文章信息
- 谭小云, 贾辛怡, 鲍依群
- TAN Xiaoyun, JIA Xinyi, BAO Yiqun
- 植物胞质分裂中成膜体的动态调控及囊泡运输机制
- Mechanisms of phragmoplast dynamics and vesicular trafficking during plant cytokinesis
- 南京农业大学学报, 2020, 43(2): 204-212
- Journal of Nanjing Agricultural University, 2020, 43(2): 204-212.
- http://dx.doi.org/10.7685/jnau.201903071
-
文章历史
- 收稿日期: 2019-03-30
细胞分裂是指1个亲代细胞(mother cell)分裂为2个子代细胞(daughter cell)的过程, 其中包括细胞核分裂以及胞质分裂。不同生物采用不同的胞质分裂方式。在动物细胞中, 随着肌动蛋白微丝和肌球蛋白形成的收缩环(contractile ring)的收紧, 依次在早末期形成卵裂沟(cleavage furrow)、晚末期形成细胞间桥(intercellular bridge), 直至2个细胞相互分离[1]。高等植物体细胞的胞质分裂则借助成膜体(phragmoplast)和细胞板(cell plate)的协同作用来完成[2]。成膜体作为细胞动态微管骨架网络, 以轨道和“脚手架”的形式介导了囊泡到达细胞板以及细胞板组装的过程[3]。随着囊泡的不断融合, 细胞板随之向两端扩展, 直至与母细胞壁相连, 形成成熟的细胞板[4], 至此胞质分裂结束。近年来, 随着生物化学、分子生物学和显微成像手段的进步, 植物胞质分裂中的研究取得了很大进展[5-6]。本文总结了在该过程中成膜体的形成及其动态变化以及细胞板囊泡拴系、融合等分子机制, 为今后的研究提供参考。
1 成膜体:细胞板囊泡运输的轨道和细胞板组装的“脚手架” 1.1 成膜体的形成在植物细胞周期的后期向末期转变的过程中, 有丝分裂的纺锤体细胞骨架重新组织, 形成成膜体。成膜体由微管、肌动蛋白微丝以及相关的内膜组分构成[6], 它是细胞板囊泡运输的轨道以及细胞板组装的“脚手架”。成膜体含有2组微管阵列, 它们的正极(近端, proximal)朝向细胞分裂面, 而负极(远端, distal)朝向细胞核, 形成具有双极性的微管阵列(图 1)。这种结构是如何维持的呢?前人用“踏车模型(treadmilling model)”来解释这种现象, 即:微管正极朝向细胞板, 不停聚合微管单体; 微管负极朝向细胞核, 以同样的速度解聚, 以此保持成膜体的稳定性。然而这个假说与一些试验结果不符合。例如:1)标记微管正端的蛋白EB1可以标记整个成膜体, 而不只是中间部位, 还可以观察到远端的EB1向中间部位运动。2)按照踏车模型, 应该有大量的微管蛋白单体从远端(解聚的一端)向近端(聚合的一端)流动, 但实际并没有发现这个现象。最近, 基于活细胞成像观察以及计算机模拟, 人们以动态不稳定模型(dynamic instability model)能更好地解释成膜体形态维持的机制。该模型认为, 微管成束延伸可以在任何一个位置开始, 但是近端的微管束具有更高的转换速率, 更容易聚合延伸, 因此微管都是向着近端延伸[7]。在延伸的过程中, 携带着囊泡向细胞板运动。
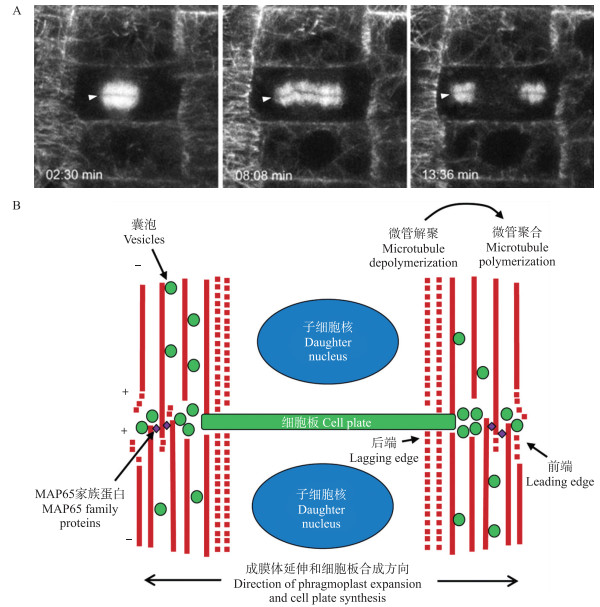
|
图 1 胞质分裂过程中成膜体形态的变化 Fig. 1 Phragmoplast dynamic during cytokinesis A.胞质分裂不同时间点的成膜体形态, 箭头所指为成膜体中区(未发表数据); B.胞质分裂期间成膜体动态变化示意图。 A. The morphologies of phragmoplast at different time during cytokinesis, arrows indicate the midzone(unpublished data); B. Schematic model of phragmoplast dynamic during cytokinesis. |
大多数的微管存在于成膜体中区(midzone)的两侧, 因而在荧光标记微管的图像中, midzone显示为一条暗线(图 1-A)。但是有一小部分的微管延伸并跨过midzone, 在中间反向交错形成重叠。此前在拟南芥中, 使用电子显微镜和断层摄影技术均未发现存在交错重叠的微管[8], 但是最近通过共聚焦显微镜在小立碗藓和拟南芥成膜体中发现微管交错的现象[9-10]。在拟南芥成膜体的中部, 反向微管的交联由保守的MAP65/Ase1/PRC1蛋白家族介导[9](表 1)。原核表达的MAP65-3/PLEIAD在体外可以形成反向平行的微管阵列[9], 如仅表达其C端的微管结合域可产生类似效果[11]。在拟南芥根中MAP65-3定位于midzone, map65-3突变会导致主根内形成残缺的细胞板以及多核细胞[9, 11-12], 成膜体及midzone都变得更宽。因此, MAP65-3通过在midzone结合并交联成膜体的反向平行微管, 来维持成膜体的正常形态。
| 名称(缩写) Name(Abbreviation) |
全称 Full name |
功能 Function |
文献 Reference |
| AtPAKRP1 | Arabidopsis phragmoplast-associated kinesin-related protein1 | 参与成膜体微管组装并维持稳定 Participate in the assembly and stability of phragmoplast |
[13] |
| KCBP | Kinesin-like calmodulin-bindingprotein | 定位于PPB、纺锤体和成膜体, 参与微管阵列组装。与钙调蛋白存在互作关系 Localized at PPB, spindle and phragmoplast, participating in microtubule array assembly. Interacting with calmodulin |
[14] |
| MAP215/GEM1/MOR1 | Microtubule-associated protein 215 | 微管结合蛋白, 定位于成膜体微管的重叠区, 在成膜体维持中起重要作用 Mircotubule associated protein 215, localized at middle of the phragmoplast, participating in phragmoplast assembly |
[15] |
| MAP65-3 | Microtubule-associated protein 65-3 | 微管结合蛋白, 定位于成膜体中线, 参与反向平行的微管的交错以及结合 Mircotubule associated protein 65-3, localized at middle of the phragmoplast, participating in the interleaving and binding of parallel microtubules |
[11] |
| BUB3 | Budding uninhibited by benzimidazole 3 | 保守的WD40蛋白, 与成膜体形成相关 Conserved WD40 proteins, participating in phragmoplast assembly |
[16] |
| Kinesin-12A/B | Kinesin-12A and Kinesin-12B | 定位在成膜体上的驱动蛋白, 对于成膜体形成很重要 Kinesins, localized at the phragmoplast, is important for phragmoplast formation |
[17] |
| KEU | KEULE | 定位在细胞板上的SEC1家族蛋白, 突变后造成成膜体动态转换缺陷 Cell plate localized SEC1 family protein, mutation in which lead to defects in phragmoplast turnover |
[18] |
| KATA | Kinesin-like protein in Arabidopsis thaliana A | 类驱动蛋白, 定位于成膜体微管中线, 与成膜体维持有关 Kinesin-like protein, localized at middle of the phragmoplast, participate in phragmoplast maintenance |
[19] |
细胞板向外扩展需要成膜体做出相应的动态变化。在已形成管网状新生细胞板结构的区域, 即成膜体中央区域的微管解聚, 解聚的微管蛋白单体在成膜体的前端(leading edge)重新聚合成微管(图 1-B)[5]。因此, 一开始实心的成膜体微管阵列逐渐空心化, 形成直径不断增大的圆环(图 1)[5]。成膜体后端(lagging edge)微管的解聚受丝裂原活化蛋白激酶(MAPK)级联信号途径调控[20]。MAPK级联信号途径的激活依赖于一个定位于细胞核以及成膜体的蛋白激酶NPK1(nucleus- and phragmoplast-localized protein kinase 1)与驱动蛋白NACK1(NPK1-activating kinesin-like protein 1)的互作。在有丝分裂前中期, 依赖于周期蛋白的激酶(cyclin-dependent kinases, CDKs)对NPK1进行的磷酸化修饰抑制NPK1与NACK1的互作, 从而抑制MAPK途径。在有丝分裂的后期和末期, CDKs降解, NPK1去磷酸化后可以与NACK1进行互作, 从而激活MAPK途径, 将MAP65蛋白磷酸化, 使其MT-crosslinking的活性丧失, 从而造成成膜体后端的微管解聚[21]。
1.3 成膜体前端的动态变化微管蛋白成核是微管形成并延伸的前提。在植物中, 微管成核作用由保守的γ-微管蛋白环状复合体(γ-tubulin ring complexes, γ-TuRC)催化。在胞质分裂过程中, γ-TuRC更多地存在于成膜体前端[22], 微管从含有γ-TuRC的成核位点处开始聚合, 这些微管比较稳定, 对戊炔草胺(propyzamide, 微管解聚药物)处理更不敏感[23]。另外在BY-2细胞的成膜体中, 前端微管被MAP65-1a优先修饰, 表明MAP65-1a在成膜体前端起交联作用, 进而稳定成膜体前端(图 1-B)[21]。
运输囊泡一般认为是结合在驱动蛋白上沿着成膜体微管运输到细胞板上, 但目前还缺乏直接的证据。拟南芥中61个驱动蛋白中, 有23个在有丝分裂过程中表达[24], 这些驱动蛋白在维持成膜体形态上发挥重要功能(表 1), 但是对于它们的动力学数据以及作用机制还了解不多[25]。
总体而言, 成膜体是一个动态的、以双极性的微管阵列为主的结构, 它在引导囊泡向细胞板运输以及细胞板的装配过程中发挥关键作用。微管结合蛋白MAP65、驱动蛋白Kinesin甚至囊泡运输相关蛋白都参与了成膜体的形成以及动态维持(表 1)。
2 细胞板囊泡的来源早期, 人们普遍认为组成细胞板的囊泡来自高尔基体。这些囊泡携带着多糖、蛋白质和脂质等物质, 从高尔基体运往反式高尔基体网络(trans Golgi network, TGN, 位于高尔基体反面但独立于高尔基体的管网状细胞器), 再运往细胞分裂面互相融合, 形成早期细胞板[2]。因此细胞板的形成被认为是一种特殊的外泌过程。近年来, 有研究发现细胞膜物质内吞后也可以运输至细胞板[26]。然而, 特异地干扰内质网到高尔基体的运输或者细胞膜内吞过程, 提示是外泌途径而非内吞途径在胞质分裂中起主要作用[27]。在植物细胞中, 外泌和内吞都可以经过TGN, 因此TGN也被称为早期内体[28]。有些蛋白(如细胞板特有的Qa-SNARE)只在胞质分裂时期表达, 合成后从高尔基体运往TGN, 然后直接运往细胞板, 这是一个外泌过程。有些质膜蛋白则是组成型表达, 间期在质膜和TGN之间循环, 不停地外泌和内吞, 但在胞质分裂期间, 这些内吞进来的蛋白被优先运往细胞板[29-30]。
运向细胞板的囊泡是如何在TGN上形成呢?近年, 人们发现ARF1的激活蛋白鸟苷酸交换因子(guanine nucleotide exchange factor, GEF)BIG1至BIG4(BIG1—BIG4)调控TGN上细胞板囊泡的形成。外泌和内吞蛋白被运输至TGN上后, BIG1—BIG4激活ARF1家族蛋白, 激活的ARF1蛋白募集“货物”并将其包裹到含有衔接蛋白复合体1(adaptor protein complex 1, AP1)的网格蛋白(Clathrin)包被的囊泡中, 并向细胞板运输[31]。另一个试验结果表明敲除AP1复合体的μ-亚基(AP1μ2)基因阻断了外泌蛋白和内吞蛋白向细胞板转运, 导致二核细胞的形成[32-33]。因此, TGN可以接受来自高尔基体的外泌囊泡和来自质膜的内吞囊泡, 并在BIG1—BIG4和AP1复合体的作用下, 将“货物”包裹在Clathrin囊泡中, 运向细胞板。
3 细胞板形成过程中囊泡拴系和融合 3.1 胞质分裂过程中的囊泡拴系机制拴系是膜融合之前的囊泡相互识别过程, 由拴系因子介导[34]。目前发现有2种拴系因子复合体(TRAPPⅡ和exocyst)参与了植物胞质分裂过程(表 2)。拟南芥TRAPPⅡ是由9个亚基组成的复合体, 它在TRAPPⅠ复合体(含Trs20、Trs23、Trs31、Trs33、Trs85、Bet3和Bet5亚基)的基础上, 增加了2个特有的亚基(Trs120和Trs130)。这2个亚基定位在TGN和细胞板上, 它们的基因突变导致胞质分裂的缺陷(表 2)[35-37]。exocyst复合体由8个亚基(SEC3、SEC5、SEC6、SEC8、SEC10、SEC15、EXO84和EXO70)构成, 其中5个亚基已被发现定位在细胞板上(表 2)[38]。EXO70A1基因突变影响了细胞板的装配过程[38]; SEC6可以和SEC1/Munc18蛋白KEULE直接互作, 意味着细胞板囊泡拴系过程与融合过程存在直接的关联[39]。TRAPPⅡ和exocyst可以相互作用, 并且Trs120和Trs130基因突变导致exocyst复合体错误定位[40], 意味着TRAPPⅡ复合物作用于exocyst的上游(图 2)。
| 蛋白名称 Protein name |
功能 Function |
亚细胞定位 Subcellular localization |
文献 Reference |
| Vamp721/722 | R-SNARE, 参与经由TGN/早期内体区室的分泌囊泡的运输, 在胞质分裂过程中参与细胞板形成 R-SNARE, participating in vesicle trafficking from TGN/EE to PM or cell plate |
细胞板、早期内体、质膜 Cell plate, early endosome, plasma membrane |
[41] |
| KNOLLE | 是拟南芥中的Qa-SNARE蛋白, 在胞质分裂过程中参与特异的囊泡融合 Qa-SNARE protein, mediating vesicle fusion during cytokinesis |
细胞板 Cell plate |
[42] |
| AtTomosyn | 是拟南芥中的Tomosyn蛋白, 可以与KNOLLE互作, 抑制其与其他SNARE亚基的互作, 进而抑制细胞板囊泡融合过程 Tomosyn protein in Arabidopsis, it can interact with KNOLLE, this interaction prevent KNOLLE interact with other SNARE subunits, then disturbed membrane fusion on cell plate |
细胞膜、TGN Plasma membrane, TGN |
[43] |
| KEULE | 与exocyst复合体和KNOLLE存在互作关系, 参与囊泡融合以及细胞板形成 Sec1/Munc18 family G protein, interacts with exocyst complex and KNOLLE, regulates membrane fusion on cell plate |
细胞板、细胞质 Cell plate, cytosol |
[18, 39] |
| RabA1a/b/c | RabA亚家族小G蛋白, 处于TRAPPⅡ复合体的下游, 与胞质分裂相关 RabA subfamily small G protein, functions at downstream of TRAPPⅡ complex |
细胞板、TGN Cell plate, TGN |
[44] |
| TRAPPⅡ 复合体 |
拴系因子复合体, 可能是RabA亚家族小G蛋白的激活蛋白, 其缺失导致细胞板不能正常形成 Tethering factor complex, it might be RabA family G protein activator, loss-of-function of TRAPPⅡ lead to cell plate defection |
细胞板、TGN Cell plate, TGN |
[37] |
| SCD1/SCD2 | 与exocyst复合体和RabE1互作, 可能是RabE1的激活蛋白 SCD1/SCD2 interacts with subunits of the exocyst complex and the RabE1 family of GTPases, it might activate RabE1 proteins |
细胞质、细胞板 Cytosol, cell plate |
[45] |
| RabE1d | RabE1家族小G蛋白, 与exocyst共定位, 可能募集ecocyst复合体 RabE1 family G protein, co-localized with exocyst, it might recruit exocyst complex |
细胞质、细胞板 Cytosol, cell plate |
[45] |
| NPSN11 | Qb-SNARE, 可以与Qa-SNARE KNOLLE和Qc-SNARE SYP71形成复合体 Qb-SNARE, interacts with KNOLLE to form a SNARE complex during cytokinesis |
细胞板 Cell plate |
[46-48] |
| SYP71 | Qc-SNARE, 可以与Qa-SNARE KNOLLE和Qb-SNARE NPSN11形成复合体 Qc-SNARE, interacts with KNOLLE to form a SNARE complex during cytokinesis |
细胞板 Cell plate |
[46-48] |
| SNAP33 | Qbc-SNARE, 可以与Qa-SNARE KNOLLE形成复合体Qbc-SNARE, interacts with KNOLLE to form a SNARE complex during cytokinesis | 细胞板 Cell plate |
[46-48] |
| exocyst 复合体 |
拴系因子复合体, 参与外泌囊泡在细胞板上的的拴系 Tethering factor, involved in vesicles tethering on cell plate |
质膜、细胞板 Plasma membrane, cell plate |
[38-39] |
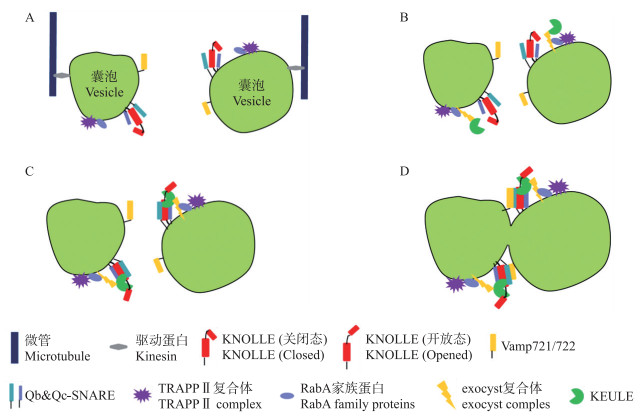
|
图 2 细胞板形成中的囊泡融合过程 Fig. 2 Membrane-vesicle fusion during cytokinesis A.运输囊泡在驱动蛋白的作用下, 沿着微管运往细胞分裂面。此时的囊泡上携带有SNARE复合物的亚基, 包括Qa-SNARE KNOLLE(关闭态)、Qb-SNARE和Qc-SNARE(或Qbc-SNARE)以及R-SNARE Vamp721/722。还携带RabA家族G蛋白及其激活蛋白TRAPⅡ复合物。B.到达细胞分裂面后, TRAPPⅡ复合物募集exocyst复合物和SM蛋白KEULE(可能通过激活RabA, 也可能直接募集)。C. KEULE与KNOLLE结合, 使其处于开放态, 开放的KNOLLE与Qb-和Qc-SNARE结合, 形成t-SNARE复合物。D. t-SNARE复合物与R-SNARE Vamp721/722结合, 形成完整的SNARE复合物, 导致囊泡融合。 A. Vesicles were transported to the position of cell plate formation(cell division zone), mediated by kinesins. Subunits of SNARE complex(Qa-SNARE KNOLLE, Qb-&Qc-SNAREs and R-SNAREs Vamp721/722), RabA family G proteins and it activator TRAPPⅡ complex were attached to the vesicles. B. When the vesicles arrived to cell division zone, TRAPPⅡ complex recruits exocyst complex and SM protein KEULE(by itself or by activating RabA proteins). C. When recruited to the vesicle, KEULE interacted with KNOLLE, promoted it turned to the opened conformation, the opened KNOLLE then interacts with Qb-and Qc-SNARE, formed a t-SNARE complex. D. Vesicles closed to each other, R-SNARE and t-SNARE complex formed a SNARE complex, lead to vesicle fusion. |
Rab家族小G蛋白往往参与调控多个囊泡运输过程, 其中RabA1—RabA3亚家族被证实与胞质分裂相关。RabA2a和RabA2d定位在细胞板的边缘和TGN上, 诱导表达显性失活态的RabA2a导致胞质分裂缺陷(表 2)[49]。RabA1c和TRAPPⅡ复合体亚基Trs130共定位在细胞板和TGN上。在trs130突变体中, RabA1c定位在细胞质中, 而且GTP结合态的RabA1c(Q72L)能够部分回补trs130的表型[37], 因此TRAPPⅡ可能是RabA1c的GEF[50]。另外一个可能参与胞质分裂的是RabE亚家族蛋白, RabE1d定位在细胞板上。SCD1和SCD2作为RabE可能的GEF, 其缺失导致胞质分裂的缺陷(表 2), RabE的下游可能是exocyst复合体[45]。
3.2 胞质分裂过程中的囊泡融合机制囊泡运输至细胞分裂面之后, 将互相融合形成细胞板。膜融合过程主要由SNARE蛋白复合体介导。SNARE复合体一般由4个α-螺旋组成, 这4个α-螺旋可能来自3~4个SNARE亚基[51]。SNARE亚基依据它们在SNARE基序上的核心氨基酸进行分类, 包括:Qa-SNARE、Qb-SNARE、Qc-SNARE和R-SNARE, 在某些情况下Qb-SNARE和Qc-SNARE可以被同时具有Qb-和Qc-SNARE基序的Qbc-SNARE取代(表 2)[51]。
在植物胞质分裂过程中, 有一个被称为KNOLLE的Qa-SNARE发挥了关键作用(表 2)[42]。它在细胞分裂中期特异表达, 在胞质分裂结束后被降解。KNOLLE参与形成2种SNARE复合体。一种是由它和Qbc-SNARE SNAP33以及R-SNARE VAMP721/722组成[46]; 另一种是由它和Qb-SNARE NPSN11、Qc-SNARE SYP71以及R-SNARE VAMP721/722组成。2种SNARE复合体对胞质分裂都有贡献(图 2)[47-48]。
SM(SEC1/Munc18)蛋白是膜融合的主要调控蛋白之一, 它可以与Qa-SNARE互作并且促使SNARE复合体的形成[52-53]。尽管SM-SNARE互作模式可能具有多种[54], 一般来讲, SM蛋白是和Qa-SNARE的N端互作, 并且进一步与形成的SNARE复合体互作[55]。在植物胞质分裂的过程中, SM蛋白KEULE可以和KNOLLE蛋白N端与SNARE基序之间的“铰链”区域互作, 并且KEULE能够更好地与处于开放构象的KNOLLE单体互作, 而不是与SNARE复合体互作[56]。这种互作能够促使KNOLLE蛋白处于稳定的开放构象, 有利于SNARE复合体的形成和细胞板囊泡融合的过程。除了与KNOLLE蛋白互作之外, KEULE还与成膜体形态的转换相关。有研究表明, 在keule突变体中, 很多处于分裂期的细胞成膜体不能从实体向空心的环状进行转换[18], 但其中的分子机制还有待进一步研究。
在酵母和果蝇中有一类叫做Tomosyn的蛋白在膜融合过程中起负调控作用, 拟南芥Tomosyn蛋白(AtTMS)可以和KNOLLE互作, 这种互作导致KNOLLE不能和其他的SNARE亚基互作, 进而抑制细胞板的囊泡膜融合过程, 导致主根中细胞板形态缺陷[43]。
4 细胞板边缘与母细胞质膜的融合过程不断伸展的细胞板最终会接触到母细胞的质膜。此时囊泡是仅与细胞板边缘融合还是同时和质膜融合, 目前还不清楚。一般而言, 介导细胞板膜融合的SNARE复合体中的Qa-SNARE是KNOLLE和SYP132, 而介导囊泡与质膜融合的SNARE复合体中的Qa-SNARE是PEN1(SYP121)、SYP132等[57-58]。在keule和knolle突变体中, 细胞分裂区积累了大量的囊泡, 意味着这些囊泡不能通过其他的SNARE复合体来与质膜融合[30, 57]。细胞板与质膜的融合是哪些SNARE复合体在发挥作用?目前还不清楚。
TPLATE基因突变导致细胞板不能与母细胞膜融合[59]。TPLATE是一类衔接蛋白, 它定位在细胞板以及皮质分裂区(cortical division zone, CDZ)。咖啡因处理会扰乱TPLATE定位到细胞板而非CDZ, 而wortmannin(内吞抑制剂)处理会扰乱TPLATE定位到CDZ而非细胞板的过程, 因此TPLATE定位至2个部位的机制不一样[60]。进一步研究表明TPLATE蛋白是内吞衔接蛋白复合体(TPLATE complex)的一个亚基, 它参与了植物细胞网格蛋白(Clathrin)包被囊泡的形成[61]。网格蛋白包被囊泡的衔接蛋白除TPLATE之外还有AP2, 植物细胞内吞主要依赖前者, 而非植物细胞内吞主要依赖于后者。因此, 植物AP2亚基的基因突变体并没有出现严重的表型[62-66]。目前, TPLATE通过内吞作用介导细胞板与母细胞膜融合的机制还不清楚。
5 细胞板的成熟细胞板在形成过程中会经历一系列形态变化, 最终形成一个扁平的成熟细胞板。这一系列变化过程主要包括囊泡管网化, 通过内吞除去细胞板多余的膜组分, 以及置换其中的多糖成分[6]。
在细胞板成熟过程中, 网格蛋白包被的囊泡以及发动蛋白相关蛋白(dynamin-related proteins, DRP)发挥重要作用。DRP是一类大分子的GTPase, 拟南芥DRP1家族有5个成员(DRP1a—DRP1e)。DRP1a和DRP1e定位在细胞板上, drp1a与drp1e双突变导致胚胎致死, 突变体胚胎出现多核细胞以及肿胀、不规则的细胞板残端[67]。DRP2家族含有2个成员(DRP2a和DRP2b), drp2a与drp2b双突变导致拟南芥配子体致死, 其中雄配子体发育在第1次有丝分裂时期受阻, 细胞板呈现分支、卷曲的形态[68]。研究表明在细胞板的前端, DRP能将囊泡束紧, 变成管状结构, 有利于二维格栅(lattice)的形成。而在细胞板的中间, DRP能通过水解GTP, 缢裂网格蛋白包被囊泡, 使之脱离细胞板。因此, DRP1和DRP2家族成员在细胞板成熟过程中均发挥功能, 但其功能是不冗余的。
除了细胞板膜组分的内吞, 细胞板囊腔中的多糖组分在后期也会发生改变。在细胞板形成的早中期, 其中的多糖主要是胼胝质, 它由被递送至细胞板的胼胝质合酶合成。胼胝质能提供一定的机械强度, 稳定细胞板管网结构, 并在细胞板插入到母细胞膜的过程发挥功能。在细胞板成熟期间, 胼胝质水解后被纤维素取代, 这使得成熟细胞板更加牢固[69]。
6 总结和展望基于正向和反向遗传学的研究策略, 以及采用遗传学、生物化学、电子显微镜、激光共聚焦显微镜等研究技术, 前人在植物特别是拟南芥中分离了许多与成膜体的形态建成以及维持、细胞板囊泡拴系/融合的相关基因, 并对其作用机制进行了解析。但是, 植物胞质分裂过程的分子机制中还有很多关键问题有待回答。这些问题包括:1)囊泡如何沿着成膜体运往细胞分裂面?尽管目前发现很多驱动蛋白突变体确实有胞质分裂缺陷, 但是它们往往是影响了成膜体形态, 未造成囊泡堆积, 到底是哪一类驱动蛋白和囊泡结合并运输囊泡, 目前还不清楚。2)细胞板上囊泡的拴系和融合如何协调?目前尽管发现KEULE既可与拴系因子exocyst复合体互作, 也可与融合蛋白KNOLLE互作, 拴系因子TRAPPⅡ可以与exocyst互作, 但四者如何协调还很不清楚。3)细胞板如何与母细胞膜进行融合?4)细胞板囊泡融合与成膜体动态变化如何协调?其中的具体分子机制是什么?随着超高分辨率荧光显微镜技术、三维电子显微镜技术和蛋白质互作研究等的应用, 人们对于植物胞质分裂中的囊泡运输机制了解将会越来越清晰。例如, 通过超高分辨率荧光显微镜可能直接观察囊泡在成膜体微管上的运输; 通过免疫共沉淀结合质谱(IP-MS)技术可以大量鉴定细胞板运输相关蛋白的互作蛋白; 利用酵母三杂交技术可以观察多对蛋白的互作关系。通过上述技术, 人们有望在不远的将来更加清晰地解析植物胞质分裂过程中的分子调控机制。
| [1] |
Fukagawa T. Cell division:a new role for the kine to chore in central spindle assembly[J]. Current Biology, 2015, 25(13): R554-R557. DOI:10.1016/j.cub.2015.05.016 |
| [2] |
Livanos P, Müller S. Division plane establishment and cytokinesis[J]. Annual Review of Plant Biology, 2019, 70: 239-267. DOI:10.1146/annurev-arplant-050718-100444 |
| [3] |
Smertenko A, Hewitt S L, Jacques C N, et al. Phragmoplast microtubule dynamics:a game of zones[J]. Journal of Cell Science, 2018, 131(2): jcs203331. DOI:10.1242/jcs.203331 |
| [4] |
Smertenko A. Phragmoplast expansion:the four-stroke engine that powers plant cytokinesis[J]. Current Opinion in Plant Biology, 2018, 46: 130-137. DOI:10.1016/j.pbi.2018.07.011 |
| [5] |
Müller S, Jürgens G. Plant cytokinesis:no ring, no constriction but centrifugal construction of the partitioning membrane[J]. Seminars in Cell & Developmental Biology, 2016, 53: 10-18. |
| [6] |
Smertenko A, Assaad F, Baluška F, et al. Plant cytokinesis:terminology for structures and processes[J]. Trends in Cell Biology, 2017, 27(12): 885-894. DOI:10.1016/j.tcb.2017.08.008 |
| [7] |
Smertenko A P, Piette B, Hussey P J. The origin of phragmoplast asymmetry[J]. Current Biology, 2011, 21(22): 1924-1930. DOI:10.1016/j.cub.2011.10.012 |
| [8] |
Austin J R, Seguí-Simarro J M, Staehelin L A. Quantitative analysis of changes in spatial distribution and plus-end geometry of microtubules involved in plant-cell cytokinesis[J]. Journal of Cell Science, 2005, 118(17): 3895-3903. DOI:10.1242/jcs.02512 |
| [9] |
Ho C-MK, Hotta T, Guo F, et al. Interaction of antiparallel microtubules in the phragmoplast is mediated by the microtubule-associated protein MAP65-3 in Arabidopsis[J]. The Plant Cell, 2011, 23(8): 2909-2923. DOI:10.1105/tpc.110.078204 |
| [10] |
Yamada M, Miki T, Goshima G. Imaging mitosis in the moss Physcomitrella patens[M]//Methods in Molecular Biology. New York: Springer, 2016: 263-282.
|
| [11] |
Ho Chin-Min K, Lee Yuh-Ru J, Kiyama L D, et al. Arabidopsis microtubule-associated protein MAP65-3 cross-links antiparallel microtubules toward their plus ends in the phragmoplast via its distinct C-terminal microtubule binding domain[J]. The Plant Cell, 2012, 24(5): 2071-2085. DOI:10.1105/tpc.111.092569 |
| [12] |
Müller S, Smertenko A, Wagner V, et al. The plant microtubule-associated protein AtMAP65-3/PLE is essential for cytokinetic phragmoplast function[J]. Current Biology, 2004, 14(5): 412-417. DOI:10.1016/j.cub.2004.02.032 |
| [13] |
Lee Y R J, Giang H M, Liu B. A novel plant kinesin-related protein specifically associates with the phragmoplast organelles[J]. The Plant Cell, 2001, 13(11): 2427-2439. DOI:10.1105/tpc.010225 |
| [14] |
Bowser J, Reddy A S N. Localization of a kinesin-like calmodulin-binding protein in dividing cells of Arabidopsis and tobacco[J]. The Plant Journal, 1997, 12(6): 1429-1437. DOI:10.1046/j.1365-313x.1997.12061429.x |
| [15] |
Kawamura E, Wasteneys G O. MOR1, the Arabidopsis thaliana homologue of Xenopus MAP215, promotes rapid growth and shrinkage, and suppresses the pausing of microtubules in vivo[J]. Journal of Cell Science, 2008, 121(24): 4114-4123. DOI:10.1242/jcs.039065 |
| [16] |
Zhang H C, Deng X G, Sun B J, et al. Role of the BUB3 protein in phragmoplast microtubule reorganization during cytokinesis[J]. Nature Plants, 2018, 4(7): 485-494. DOI:10.1038/s41477-018-0192-z |
| [17] |
Lee Yuh-Ru J, Li Y, Liu B. Two Arabidopsis phragmoplast-associated kinesins play a critical role in cytokinesis during male gametogenesis[J]. The Plant Cell, 2007, 19(8): 2595-2605. DOI:10.1105/tpc.107.050716 |
| [18] |
Steiner A, Müller L, Rybak K, et al. The membrane-associated Sec1/Munc18 KEULE is required for phragmoplast microtubule reorganization during cytokinesis in Arabidopsis[J]. Molecular Plant, 2016, 9(4): 528-540. DOI:10.1016/j.molp.2015.12.005 |
| [19] |
Marcus A I, Ambrose J C, Blickley L, et al. Arabidopsis thaliana protein, ATK1, is a minus-end directed kinesin that exhibits non-processive movement[J]. Cell Motility and the Cytoskeleton, 2002, 52(3): 144-150. DOI:10.1002/cm.10045 |
| [20] |
Sasabe M, Machida Y. MAP65:a bridge linking a MAP kinase to microtubule turnover[J]. Current Opinion in Plant Biology, 2006, 9(6): 563-570. DOI:10.1016/j.pbi.2006.09.010 |
| [21] |
Sasabe M. Phosphorylation of NtMAP65-1 by a MAP kinase down-regulates its activity of microtubule bundling and stimulates progression of cytokinesis of tobacco cells[J]. Genes & Development, 2006, 20(8): 1004-1014. |
| [22] |
Ehrhardt D W, Shaw S L. Microtubule dynamics and organization in the plant cortical array[J]. Annual Review of Plant Biology, 2006, 57(1): 859-875. DOI:10.1146/annurev.arplant.57.032905.105329 |
| [23] |
Murata T, Sano T, Sasabe M, et al. Mechanism of microtubule array expansion in the cytokinetic phragmoplast[J]. Nature Communications, 2013, 4: 1967. DOI:10.1038/ncomms2967 |
| [24] |
Vanstraelen M, Inzé D, Geelen D. Mitosis-specific kinesins in Arabidopsis[J]. Trends in Plant Science, 2006, 11(4): 167-175. DOI:10.1016/j.tplants.2006.02.004 |
| [25] |
Lee Yuh-Ru J, Qiu W H, Liu B. Kinesin motors in plants:from subcellular dynamics to motility regulation[J]. Current Opinion in Plant Biology, 2015, 28: 120-126. DOI:10.1016/j.pbi.2015.10.003 |
| [26] |
Dhonukshe P, Baluška F, Schlicht M, et al. Endocytosis of cell surface material mediates cell plate formation during plant cytokinesis[J]. Developmental Cell, 2006, 10(1): 137-150. DOI:10.1016/j.devcel.2005.11.015 |
| [27] |
Reichardt I, Stierhof Y D, Mayer U, et al. Plant cytokinesis requires de novo secretory trafficking but not endocytosis[J]. Current Biology, 2007, 17(23): 2047-2053. DOI:10.1016/j.cub.2007.10.040 |
| [28] |
Viotti C, Bubeck J, Stierhof Y D, et al. Endocytic and secretory traffic in Arabidopsis merge in the trans-Golgi network/early endosome, an independent and highly dynamic organelle[J]. The Plant Cell, 2010, 22: 1344-1357. DOI:10.1105/tpc.109.072637 |
| [29] |
Reichardt I, Slane D, El Kasmi F, et al. Mechanisms of functional specificity among plasma-membrane syntaxins in Arabidopsis[J]. Traffic, 2011, 12(9): 1269-1280. DOI:10.1111/j.1600-0854.2011.01222.x |
| [30] |
Touihri S, Knöll C, Stierhof Y D, et al. Functional anatomy of the Arabidopsis cytokinesis-specific syntaxin KNOLLE[J]. The Plant Journal, 2011, 68(5): 755-764. DOI:10.1111/j.1365-313X.2011.04736.x |
| [31] |
Richter S, Kientz M, Brumm S, et al. Delivery of endocytosed proteins to the cell-division plane requires change of pathway from recycling to secretion[J]. eLife, 2014, 3: e02131. DOI:10.7554/eLife.02131 |
| [32] |
Park M, Song K, Reichardt I, et al. Arabidopsis-adaptin subunit AP1M of adaptor protein complex 1 mediates late secretory and vacuolar traffic and is required for growth[J]. Proc Natl Acad Sci USA, 2013, 110(25): 10318-10323. DOI:10.1073/pnas.1300460110 |
| [33] |
Teh O K, Shimono Y, Shirakawa M, et al. The AP-1μ adaptin is required for KNOLLE localization at the cell plate to mediate cytokinesis in Arabidopsis[J]. Plant and Cell Physiology, 2013, 54(6): 838-847. DOI:10.1093/pcp/pct048 |
| [34] |
Cai H Q, Reinisch K, Ferro-Novick S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle[J]. Developmental Cell, 2007, 12(5): 671-682. DOI:10.1016/j.devcel.2007.04.005 |
| [35] |
Jaber E, Thiele K, Kindzierski V, et al. A putative TRAPPⅡ tethering factor is required for cell plate assembly during cytokinesis in Arabidopsis[J]. New Phytologist, 2010, 187(3): 751-763. DOI:10.1111/j.1469-8137.2010.03331.x |
| [36] |
Thellmann M, Rybak K, Thiele K, et al. Tethering factors required for cytokinesis in Arabidopsis[J]. Plant Physiology, 2010, 154(2): 720-732. DOI:10.1104/pp.110.154286 |
| [37] |
Qi X Y, Kaneda M, Chen J, et al. A specific role for Arabidopsis TRAPPⅡ in post-Golgi trafficking that is crucial for cytokinesis and cell polarity[J]. The Plant Journal, 2011, 68(2): 234-248. DOI:10.1111/j.1365-313X.2011.04681.x |
| [38] |
Fendrych M, Synek L, Pečenková T, et al. The Arabidopsis exocyst complex is involved in cytokinesis and cell plate maturation[J]. The Plant Cell, 2010, 22(9): 3053-3065. DOI:10.1105/tpc.110.074351 |
| [39] |
Wu J D, Tan X Y, Wu C Y, et al. Regulation of cytokinesis by exocyst subunit SEC6 and KEULE in Arabidopsis thaliana[J]. Molecular Plant, 2013, 6(6): 1863-1876. DOI:10.1093/mp/sst082 |
| [40] |
Rybak K, Steiner A, Synek L, et al. Plant cytokinesis is orchestrated by the sequential action of the TRAPPⅡ and exocyst tethering complexes[J]. Developmental Cell, 2014, 29: 607-20. DOI:10.1016/j.devcel.2014.04.029 |
| [41] |
Zhang L, Zhang H Y, Liu P, et al. Arabidopsis R-SNARE proteins VAMP721 and VAMP722 are required for cell plate formation[J]. PLoS One, 2011, 6(10): e26129. DOI:10.1371/journal.pone.0026129 |
| [42] |
Lauber M H, Waizenegger I, Steinmann T, et al. The Arabidopsis KNOLLE protein is a cytokinesis-specific syntaxin[J]. The Journal of Cell Biology, 1997, 139(6): 1485-1493. DOI:10.1083/jcb.139.6.1485 |
| [43] |
Li B X, Li Y B, Liu F, et al. Overexpressed tomosyn binds syntaxins and blocks secretion during pollen development[J]. Plant Physiology, 2019, 181(3): 1114-1126. DOI:10.1104/pp.19.00965 |
| [44] |
Qi X Y, Zheng H Q. Rab-A1c GTPase defines a population of the trans-Golgi network that is sensitive to endosidin1 during cytokinesis in Arabidopsis[J]. Molecular Plant, 2013, 6(3): 847-859. DOI:10.1093/mp/sss116 |
| [45] |
Mayers J R, Hu T W, Wang C, et al. SCD1 and SCD2 form a complex that functions with the exocyst and RabE1 in exocytosis and cytokinesis[J]. The Plant Cell, 2017, 29(10): 2610-2625. DOI:10.1105/tpc.17.00409 |
| [46] |
Kasmi F E, Krause C, Hiller U, et al. SNARE complexes of different composition jointly mediate membrane fusion in Arabidopsis cytokinesis[J]. Molecular Biology of the Cell, 2013, 24(10): 1593-1601. DOI:10.1091/mbc.e13-02-0074 |
| [47] |
Zheng H Y, Bednarek S Y, Sanderfoot A A, et al. NPSN11 is a cell plate-associated SNARE protein that interacts with the syntaxin KNOLLE[J]. Plant Physiology, 2002, 129(2): 530-539. DOI:10.1104/pp.003970 |
| [48] |
Jürgens G, Park M, Richter S, et al. Plant cytokinesis:a tale of membrane traffic and fusion[J]. Biochemical Society Transactions, 2015, 43(1): 73-78. |
| [49] |
Chow C M, Neto H, Foucart C, et al. Rab-A2 and rab-A3 GTPases define a trans-Golgi endosomal membrane domain in Arabidopsis that contributes substantially to the cell plate[J]. The Plant Cell, 2008, 20(1): 101-123. DOI:10.1105/tpc.107.052001 |
| [50] |
Thomas L L, Fromme J C. GTPase cross talk regulates TRAPPⅡ activation of Rab11 homologues during vesicle biogenesis[J]. The Journal of Cell Biology, 2016, 215(4): 499-513. DOI:10.1083/jcb.201608123 |
| [51] |
Kliesch T T, Dietz J, Turco L, et al. Membrane tension increases fusion efficiency of model membranes in the presence of SNAREs[J]. Scientific Reports, 2017, 7: 12070. DOI:10.1038/s41598-017-12348-w |
| [52] |
Wang S, Choi U B, Gong J H, et al. Conformational change of syntaxin linker region induced by Munc13s initiates SNARE complex formation in synaptic exocytosis[J]. The EMBO Journal, 2017, 36(6): 816-829. DOI:10.15252/embj.201695775 |
| [53] |
Carr C M, Rizo J. At the junction of SNARE and SM protein function[J]. Current Opinion in Cell Biology, 2010, 22(4): 488-495. DOI:10.1016/j.ceb.2010.04.006 |
| [54] |
Rehman A, Archbold J K, Hu S H, et al. Reconciling the regulatory role of Munc18 proteins in SNARE-complex assembly[J]. IUCrJ, 2014, 1(6): 505-513. DOI:10.1107/S2052252514020727 |
| [55] |
Bacaj T, Pang Z P, Sudhof T C. Testing the SNARE/SM protein model of membrane fusion[J]. Proc Natl Acad Sci USA, 2010, 107(52): 22365-22366. DOI:10.1073/pnas.1017268108 |
| [56] |
Park M, Touihri S, Muller I, et al. Sec1/Munc18 proteins stabilizes fusion-competent syntaxin for membrane fusion in Arabidopsis cytokinesis[J]. Developmental Cell, 2012, 22(5): 989-1000. DOI:10.1016/j.devcel.2012.03.002 |
| [57] |
Karnahl M, Park M, Krause C, et al. Functional diversification of Arabidopsis SEC1-related SM proteins in cytokinetic and secretory membrane fusion[J]. Proc Natl Acad Sci USA, 2018, 115(24): 6309-6314. DOI:10.1073/pnas.1722611115 |
| [58] |
Park M, Krause C, Karnahl M. Concerted action of evolutionarily ancient and novel SNARE complexes in flowering-plant cytokinesis[J]. Developmental Cell, 2018, 44(4): 500-511. DOI:10.1016/j.devcel.2017.12.027 |
| [59] |
van Damme D, Coutuer S, de Rycke R, et al. Somatic cytokinesis and pollen maturation in Arabidopsis depend on TPLATE, which has domains similar to coat proteins[J]. The Plant Cell, 2006, 18(12): 3502-3518. DOI:10.1105/tpc.106.040923 |
| [60] |
van Damme D, Gadeyne A, Vanstraelen M, et al. Adaptin-like protein TPLATE and clathrin recruitment during plant somatic cytokinesis occurs via two distinct pathways[J]. Proc Natl Acad Sci USA, 2011, 108(2): 615-620. DOI:10.1073/pnas.1017890108 |
| [61] |
Gadeyne A, Sánchez-Rodríguez C, Vanneste S, et al. The TPLATE adaptor complex drives clathrin-mediated endocytosis in plants[J]. Cell, 2014, 156(4): 691-704. DOI:10.1016/j.cell.2014.01.039 |
| [62] |
Zhang Y, Persson S, Hirst J, et al. Change your TPLATE, change your fate:plant CME and beyond[J]. Trends in Plant Science, 2015, 20(1): 41-48. DOI:10.1016/j.tplants.2014.09.002 |
| [63] |
di Rubbo S, Irani N G, Kim S Y, et al. The clathrin adaptor complex AP-2 mediates endocytosis of BRASSINOSTEROID INSENSITIVE1 in Arabidopsis[J]. The Plant Cell, 2013, 25(8): 2986-2997. DOI:10.1105/tpc.113.114058 |
| [64] |
Kim S Y, Xu Z Y, Song K, et al. Adaptor protein complex 2-mediated endocytosis is crucial for male reproductive organ development in Arabidopsis[J]. The Plant Cell, 2013, 25(8): 2970-2985. DOI:10.1105/tpc.113.114264 |
| [65] |
Yamaoka S, Shimono Y, Shirakawa M, et al. Identification and dynamics of Arabidopsis adaptor protein-2 complex and its involvement in floral organ development[J]. The Plant Cell, 2013, 25(8): 2958-2969. DOI:10.1105/tpc.113.114082 |
| [66] |
Fan L, Hao H, Xue Y, et al. Dynamic analysis of Arabidopsis AP2 subunit reveals a key role in clathrin-mediated endocytosis and plant development[J]. Development, 2013, 140(18): 3826-3837. DOI:10.1242/dev.095711 |
| [67] |
Kang B H, Busse J S, Bednarek S Y. Members of the Arabidopsis dynamin-like gene family, ADL1, are essential for plant cytokinesis and polarized cell growth[J]. The Plant Cell, 2003, 15(4): 899-913. DOI:10.1105/tpc.009670 |
| [68] |
Backues S K, Korasick D A, Heese A, et al. The Arabidopsis dynamin-related protein2 family is essential for gametophyte development[J]. The Plant Cell, 2010, 22(10): 3218-3231. DOI:10.1105/tpc.110.077727 |
| [69] |
Drakakaki G. Polysaccharide deposition during cytokinesis:challenges and future perspectives[J]. Plant Science, 2015, 236: 177-184. DOI:10.1016/j.plantsci.2015.03.018 |




