文章信息
- 刘明妮, 胡云, 方茜, 马文强, 赵茹茜
- LIU Mingni, HU Yun, FANG Xi, MA Wenqiang, ZHAO Ruqian
- 慢性皮质酮暴露对肉鸡生长性能及肝脏铁稳态的影响
- Effect of chronic corticosterone exposure on growth performance and hepatic iron homeostasis in broilers
- 南京农业大学学报, 2020, 43(4): 714-719
- Journal of Nanjing Agricultural University, 2020, 43(4): 714-719.
- http://dx.doi.org/10.7685/jnau.201907008
-
文章历史
- 收稿日期: 2019-07-05
现代集约化家禽饲养模式中存在多种应激源, 如高温、高密度养殖、免疫接种、有害气体等。随着人类对家禽快速生长和高饲料报酬的过分追求, 使家禽对各种应激源更加敏感[1-2]。应激因素可激活家禽下丘脑-垂体-肾上腺轴(HPA轴), 分泌大量应激激素, 使机体对应激产生各种应答反应。热应激可显著降低肉鸡生长速度及日采食量, 影响肠道绒毛发育及肠道黏膜完整性[3-6]。研究发现, 热应激可降低肉鸡心脏、肝脏和脾脏相对质量, 进而延迟心脏、肝脏和脾脏的发育[7-8]。高密度饲养会抑制肉鸡生长及降低机体抗氧化机能, 改变肉鸡盲肠菌群多样性, 并提高肉鸡饲料报酬[9-10]。由此可见, 应激会影响家禽生产性能, 给家禽养殖业造成较大的经济损失。
铁是家禽生长发育必需的微量元素之一, 其具有重要的生物学功能, 在DNA、RNA和蛋白质的合成、电子传递、细胞呼吸、细胞增殖和分化等生化过程中起重要作用[12]。任何形式的铁过载可导致组织铁沉积, 诱发氧化应激导致组织损害[13-14]。肝脏是铁贮存和维持铁稳态的主要器官, 动物机体铁失衡可影响动物健康。有研究报道, 心理应激会降低大鼠血铁水平, 并导致肝脏铁过载, 诱发氧化应激[15-17]。与正常对照组相比, 接受束缚应激的小鼠全血中铁含量和热应激后雏鸡血清铁含量都显著降低[18-19]。泌乳奶牛的血清铁含量在热应激后也降低[20]。然而, 应激对家禽铁吸收及肝脏铁代谢的影响尚未见报道。
据报道, 应激可导致家禽血清或血浆中皮质酮水平显著升高[5, 21]。皮质酮是家禽最重要的应激激素, 常被用于模拟诱导建立家禽慢性应激模型。本试验采用肉鸡皮下注射皮质酮方式建立慢性应激模型, 检测应激对肉鸡铁代谢的影响并进一步讨论其机制。
1 材料与方法 1.1 试验仪器与试剂 1.1.1 试验仪器冷冻离心机(AllegraTM64R, USA), 超声波破碎仪(Sonics Vcx130, USA), 匀浆仪(MS-100R, TOMY), 电消解仪(DigiBlock ED54, LabTech, 北京), 原子吸收分光光度计(ICE3500, Thermo Fisher, 美国), 全自动生化分析仪(7020, Hitachi High-Tech Cropration, 日本), Real-time PCR仪(Mx3000, Stratagene, 美国), 核酸定量仪(Nanodrop ND-1000, USA), 凝胶成像系统(VersaDoc 4000MP, BIO-RAD, 美国)等。
1.1.2 试验试剂Trizol试剂(普飞, 上海), 反转录试剂盒(D6110A, 诺唯赞, 南京), 血清铁检测试剂盒(6063-2012, Sigma, 美国), 不饱和铁结合力检测试剂盒(6062-2012, Shino-test, 日本), 实时荧光定量PCR试剂盒(TaKaRa, 大连)等。
1.2 试验方法选取30只49日龄如皋黄羽雄性肉鸡, 称体质量后随机分为2组:对照组(control group, Con)和皮质酮组(corticosterone group, Cort), 在50日龄时对照组肉鸡皮下注射15%(体积分数)乙醇0.4 mL·kg-1, 试验组肉鸡皮下注射皮质酮(C2505, Sigma-Aldrich)4.0 mg·kg-1, 每天2次, 持续7 d。试验期间, 肉鸡自由饮水和采食。试验结束后, 在57日龄时静脉采血, 采血管静置后以3 000 r·min-1离心, 吸取血清后-20 ℃保存备用。肉鸡剪断颈静脉处死, 全身消毒后剖开腹腔分离肉鸡十二指肠和肝脏样品, 快速置于液氮中, -80 ℃保存备用。分离肉鸡脾脏, -80 ℃保存备用。
1.3 测定指标 1.3.1 生长性能测定记录如皋黄羽雄鸡49日龄初始体质量及57日龄的终末体质量。
1.3.2 血液中铁代谢相关指标及组织铁含量测定用电消解仪消解血清和肝脏样品, 使用原子吸收分光光度计检测血清及肝脏中铁水平, 使用全自动生化分析仪检测血清中的转铁蛋白结合铁(transferrin-bound iron, Tf-Fe)以及不饱和铁结合力(unsaturated iron binding capacity, UIBC), 然后通过计算得出血清中的总铁结合力(total iron binding capacity, TIBC)以及转铁蛋白饱和度(transferrin saturation, TS)。
1.3.3 实时荧光定量PCR检测十二指肠和肝脏铁代谢相关基因表达称取40 mg左右的组织用Trizol试剂提取总RNA, 生物分光光度法测定RNA浓度和纯度(1.8 < D260/D280 < 2.0), 纯度达标后进行总RNA琼脂糖凝胶电泳, 没有DNA污染且28S/18S值约为2, 表明RNA没有降解, 然后反转录合成cDNA, 用实时荧光定量PCR检测十二指肠和肝脏中铁代谢相关基因的表达。PCR反应条件:95 ℃ 5 min, 95 ℃ 10 s, 60 ℃ 30 s, 95 ℃ 15 s, 60 ℃ 60 s, 95 ℃ 15 s。十二指肠及肝脏中铁代谢相关基因:二价金属离子转运体基因(divalent metal transporter 1 gene, Dmt1)、转铁蛋白基因(transferrin gene, Tf)、转铁蛋白受体1基因(transferrin receptor 1 gene, Tfr1)、重链铁蛋白基因(ferritin heavy chain gene, Fth)、轻链铁蛋白基因(ferritin light chain gene, Ftl)以及膜铁转运蛋白基因(ferroportin gene, Fpn), 内参基因为18S rRNA及β-actin。以试验组目的基因的表达为基准, 用2-ΔΔCT方法计算各目的基因的相对表达量。引物由北京擎科生物工程有限公司合成。引物序列见表 1。
| 基因 Gene |
GenBank登录号 GenBank accession No. |
引物对序列 Primer pairs sequence(5′→3′) |
片段长度/bp Fragment size |
退火温度/℃ Annealing temperature |
| Tf | NM_205304 | CCATTGCTGCTGAGATTTA/GATTCTATGCCTTCCCACT | 208 | 62 |
| Tfr1 | XM_015291382 | GTGACAACAAAGCACGAGA/CCTGGATAGCACCGAAG | 97 | 62 |
| Dmt1 | NM_001128102 | TCGGGCAGTCGGAACG/CGGGGATGGGGATTTTG | 91 | 62 |
| Fth | NM_205086 | GCAGCCGTCGTATCCAC/TCAGAGCCACATCATCCC | 193 | 62 |
| Ftl | NM_204383 | GCCAGCACTATCAAGAAACA/CGGTCCATAAAGTAATCCC | 137 | 62 |
| Fpn | NM_001012913 | GGGTTTGAGTGGTTCTGTG/CGACGAAGCCAAGTGA | 90 | 62 |
| 18S | NR_046237.1 | GAAACGGCTACCACATCCA/CACCAGACTTGCCCTCCA | 168 | 62 |
| β-actin | L08165.1 | ATCTTTCTTGGGTATGGAGTC/TCAGCAATGCCAGGGTA | 141 | 62 |
| 注:Dmt1:二价金属离子转运体基因Divalent metal transporter 1 gene; Tf:转铁蛋白基因Transferrin gene; Tfr1:转铁蛋白受体1基因Transferrin receptor 1 gene; Fth:重链铁蛋白基因Ferritin heavy chain gene; Ftl:轻链铁蛋白基因Ferritin light chain gene; Fpn:膜铁转运蛋白基因Ferroportin gene. | ||||
在肉鸡十二指肠和肝脏样品中加入蛋白裂解液RIPA, 采用BCA法测定蛋白浓度, 95 ℃水浴10 min, 所提蛋白样品全部置于-80 ℃冰箱备用。选取Tubulin-α及β-actin为内参, 经过SDS-PAGE凝胶电泳、硝酸纤维膜转膜、封闭(4%脱脂奶粉, 2 h), 4 ℃一抗孵育过夜, TBST缓冲溶液摇床清洗(10 min, 3次), 用辣根氧化物酶标记的二抗室温孵育2 h, 再经TBST洗膜后显影, 凝胶成像系统检测目的蛋白条带灰度值。组织中铁代谢相关蛋白表达用目的蛋白与内参蛋白灰度值的比值来表示[22]。铁代谢相关抗体如下:TF(HPA001527, Sigma)、TFR1(10084-2-AP, Proteintech, 英国)、DMT1(20507-1-AP, Proteintech)、FTH(ab183781, Abcam)、FTL(ab69090, Abcam)、FPN(sc-49668, Anta cruz)、Tubulin-α(BS1966, Bioworld), 辣根过氧化物酶标记的羊抗兔的兔二抗(BS10043)、兔抗羊的羊二抗(BS30503)。
1.4 数据统计试验数据采用SPSS 19.0软件进行处理, 表示为均值±标准误(x±SE)。
2 结果与分析 2.1 皮质酮处理对如皋黄羽雄性肉鸡生长性能的影响如表 2所示:皮质酮处理抑制了肉鸡生长, 极显著降低肉鸡终末体质量(P < 0.01)。
| 指标Iems | 对照组Control group | 皮质酮组Corticosterone group |
| 初始体质量Initial body weight | 577.34±5.39 | 579.34±4.08 |
| 终末体质量Final body weight | 652.54±6.06 | 623.00±5.85** |
| 注:*和**分别表示与对照组相比, 差异显著(P < 0.05)和差异极显著(P < 0.01)。下同。 Note:* and ** mean the significant difference(P < 0.05)and extremely significant difference(P < 0.01)compared with the control group. The same below. |
||
如表 3所示:与对照组相比, 7 d连续皮质酮处理极显著提高肉鸡血清铁含量、总铁结合力、不饱和铁结合力及转铁蛋白饱和度(P < 0.01), 但极显著降低肉鸡肝脏铁含量(P < 0.01)。皮质酮处理对肉鸡脾脏中铁含量无显著影响(P > 0.05)。
| 指标 Items |
对照组 Control group |
皮质酮组 Corticosterone group |
| 血清铁代谢指标(n=12) Serum iron metabolism index | ||
| 血清铁含量/(μg·mL-1) Serum iron content | 5.74±0.74 | 15.95±1.74** |
| 总铁结合力/(μmol·L-1) Total iron binding capacity | 22.47±1.53 | 51.43±5.32** |
| 不饱和铁结合力/(μmol·L-1) Unsaturated iron bonding capacity | 16.73±1.86 | 35.48±5.19** |
| 转铁蛋白饱和度/% Transferrin saturation | 19.12±3.44 | 35.83±3.30** |
| 组织中铁含量(n=8) Tissue iron content | ||
| 肝脏/(μg·g-1) Liver | 99.98±9.31 | 70.28±2.68** |
| 脾脏/(μg·g-1) Spleen | 111.13±6.08 | 126.48±10.18 |
由图 1可知:与对照组相比, 皮质酮处理未影响肉鸡十二指肠Fth、Ftl及Fpn mRNA水平, 但显著下调Dmt1 mRNA的表达水平(P < 0.05), 且使十二指肠中的FTH蛋白表达水平显著上调(P < 0.05)。
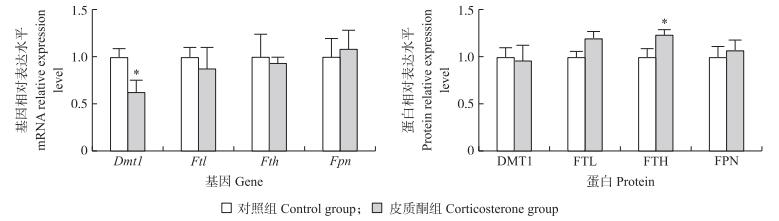
|
图 1 皮质酮处理对如皋黄羽雄性肉鸡十二指肠铁代谢相关基因mRNA及蛋白表达的影响 Fig. 1 Effect of chronic corticosterone exposure on the expression of duodenumal iron-metabolism-related genes and protiens in Rugao yellow-feathered male broilers |
如图 2所示:与对照组相比, 皮质酮处理极显著提高肉鸡肝脏TF蛋白表达水平(P < 0.01), 且降低肝脏Tfr1 mRNA水平(P < 0.05)和蛋白表达(P=0.06)。
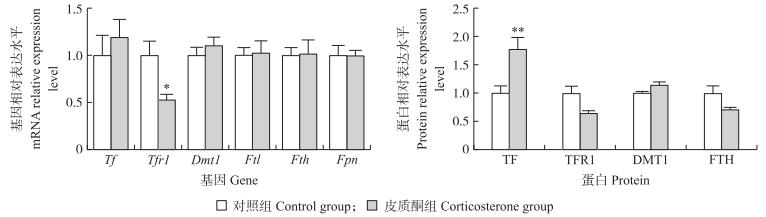
|
图 2 皮质酮处理对如皋黄羽雄性肉鸡肝脏铁代谢相关基因mRNA及蛋白表达的影响 Fig. 2 Effect of chronic corticosterone exposure on the expression of hepatic iron-metabolism-related genes in Rugao yellow-feathered male broilers |
应激可降低肉鸡生产性能。研究发现, 高温、高密度饲养、免疫等应激均可抑制肉鸡生长[23-25]。应激激素皮质酮暴露(注射或饮水)可显著抑制家禽生长、增加腹脂和肝脏脂肪沉积[26-28]。本研究结果显示, 7 d连续注射4.0 mg·kg-1皮质酮可显著抑制肉鸡生长, 降低肉鸡终末体质量。研究报道, 皮质酮处理可抑制大鼠或家禽采食, 降低体质量, 并促进其争斗行为[29-31]。防御性攻击行为可增加动物的基础代谢率, 摄入能和消化能均显著增加[32]。皮质酮诱导的慢性应激对家禽生长抑制可能与家禽摄食抑制及能量支出增加有关。
铁供应和支出平衡的关键在于机体血液铁水平的调节[13]。对啮齿动物的相关研究表明, 诱导的心理应激可降低血清铁水平, 增加血清中应激激素皮质酮水平, 且血清铁水平的降低与抑制十二指肠铁释放蛋白(FPN)表达密切相关[12, 33]。地塞米松(人工合成的糖皮质激素)腹腔注射可提高大鼠血清铁水平, 但十二指肠铁吸收关键蛋白(DMT1)表达明显降低, 而FPN明显增加[34]。TIBC间接反映血浆中Tf含量, UIBC反映脱铁转铁蛋白含量, 提示血液缺铁情况, 转铁蛋白结合铁直接反映血浆铁是否正常。TS反映网状内皮组织铁释放和骨髓铁摄取状态, 是机体铁储存的重要指标[35]。生理情况下, 人体约有30%饱和转铁蛋白, 当Tf饱和度小于15%表示缺铁, 大于45%表示铁过载[13]。本研究发现, 皮质酮暴露可明显增加肉鸡血清铁水平、总铁结合力、不饱和铁结合力以及转铁蛋白饱和度, 这表明皮质酮处理影响铁循环; 肉鸡十二指肠铁吸收关键基因Dmt1显著降低而DMT1蛋白无影响可能与miRNA有关; 重链铁蛋白(FTH)的表达显著升高, 这与血清铁升高结果不一致。应激对铁代谢的影响可能与应激类型、强度、作用时间及动物类别有关[36-37]。
肝脏是机体贮存铁的主要部位[13], 是铁代谢中心和枢纽, 而肝脏铁代谢紊乱可影响机体健康。肝脏中铁摄取的机制分别通过转铁蛋白受体(TFR)依赖性途径(TFR1和TFR2介导)和非TFR依赖性途径(DMT1和ZIP14介导)[38-39]。TF主要在肝脏中表达并被分泌释放至血液, 与三价铁结合形成转铁蛋白结合铁, 在组织细胞表面通过与TFR结合介导进入组织。TIBC是每升血清中可由转铁蛋白结合的铁最大量, 皮质酮暴露增加了肉鸡血清TIBC水平也反映了肝脏转铁蛋白的高表达结果。Zhao等[40]研究发现, 持续的心理应激可显著上调肝脏中铁摄取关键载体转铁蛋白受体2(TFR2)的表达, 导致大鼠肝脏铁蓄积, 并造成氧化应激。本研究发现, 皮质酮暴露下调了肉鸡肝脏TFR1表达, 降低肉鸡肝脏铁沉积, 但显著增加肉鸡肝脏TF的表达。皮质酮暴露造成的肝脏铁含量降低与肝脏TFR1的低表达密切相关。
综上, 皮质酮暴露显著降低肉鸡生长性能, 提高血清铁水平、总铁结合力和肝脏TF蛋白表达, 但降低肝脏铁含量和TFR1的表达。
| [1] |
Yalçin S, Ozkan S, Türkmut L, et al. Responses to heat stress in commercial and local broiler stocks.2.Developmental stability of bilateral traits[J]. British Poultry Science, 2001, 42(2): 153-160. DOI:10.1080/00071660120048384 |
| [2] |
Deeb N, Cahaner A. Genotype-by-environment interaction with broiler genotypes differing in growth rate. 3.Growth rate and water consumption of broiler progeny from weight-selected versus nonselected parents under normal and high ambient temperatures[J]. Poultry Science, 2002, 81(3): 293-301. DOI:10.1093/ps/81.3.293 |
| [3] |
Deng W, Dong X F, Tong J M, et al. The probiotic Bacillus licheniformis ameliorates heat stress-induced impairment of egg production, gut morphology and intestinal mucosal immunity in laying hens[J]. Poultry Science, 2012, 91(3): 575-582. DOI:10.3382/ps.2010-01293 |
| [4] |
Song J, Xiao K, Ke Y L, et al. Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress[J]. Poultry Science, 2014, 93(3): 581-588. DOI:10.3382/ps.2013-03455 |
| [5] |
王松波, 邓琳, 赵婕, 等. 热应激对肉鸡抗氧化能力及腓肠肌纤维类型的影响[J]. 华南农业大学学报, 2015, 36(6): 23-28. Wang S B, Deng L, Zhao J, et al. Effects of heat stress on antioxidant capacity and gastrocnemius fiber types in broilers[J]. Journal of South China Agricultural University, 2015, 36(6): 23-28 (in Chinese with English abstract). |
| [6] |
钟光, 施寿荣, 邵丹, 等. 持续热应激对黄羽肉鸡生长性能、肉品质和血液指标的影响[J]. 动物营养学报, 2018, 30(10): 3923-3929. Zhong G, Shi S R, Shao D, et al. Effects of continuous heat stress on growth performance, meat quality and blood parameters of yellow-feathered broilers[J]. Chinese Journal of Animal Nutrition, 2018, 30(10): 3923-3929 (in Chinese with English abstract). DOI:10.3969/j.issn.1006-267x.2018.10.016 |
| [7] |
李叶涵, 周佳, 张越, 等. L-精氨酸和α-酮戊二酸对热应激肉鸡肝脏功能的影响[J]. 饲料工业, 2016, 37(16): 6-11. Li Y H, Zhou J, Zhang Y, et al. Effects of L-arginine and α-ketoglutaric acid on liver function in heat-stressed broilers[J]. Feed Industry, 2016, 37(16): 6-11 (in Chinese with English abstract). |
| [8] |
张少帅, 甄龙, 冯京海, 等. 持续偏热处理对肉仔鸡免疫器官指数、小肠形态结构和黏膜免疫指标的影响[J]. 动物营养学报, 2015, 27(12): 3887-3894. Zhang S S, Zhen L, Feng J H, et al. Effects of continuous partial heat treatment on immune organ index, intestinal morphological structure and mucosal immune index in broilers[J]. Chinese Journal of Animal Nutrition, 2015, 27(12): 3887-3894 (in Chinese with English abstract). DOI:10.3969/j.issn.1006-267x.2015.12.030 |
| [9] |
Dozier W A Ⅲ, Thaxton J P, Purswell J L, et al. Stocking density effects on male broilers grown to 1.8 kilograms of body weight[J]. Poultry Science, 2006, 85(2): 344-351. DOI:10.1093/ps/85.2.344 |
| [10] |
卢营杰, 苗志强, 李建慧, 等. 饲养密度与活动空间对肉鸡免疫和应激指标的影响[J]. 动物营养学报, 2016, 28(9): 2927-2935. Lu Y J, Miao Z Q, Li J H, et al. Effects of stocking density and activity space on immune and stress indicators in broilers[J]. Chinese Journal of Animal Nutrition, 2016, 28(9): 2927-2935 (in Chinese with English abstract). DOI:10.3969/j.issn.1006-267x.2016.09.032 |
| [11] |
常双双, 柳青秀, 张敏红, 等. 饲养密度对肉鸡盲肠菌群多样性、挥发性脂肪酸和血清脑肠肽的影响[J]. 动物营养学报, 2018, 30(3): 938-946. Chang S S, Liu Q X, Zhang M H, et al. Effects of stocking density on cecal flora diversity, volatile fatty acids and serum brain gut peptide in broilers[J]. Chinese Journal of Animal Nutrition, 2018, 30(3): 938-946 (in Chinese with English abstract). DOI:10.3969/j.issn.1006-267x.2018.03.017 |
| [12] |
Conrad M E, Umbreit J N, Moore E G, et al. Iron absorption and transport[J]. The American Journal of the Medical Sciences, 1999, 318(4): 213-229. DOI:10.1097/00000441-199910000-00002 |
| [13] |
Hentze M W, Muckenthaler M U, Galy B, et al. Two to tango:regulation of mammalian iron metabolism[J]. Cell, 2010, 142(1): 24-38. DOI:10.1016/j.cell.2010.06.028 |
| [14] |
Kramer J H, Spurney C F, Iantorno M, et al. d-propranolal protects against oxidative stress and progressive cardiac dysfunction in iron overloaded rats[J]. Canadian Journal of Physiology and Pharmacology, 2012, 90(9): 1257-1268. DOI:10.1139/y2012-091 |
| [15] |
Zhao M, Chen J B, Wang W Y, et al. Psychological stress induces hypoferremia through the IL-6-hepcidin axis in rats[J]. Biochemical and Biophysical Research Communications, 2008, 373(1): 90-93. DOI:10.1016/j.bbrc.2008.05.166 |
| [16] |
Chen J B, Shen H, Chen C J, et al. The effect of psychological stress on iron absorption in rats[J]. BMC Gastroenterology, 2009, 9(1): 83. DOI:10.1186/1471-230X-9-83 |
| [17] |
He F, Ma L, Wang H Y, et al. Glucocorticoid causes iron accumulation in liver by up-regulating expression of iron regulatory protein 1 gene through GR and STAT5[J]. Cell Biochemistry and Biophysics, 2011, 61(1): 65-71. |
| [18] |
滕文锋, 施丽飞, 侯殿东, 等. 慢性束缚应激对小鼠全血铁锌钙镁含量的影响[J]. 现代预防医学, 2007, 34(7): 1201-1203. Teng W F, Shi L F, Hou D D, et al. Effect of chronic restraint stress on the content of iron, zinc, calcium and magnesium in whole blood of mice[J]. Modern Preventive Medicine, 2007, 34(7): 1201-1203 (in Chinese with English abstract). DOI:10.3969/j.issn.1003-8507.2007.07.001 |
| [19] |
Harsini S G, Habibiyan M, Moeini M M, et al. Effects of dietary selenium, vitamin E, and their combination on growth, serum metabolites, and antioxidant defense system in skeletal muscle of broilers under heat stress[J]. Biological Trace Element Research, 2012, 148(3): 322-330. DOI:10.1007/s12011-012-9374-0 |
| [20] |
邓发清. 热应激对泌乳奶牛抗氧化性能及微量元素代谢的影响[J]. 中国畜牧杂志, 2008, 44(5): 61-63. Deng F Q. Effects of heat stress on antioxidant activity and trace element metabolism in lactating dairy cows[J]. Chinese Journal of Animal Science, 2008, 44(5): 61-63 (in Chinese with English abstract). |
| [21] |
Kamel N N, Ahmed A M H, Mehaisen G M K, et al. Depression of leukocyte protein synthesis, immune function and growth performance induced by high environmental temperature in broiler chickens[J]. International Journal of Biometeorology, 2017, 61(9): 1637-1645. DOI:10.1007/s00484-017-1342-0 |
| [22] |
高志参, 李照耀, 周静, 等. 4个种属Mx蛋白抑制水疱性口炎病毒的研究[J]. 南京农业大学学报, 2017, 40(4): 690-696. Gao Z C, Li Z Y, Zhou J, et al. Research of Mx proteins from four species against vesicular stomatitis virus[J]. Journal of Nanjing Agricultural University, 2017, 40(4): 690-696 (in Chinese with English abstract). DOI:10.7685/jnau.201703023 |
| [23] |
苏红光, 张敏红, 冯京海, 等. 持续冷热环境对肉鸡生产性能、糖代谢和解偶联蛋白mRNA表达的影响[J]. 动物营养学报, 2014, 26(11): 3276-3283. Su H G, Zhang M H, Feng J H, et al. Effects of continuous hot and cold environment on production performance, glucose metabolism and uncoupling protein mRNA expression in broilers[J]. Chinese Journal of Animal Nutrition, 2014, 26(11): 3276-3283 (in Chinese with English abstract). DOI:10.3969/j.issn.1006-267x.2014.11.013 |
| [24] |
Pesti G M, Howarth B. Effects of population density on the growth, organ weights, and plasma corticosterone of young broiler chicks[J]. Poultry Science, 1983, 62(6): 1080-1083. DOI:10.3382/ps.0621080 |
| [25] |
罗梦圆, 严思思, 李晓文, 等. 复合免疫应激对肉鸡生产性能的影响[J]. 中兽医医药杂志, 2017, 36(1): 34-36. Luo M Y, Yan S S, Li X W, et al. Effects of compound immune stress on performance of broilers[J]. Journal of Traditional Chinese Veterinary Medicine, 2017, 36(1): 34-36 (in Chinese with English abstract). |
| [26] |
Bartov I. Corticosterone and fat deposition in broiler chicks:effect of injection time, breed, sex and age[J]. British Poultry Science, 1982, 23(2): 161-170. DOI:10.1080/00071688208447942 |
| [27] |
Bartov I. Effects of dietary protein concentration and corticosterone injections on energy and nitrogen balances and fat deposition in broiler chicks[J]. British Poultry Science, 1985, 26(3): 311-324. DOI:10.1080/00071668508416819 |
| [28] |
Shini S, Shini A, Huff G R. Effects of chronic and repeated corticosterone administration in rearing chickens on physiology, the onset of lay and egg production of hens[J]. Physiology & Behavior, 2009, 98(1/2): 73-77. |
| [29] |
Jahng J W, Kim N Y, Ryu V, et al. Dexamethasone reduces food intake, weight gain and the hypothalamic 5-HT concentration and increases plasma leptin in rats[J]. European Journal of Pharmacology, 2008, 581(1/2): 64-70. |
| [30] |
Wang S, Ni Y D, Guo F, et al. Effect of corticosterone on growth and welfare of broiler chickens showing long or short tonic immobility[J]. Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology, 2013, 164(3): 537-543. |
| [31] |
Ahmed A A, Ma W Q, Ni Y D, et al. Corticosterone in ovo modifies aggressive behaviors and reproductive performances through alterations of the hypothalamic-pituitary-gonadal axis in the chicken[J]. Animal Reproduction Science, 2014, 146(3/4): 193-201. |
| [32] |
闵红霞, 毛思思, 黄奕鑫, 等. 防御性攻击行为对黑线仓鼠能量收支的影响[J]. 兽类学报, 2018, 38(2): 166-173. Min H X, Mao S S, Huang Y X, et al. Effects of defensive aggression on energy budget of black-line hamsters[J]. Acta Theriologica Sinica, 2018, 38(2): 166-173 (in Chinese with English abstract). |
| [33] |
Teng W F, Sun W M, Shi L F, et al. Effects of restraint stress on iron, zinc, calcium, and magnesium whole blood levels in mice[J]. Biological Trace Element Research, 2008, 121(3): 243-248. DOI:10.1007/s12011-007-8047-x |
| [34] |
Li H F, Jiang S X, Yang C, et al. Long-term dexamethasone exposure down-regulates hepatic TFR1 and reduces liver iron concentration in rats[J]. Nutrients, 2017, 9(6): 617. DOI:10.3390/nu9060617 |
| [35] |
Gkouvatsos K, Papanikolaou G, Pantopoulos K. Regulation of iron transport and the role of transferrin[J]. Biochimica et Biophysica Acta(BBA):General Subjects, 2012, 1820(3): 188-202. DOI:10.1016/j.bbagen.2011.10.013 |
| [36] |
Dhabhar F S. Effects of stress on immune function:the good, the bad, and the beautiful[J]. Immunologic Research, 2014, 58(2/3): 193-210. |
| [37] |
王免免, 周艳婷, 张蒙, 等. 外源皮质酮对雏鸡法氏囊microRNA表达的影响[J]. 畜牧与兽医, 2018, 50(5): 71-76. Wang M M, Zhou Y T, Zhang M, et al. Effect of exogenous corticosterone on the expression of miRNAs in bursa of fabricius in chicken[J]. Animal Husbandry & Veterinary Medicine, 2018, 50(5): 71-76. |
| [38] |
Graham R M, Chua A C G, Herbison C E, et al. Liver iron transport[J]. World Journal of Gastroenterology, 2007, 13(35): 4725-4736. DOI:10.3748/wjg.v13.i35.4725 |
| [39] |
Anderson E R, Shah Y M. Iron homeostasis in the liver[J]. Comprehensive Physiology, 2013, 3(1): 315-330. |
| [40] |
Zhao M, Liu L J, Li X G, et al. Psychological stress leads to hepatic iron accumulation and disturbs iron homeostasis[J]. Journal of Chemical and Pharmaceutical Research, 2014, 6(5): 1128-1134. |




