏中MDA的含量(P < 0.05), 并且还显著提高低氧孵化藏鸡肝脏中Cu/Zn-SOD活性(P < 0.05)和显著降低MDA含量及GSH-Px活性(P < 0.05);肉鸡低剂量组Cu/Zn-SOD活性、MDA含量和高剂量组GSH-Px活性都显著高于同组藏鸡。
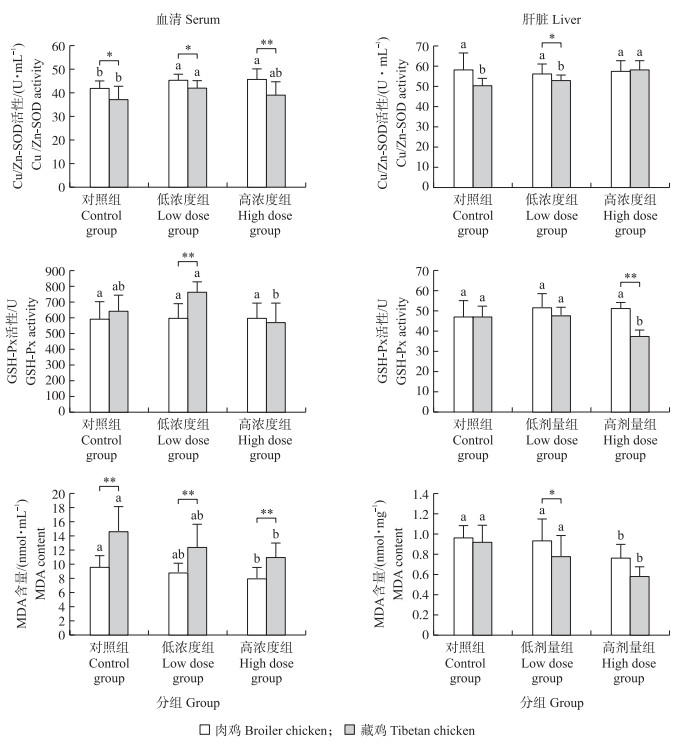
|
图 3 低氧环境下种蛋内注射CuSO4对1日龄藏鸡、肉鸡血清和肝脏中Cu/Zn-SOD、GSH-Px活性及MDA含量的影响 Fig. 3 Effects of in ovo injection of CuSO4on the activities of Cu/Zn-SOD, GSH-Px and content of MDA in serum and liver of Tibetan chickens and broilers at one-day-old under hypoxia condition 不同小写字母表示同一品种不同处理组间差异显著(P < 0.05);*和**表示两品种间差异显著(P < 0.05)和极显著(P < 0.01)。下同。 The data with different lowercase letters indicate significant difference in the different of the same breed(P < 0.05);* and ** indicate significant difference in the two breeds at 0.05 and 0.01 levels. The same as follows. |
蛋内注射CuSO4对低氧孵化下12胚龄藏鸡和肉鸡尿囊膜上VEGF基因的表达均有影响。其中肉鸡低剂量组VEGF基因表达量显著高于对照组(P < 0.05), 藏鸡低、高剂量组VEGF基因表达量均显著高于对照组(P < 0.05)。各组藏鸡尿囊膜VEGF基因的表达量均高于肉鸡。在对照组两品种间比较有显著差异(P < 0.05), 在低剂量组和高剂量组两品种间比较差异为极显著(P < 0.01)(图 4-A)。
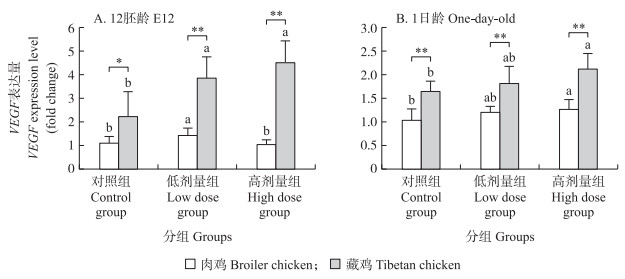
|
图 4 种蛋内注射CuSO4对12胚龄肉鸡、藏鸡尿囊膜和1日龄肉鸡、藏鸡肝脏VEGF基因表达量的影响 Fig. 4 Effects of in ovo injection of CuSO4on the expression level of VEGF gene in CAM and liver of broiler chickens and Tibetan chickens of E12 and one-day-old under hypoxia condition |
与对照组相比, 高剂量CuSO4能显著上调低氧孵化1日龄藏鸡和肉鸡肝脏中VEGF基因的表达量(P < 0.05), 而低剂量CuSO4对其影响不大(P>0.05)。3组中藏鸡VEGF基因表达量均极显著高于肉鸡(P < 0.01)(图 4-B)。
3 讨论氧气是影响鸡胚发育和出雏的重要因素, 低氧不仅影响藏鸡的繁殖和生产性能, 也制约着高原地区平原鸡种的养殖[13]。本试验结果显示, 蛋内注射CuSO4能有效提高低海拔肉鸡在高原环境的出雏率, 增加肉鸡的出雏质量及肝体比和腿体比, 促进胚胎的生长, 但Cu2+对长期生活在低氧环境下藏鸡的作用效果不如肉鸡明显, 仅对鸡胚的肝体比有影响。本试验中, 藏鸡孵化率的增高趋势不明显, 可能是由于藏鸡的受精率较低导致用于孵化率统计的样本数减少, 致使试验组与对照组孵化率差异不显著。因此提高藏鸡种蛋受精率也是未来藏鸡生产中应着重关注的问题。
Cu2+能降低低氧孵化的1日龄肉鸡和藏鸡血清中MDA含量, 表明低氧环境下, Cu2+在鸡胚机体抗低氧过程中起着重要作用, 而血清中Cu/Zn-SOD和GSH-Px活性的变化情况显示这种作用是通过增强血清中Cu/Zn-SOD活性而不是GSH-Px活性来实现的[14-17]。低剂量CuSO4对藏鸡和肉鸡肝脏Cu/Zn-SOD、GSH-Px活性和MDA含量均无显著影响, 而高剂量CuSO4能提升藏鸡Cu/Zn-SOD活性、降低藏鸡和肉鸡MDA含量, 但藏鸡GSH-Px活性下降, 分析原因可能是由于Cu2+会影响硒的吸收, 而硒是GSH-Px的重要组成部分[18], 所以GSH-Px活性因此而受到影响。这进一步证实了Cu2+对低氧环境下机体抗氧化能力的提升是通过增强Cu/Zn-SOD活性来实现的。藏鸡和肉鸡的对比结果显示, 肉鸡血清各组中Cu/Zn-SOD活性均高于藏鸡, 同时MDA含量均低于藏鸡, 说明在低氧环境下肉鸡比藏鸡具有更强的抗氧化能力, 这可能与肉鸡是生活在氧含量较高的平原环境中有关。
本试验结果显示藏鸡、肉鸡种蛋内注射高剂量的CuSO4, 能增加尿囊膜上的血管面积和中血管数, 同时对藏鸡的大、小血管的血管生成也具有促进作用, 证实了在鸡胚发育过程中, Cu2+对血管生成的调控作用。鸡胚尿囊膜是血管生成试验的常用模型[19-22], 大量研究证实尿囊膜上血管面积和数量的改变能有效调节鸡胚死亡率、出雏率和胚胎发育[23-25]。所以, 本试验中Cu2+能提高低氧环境下肉鸡出雏率、出雏质量和关键组织质量, 可能是其通过促进肉鸡血管生成来实现的。
VEGF调节内皮细胞的有丝分裂和增殖、血管的修复和生成[26]。低氧能使低氧诱导因子(hypoxia-inducible factor, HIF)逃脱PHD酶的降解, 造成HIF-1大量蓄积, 从而使低氧下VEGF基因的表达发生变化[27-29]。在此过程中铜离子发挥着重要作用, 包括调控低氧诱导因子抑制因子(HIF inhibiting factor, FIH)的活性和促进HIF-1与VEGF基因间的亲和力等[19]。本试验结果显示, 低、高剂量的CuSO4能有效上调藏鸡尿囊膜中的VEGF基因表达, 这与尿囊膜上中、小血管数量的研究结果一致; 此外高剂量的CuSO4能有效上调藏鸡和肉鸡肝脏中VEGF基因的表达量。本试验还发现, 无论在尿囊膜还是肝脏组织中, 3个组藏鸡VEGF基因的表达量均高于肉鸡, 这可能是由于藏鸡长期生活在高原低氧环境中, 形成了通过上调血管生成相关基因的表达量来促进血管生成, 提高血氧运输效率, 从而增强其对低氧环境的适应能力。
综上所述, 低氧下在种蛋内注射CuSO4能提高肉鸡孵化率, 促进藏鸡、肉鸡鸡胚尿囊膜血管生成及藏鸡、肉鸡鸡胚肝脏的发育。推测这与Cu2+上调藏鸡、肉鸡鸡胚肝脏和尿囊膜组织VEGF基因表达量及提高藏鸡、肉鸡机体的的抗氧化能力有关。在低氧下蛋内注射CuSO4使藏鸡的血管生成能力强于肉鸡, 而肉鸡机体抗氧化能力强于藏鸡。
| [1] |
郑丕留, 张仲葛, 陈效华, 等. 中国家禽品种志[M]. 上海: 上海科学技术出版社, 1989. Zheng P L, Zhang Z G, Chen X H, et al. China Poultry Species Records[M]. Shanghai: Shanghai Science and Technology Press, 1989 (in Chinese). |
| [2] |
强巴央宗, 谢庄, 商鹏, 等. 藏鸡血管内皮生长因子基因表达与低氧适应[J]. 畜牧兽医学报, 2009, 40(7): 1101-1105. Yang-Zom C, Xie Z, Shang P, et al. Quantitative expression of vascular endothelial growth factor gene and hypoxic adaptation in Tibetan chicken[J]. Acta Veterinaria et Zootechnica Sinica, 2009, 40(7): 1101-1105 (in Chinese with English abstract). |
| [3] |
王翔宇.藏鸡胚胎低氧适应的转录组差异表达分析[D].北京: 中国农业大学, 2014. Wang X Y. Transcriptomic difference analysis in Tibetan chicken embryo at hypoxia[D]. Beijing: China Agricultural University, 2014(in Chinese with English abstract). |
| [4] |
樊银珍, 俸艳萍, 徐双贵, 等. 受精蛋和无精蛋、死胚及正常发育胚的种蛋品质差异研究[J]. 中国家禽, 2008, 30(11): 15-19. Fan Y Z, Feng Y P, Xu S G, et al. Quality difference on hatching eggs of fertile eggs, aspermatism eggs, dead embryos and normal development embryos of chicken[J]. China Poultry, 2008, 30(11): 15-19 (in Chinese with English abstract). |
| [5] |
姜云霞. 微量元素铜的研究进展及其对动物健康的影响[J]. 微量元素与健康研究, 2007, 24(5): 58-61. Jiang Y X. Research progress of trace element copper and its effect on animal health[J]. Studies of Trace Elements and Health, 2007, 24(5): 58-61 (in Chinese with English abstract). |
| [6] |
杨海明, 王志跃, 陈五湖, 等. 鸡蛋孵化期间钙磷含量的变化[J]. 江苏农业科学, 2006, 12(3): 128-130. Yang H M, Wang Z Y, Chen W H, et al. Changes of calcium and phosphorus content during egg incubation[J]. Jiangsu Agricultural Sciences, 2006, 12(3): 128-130 (in Chinese with English abstract). |
| [7] |
李小迪, 覃杨, 何蓉蓉, 等. 鸡胚氧化应激模型研究进展[J]. 中国药理学通报, 2011, 27(1): 15-19. Li X D, Qin Y, He R R, et al. Research progress on oxidative stress models of chicken embryo[J]. Chinese Pharmacological Bulletin, 2011, 27(1): 15-19 (in Chinese with English abstract). |
| [8] |
Golde J C V, Borm P J, Wolfs M C, et al. Induction of antioxidant enzyme activity by hyperoxia(60% O2)in the developing chick embryo[J]. Journal of Physiology, 1998, 509: 289-296. DOI:10.1111/j.1469-7793.1998.289bo.x |
| [9] |
Oliveira T F B, Bertechini A G, Bricka R M, et al. Effects of in ovo injection of organic zinc, manganese, and copper on the hatchability and bone parameters of broiler hatchlings[J]. Poultry Science, 2015, 94(10): 2488-2494. DOI:10.3382/ps/pev248 |
| [10] |
Urso E, Maffia M. Behind the link between copper and angiogenesis:established mechanisms and an overview on the role of vascular copper transport systems[J]. Journal of Vascular Research, 2015, 52(3): 172-196. DOI:10.1159/000438485 |
| [11] |
Jiang Y, Reynolds C C, Feng W, et al. Dietary copper supplementation reverses hypertrophic cardiomyopathy induced by chronic pressure overload in mice[J]. Journal of Experimental Medicine, 2007, 204(3): 657-666. DOI:10.1084/jem.20061943 |
| [12] |
Natalia M S, Ewa S, Krishna Prasad V, et al. Nanoparticles of copper stimulate angiogenesis at systemic and molecular level[J]. International Journal of Molecular Sciences, 2015, 16(3): 4838-4839. DOI:10.3390/ijms16034838 |
| [13] |
朱凤艳. 鸡胚胎的21天[J]. 农民致富之友, 2005(9): 31. Zhu F Y. The 21 days of the chicken embryo[J]. Nongmin Zhifu Zhiyou, 2005(9): 31 (in Chinese). |
| [14] |
李海英, 赵娟, 佟长青. 低氧及复合运动对大鼠血清超氧化物歧化酶活性及丙二醛含量的影响[J]. 现代中西医结合杂志, 2008, 17(15): 2284-2285. Li H Y, Zhao J, Tong C Q. Effects of hypoxia and exercise training combined on superoxide dismutase and concentrations of malondialdehyde in serum of rats[J]. Modern Journal of Integrated Traditional Chinese and Western Medicine, 2008, 17(15): 2284-2285 (in Chinese with English abstract). |
| [15] |
蒲俊华, 吴敏, 张小燕, 等. 不同种类禽蛋微量元素铁铜锰锌含量比较[J]. 畜牧与兽医, 2011, 43(5): 11-13. Pu J H, Wu M, Zhang X Y, et al. Comparison of trace elements Fe, Cu, Mn and Zn in eggs of different species[J]. Animal Husbandry and Veterinary Medicine, 2011, 43(5): 11-13 (in Chinese with English abstract). |
| [16] |
袁惠娟, 陈忆文, 常海清, 等. 禽蛋中铜铁锌钙镁硒等元素分析[J]. 微量元素与健康研究, 2000(1): 47-48. Yuan H J, Chen Y W, Chang H Q, et al. Analysis of copper, iron, zinc, calcium, magnesium and selenium in poultry eggs[J]. Studies of Trace Elements and Health, 2000(1): 47-48 (in Chinese). |
| [17] |
Carmeliet P, Ferreira V, Breier G, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele[J]. Nature, 1996, 380(6573): 435-439. DOI:10.1038/380435a0 |
| [18] |
Feng W, Ye F W, Zhou Z, et al. Copper regulation of hypoxia-inducible factor-1 activity[J]. Molecular Pharmacology, 2008, 75(1): 174-182. |
| [19] |
Bellairs R, Osmond M. The Atlas of Chick Development[M]. London: Elsevier Academic Press, 2005: 110-117.
|
| [20] |
王世军, 孙静, 张栋, 等. 鸡胚尿囊膜血管生长的特点及观测方法[J]. 生物医学工程研究, 2004, 23(1): 38-40. Wang S J, Sun J, Zhang D, et al. Characteristics and observing method of microvessels of chorioallantoic membrane in chicken embryo[J]. Journal of Biomedical Engineering Research, 2004, 23(1): 38-40 (in Chinese with English abstract). |
| [21] |
苏兰利, 王金荣, 王卫国, 等.黄芪多糖浸泡种蛋对鸡胚生长发育和抗氧化性能的影响[C]//2013中国粮油学会饲料分会饲料科技论坛暨学术年会、第二届天目湖论坛、中国饲料工业生产管理与工艺创新论坛论文集.常州, 2013: 112-117. Su L L, Wang J R, Wang W G, et al. Effects of seed eggs soaked with astragalus polysaccharide on the growth and antioxidant properties of chicken embryo[C]//2013 Feed Science and Technology Forum and Academic Annual Conference of Feed Science and Technology of Feed Branch of China Grain and Oil Society, the Second Tianmu Lake Forum, Forum of Production Management and Technological Innovation of China Feed Industry. Changzhou, 2013: 112-117(in Chinese with English abstract). |
| [22] |
饶开晴. Leptin对鸡胚卵黄膜和尿囊膜基因表达及血管形成的影响[D].南京: 南京农业大学, 2009. Rao K Q. Effect of leptin on yolk SAC and chorioallantoic membrane gene expression and angiogenesis in the chicken embryo[D]. Nanjing: Nanjing Agricultural University, 2009(in Chinese with English abstract). |
| [23] |
赵丽, 杨帆, 彭西, 等. 高铜对雏鸭抗氧化酶活性的影响[J]. 中国兽医学报, 2009, 29(2): 210-213. Zhao L, Yang F, Peng X, et al. Effect of high copper diet on the antioxidase activities in ducklings[J]. Chinese Journal of Veterinary Science, 2009, 29(2): 210-213 (in Chinese with English abstract). |
| [24] |
Dumont D J, Jussila L, Taipale J, et al. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3[J]. Science, 1998, 282(5390): 946-949. DOI:10.1126/science.282.5390.946 |
| [25] |
Ferrara N, Carver-Moore K, Chen H, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene[J]. Nature, 1996, 380(6573): 439-442. DOI:10.1038/380439a0 |
| [26] |
des Rieux A, Ucakar B, Mupendwa B P, et al. 3D systems delivering VEGF to promote angiogenesis for tissue engineering[J]. J Control Release, 2011, 150(3): 272-278. DOI:10.1016/j.jconrel.2010.11.028 |
| [27] |
Gaál T, Mézes M, Noble R C, et al. Development of antioxidant capacity in the chick embryo[J]. Comparative Biochemistry & Physiology B:Comparative Biochemistry, 1995, 112(4): 711-716. |
| [28] |
Golde J C V, Borm P J, Wolfs M C, et al. Induction of antioxidant enzyme activity by hyperoxia(60% O2)in the developing chick embryo[J]. Journal of Physiology, 1998, 509(1): 289-296. DOI:10.1111/j.1469-7793.1998.289bo.x |
| [29] |
李敏, 崔伟, 彭西, 等. 高铜对雏鸡脑组织抗氧化酶活性的影响[J]. 畜牧兽医学报, 2010, 41(2): 220-223. Li M, Cui W, Peng X, et al. Effect of dietary high copper on the antioxidase activities of brain tissue in chickens[J]. Acta Veterinaria et Zootechnica Sinica, 2010, 41(2): 220-223 (in Chinese with English abstract). |




