文章信息
- 张娅, 黄天虹, 张西林, 刘同坤, 侯喜林, 李英
- ZHANG Ya, HUANG Tianhong, ZHANG Xilin, LIU Tongkun, HOU Xilin, LI Ying
- 不结球白菜BcSERK1基因的克隆及表达分析
- Cloning and expression analysis of BcSERK1 from non-heading Chinese cabbage
- 南京农业大学学报, 2019, 42(6): 1014-1021
- Journal of Nanjing Agricultural University, 2019, 42(6): 1014-1021.
- http://dx.doi.org/10.7685/jnau.201901034
-
文章历史
- 收稿日期: 2019-01-19
游离小孢子培养在诱变育种[1-2]、转基因育种[3-5]、分子标记和基因定位[6-8]等方面具有重要的应用价值, 然而小孢子培养胚胎发生的机制尚不清楚。体细胞胚胎发生受体激酶(somatic embryogenesis receptor-like kinase, SERK)属于富含亮氨酸重复序列类受体蛋白激酶(leucine-rich repeat receptor-like kinases, LRR-RLKs)[9], 该激酶含有细胞外结构域、跨膜结构域以及能够将细胞外信号转到细胞内的细胞质激酶结构域[10], 这样的结构使其在细胞间和细胞与环境间的信号转导方面发挥着重要作用。SERK基因最早在胡萝卜组织中克隆得到, Schmidt等[11]在对其表达特异性进行研究时发现该基因仅在胚性细胞中表达, 而在其他组织中不表达, 因此将其作为胚胎发育的标记基因。随后SERK基因在拟南芥、玉米中也被分离出来[12-13], 且在双子叶植物、单子叶植物中均有发现。此外, 通过RNA干扰沉默莴苣LsSERK基因、中粒咖啡CcSERK1基因、水稻OsSERK1基因均会导致其体细胞胚胎发生能力降低[14-16]; 过表达拟南芥AtSERK1基因可以使拟南芥体细胞胚胎生成率提高3~4倍; 过表达水稻OsSERK1基因, 其细胞胚胎发生能力从72%增加到86%[12, 16]。研究还表明, 通过添加生长素, 硼和钙诱导体细胞胚胎发生是由SERK途径介导的[17-18]。
不结球白菜(Brassica campestris ssp. chinensis)在长江中下游种植面积广泛, 在农业生产中占有重要位置[19]。目前, 小孢子培养技术已在不结球白菜种质创制中得到成熟应用, 然而对于其发生机制的研究较少涉及。本研究对不结球白菜BcSERK1基因进行了生物信息学及表达分析, 旨在为深入研究BcSERK1基因在不结球白菜胚胎发生过程中的作用提供理论依据。
1 材料与方法 1.1 不结球白菜BcSERK1基因的克隆参照RNA Simple Total RNA Kit(TaKaRa)说明书提取不结球白菜‘二桩白’总RNA, 使用PrimeScriptTM Ⅱ 1st Strand cDNA Synthesis Kit(TaKaRa)反转录合成cDNA, 作为模板。参照大白菜Bra016112基因序列, 采用同源克隆的方法, 用Primer Premier 5.0设计基因克隆引物(表 1), 由南京擎科生物有限公司合成。反应体系(20 μL):cDNA模板1 μL, 正、反引物各1 μL, 2×Primer STAR Max DNA聚合酶10 μL, ddH2O 7 μL。反应程序:95 ℃ 5 min; 95 ℃ 30 s, 58 ℃ 2 min, 72 ℃ 10 min, 30个循环, 4 ℃保存。PCR产物用12 g·L-1的琼脂糖凝胶电泳进行检测, 目的片段胶回收后连接到pEAZY-Blunt载体上, 转化到大肠杆菌感受态细胞Transl-T1中, 挑取阳性单克隆测序验证。
| 引物名称 Primer name |
引物序列(5′→3′) Primer sequences |
引物用途 Usage of primers |
| BcSERK1-F/R | ATGGAAACGATTCATCTGGTGTTTATC/ TTACCTAGGACCAGATAACTCAACAGC |
BcSERK1基因克隆Cloning of BcSERK1 |
| Cell-F/R | NNNGGATCCATGTCTCCTGAAGCTTACGTTCTGT/ NNNGTCGACACCAAAACATACATTCCTTGGAACA |
亚细胞定位Subcellular localization |
| RT-qPCR-F/R | TCTGGTTCGGTTCCTGATAATG/ CAGGACATGGGTGACTTGTT |
BcSERK1的RT-qPCR RT-qPCR of BcSERK1 |
| Actin-F/R | CTCAGTCCAAAAGAGGTATTCT/ GTAGAATGTGTGATGCCAGATC |
RT-qPCR内参 Reference gene in RT-qPCR |
| 注:下划线表示酶切位点。 Note:Underlined sites are the sequences of restriction enzyme cutting site. |
||
用ORF Finder预测序列的开放阅读框, 蛋白质的一级序列用ProtParam进行分析, 蛋白质的跨膜区采用TMHMM进行预测, 用Signalp 4.0预测信号肽, 用NCBI的CD-Search预测氨基酸的序列保守结构域。利用DNAMAN 6.0软件进行氨基酸同源性比对, 用MEGA 5.2软件中的邻接法(NJ)构建系统进化树。
1.3 BcSERK1亚细胞定位分析根据BcSERK1基因的序列和PC1300-N1-YFP载体序列, 设计含有BamHⅠ和SalⅠ酶切位点的特异性引物(表 1)。采用双酶切的方法构建BcSERK1与PC1300-N1-YFP的融合表达载体。采用申浩冉等[20]的方法将PC1300-BcSERK1-YFP和空载PC1300-N1-YFP转化到洋葱表皮细胞中, 暗培养16 h后, 用激光共聚焦显微镜观察并拍照, 以PC1300-N1-YFP作为对照。
1.4 游离小孢子培养采用黄天虹等[21]的方法并略有改进。将2~3 mm的花蕾用1%(有效氯浓度)次氯酸钠表面消毒20 min, 再用无菌蒸馏水洗涤3次, 利用研磨机(IKA公司)提取小孢子。小孢子洗涤离心后重悬于NLN-13培养基中, 之后用血球计数板将小孢子的密度调至约1×105 mL-1。将4.5 mL小孢子悬浮液分装到培养皿(直径60 mm, 高度15 mm)中, 置于细胞培养箱中, 33 ℃暗培养(热激)24 h后转移至25 ℃。分别在热激1 d和培养7、14和21 d后观察胚胎发育过程。游离小孢子培养2周后统计出胚情况, 计算出胚率(小孢子出胚率=出胚数/花蕾数)。
1.5 实时荧光定量PCR在不结球白菜品种‘四九菜心’和‘二桩白’盛花期取样, 包括根、茎、叶、蕾(小于2 mm, 2~3 mm和大于3 mm)以及花的不同组织样品各0.2 g。将2个品种新鲜游离的小孢子(0 d)、热激1 d及培养3、5、7 d后的小孢子培养液收集到50 mL离心管中, 100 g离心3 min, 弃上清液, 然后将小孢子转移到2.0 mL离心管中, 迅速在液氮中冷冻并置于-80 ℃冰箱中备用。用总RNA提取试剂盒(TIANGEN)提取RNA, cDNA的合成参照PrimeScriptTM RT试剂盒说明书, 用Primer Quest Tool设计定量引物(表 1)。反应体系参照SYBR PrimerScript RT PCR Kit Ⅱ(TaKaRa)说明书。PCR程序采用两步法:95 ℃ 30 s; 94 ℃ 5 s, 60 ℃ 15 s, 72 ℃ 10 s, 40个循环; 设置60 ℃~95 ℃的熔解曲线。3次生物学重复和3次技术重复。用Rotor-Gene Real-time Analysis Software 6.1和Excel 2007软件进行数据分析。基因相对表达量计算采用2-ΔΔCT法[22]。
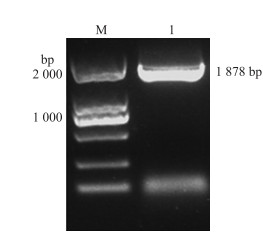
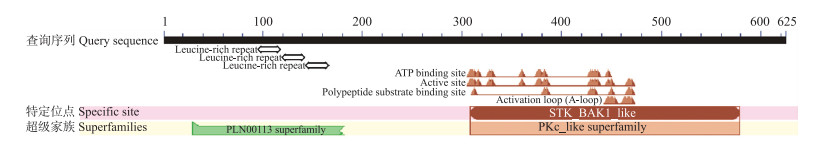
2 结果与分析 2.1 BcSERK1基因的克隆及序列分析以不结球白菜‘二桩白’cDNA为模板, 用特异性引物扩增获得的片段与预期片段大小一致(图 1)。序列分析结果表明BcSERK1基因包含1个长度为1 878 bp的开放阅读框(ORF), 编码625个氨基酸。对BcSERK1蛋白质一级序列理化性质预测表明:该蛋白质相对分子质量为155.588×103, 理论等电点为4.93, 不稳定指数为53.67, 属于不稳定蛋白。蛋白跨膜结构分析表明该蛋白具有跨膜结构域, 分别为第4~26和第240~262氨基酸的位置(图 2), 推测该蛋白很可能是1个跨膜蛋白。信号肽预测结果表明该蛋白含有信号肽(图 3), 信号肽结构域所在位置为1~26, 具有SERK家族典型的特征。BcSERK1保守结构域预测结果(图 4)显示:BcSERK1蛋白氨基酸序列前端有3个富亮氨酸重复序列, 氨基酸序列中有1个STK_BAK1_like结构, 该蛋白属于PKc_like和PLN00113超级家族。
|
图 1 BcSERK1基因PCR扩增产物 Fig. 1 PCR amplified product of BcSERK1 gene M. Marker; 1.BcSERK1扩增产物BcSERK1 PCR amplified. |

|
图 2 BcSERK1蛋白跨膜结构预测 Fig. 2 Predicted transmembrane domain of BcSERK1 protein |

|
图 3 BcSERK1蛋白的信号肽预测 Fig. 3 The prediction of signal peptide in BcSERK1 protein |
|
图 4 BcSERK1氨基酸序列的保守结构域 Fig. 4 Conserved domains of amino acid sequences of BcSERK1 |
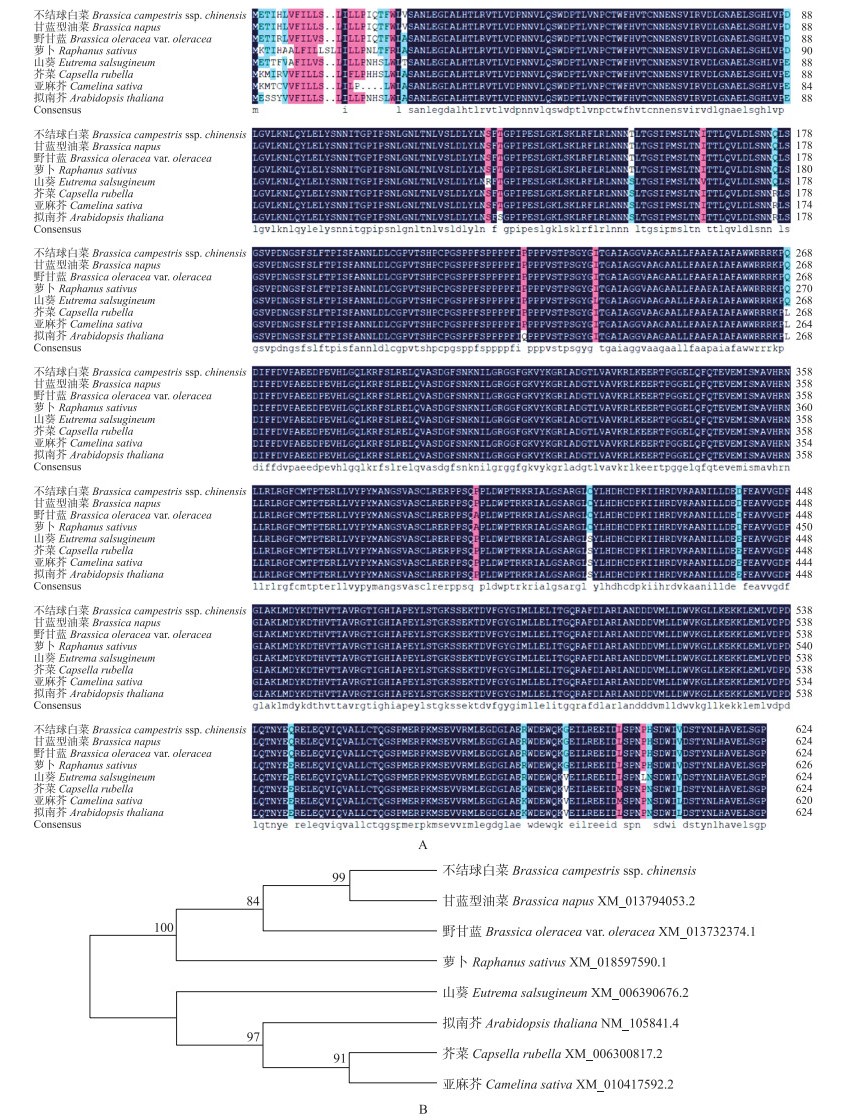
从NCBI中选择7个其他物种的SERK1蛋白序列, 与BcSERK1进行氨基酸序列比对及进化树分析, 结果(图 5)表明:BcSERK1与甘蓝型油菜、野甘蓝、萝卜、山葵、芥菜、亚麻芥、拟南芥同源性分别为99.68%、99.04%、98.09%、96.33%、96.17%、95.85%、96.17%, 显示其保守性较高, 且与甘蓝型油菜亲缘关系最近。
|
图 5 不结球白菜与其他物种SERK1同源氨基酸序列比对(A)及进化树分析(B) Fig. 5 The alignment of amino acid(A)and phylogenetic analysis(B)of SERK1 protein from non-heading Chinese cabbage and other plants |
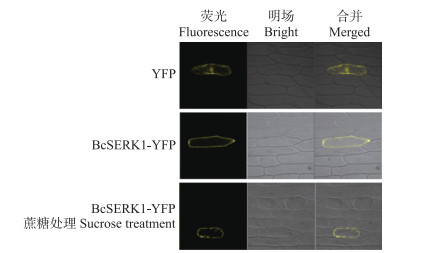
由图 6可见:在细胞膜、细胞质和细胞核中均可以观察到空载的黄色荧光; BcSERK1-YFP的黄色荧光信号全部集中在细胞膜和细胞壁上, 经过300 g·L-1蔗糖处理使其质壁分离后, 观察到黄色荧光信号在细胞膜上, 表明BcSERK1可能在细胞膜上发挥作用。
|
图 6 BcSERK1蛋白在洋葱表皮细胞中的亚细胞定位 Fig. 6 Subcellular localization of BcSERK1 protein in the onion epidermis cells |
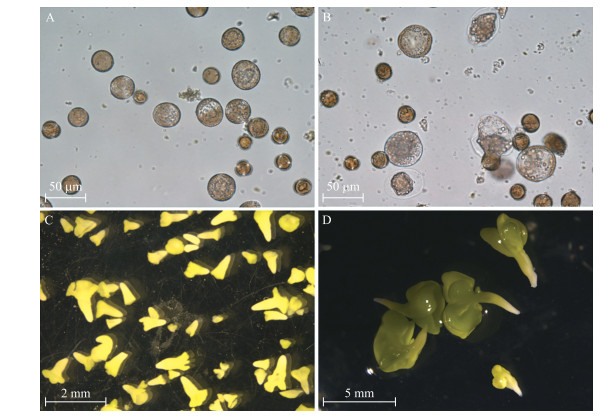
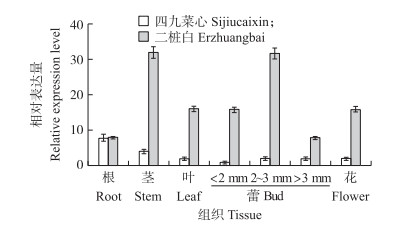
不结球白菜游离小孢子培养结果表明:‘二桩白’出胚率达到每蕾6.67胚, 而‘四九菜心’则不出胚。由图 7-A可见:33 ℃热激1 d后有些小孢子已经膨大, 培养7 d后有些小孢子形成多细胞结构(图 7-B), 培养14 d早期的子叶型胚形成, 但小孢子发育不同步, 培养基中还有球形胚、鱼雷形胚等(图 7-C), 培养21 d后则形成成熟的子叶型胚(图 7-D)。由图 8可见:该基因在2个品种的不同组织中均有表达。在‘二桩白’中, BcSERK1在茎和2~3 mm的蕾中表达量最高, 叶和小于2 mm的蕾以及花中次之, 根和大于3 mm的蕾中最低; 而在‘四九菜心’的茎、叶、花和蕾中表达量均较低, 且在2个品种根中的表达量相差不大。
|
图 7 不结球白菜‘二桩白’小孢子胚胎发育过程 Fig. 7 Microspore-derived embryos(MDE)development in non-heading Chinese cabbage of 'Erzhuangbai' A. 33 ℃热激1 d后的小孢子; B.培养7 d后形成的多细胞结构; C.培养14 d, 早期的子叶型胚和其他阶段的胚状体; D.培养21 d, 成熟的子叶型胚。 A. Microspores after heat shock at 33 ℃ for 1 d; B. Multicellular structure after 7 d of culture; C. Early cotyledon embryos and other stages of embryos after 14 d of culture; D. Mature cotyledon embryos after 21 d of culture. |
|
图 8 不结球白菜不同组织中BcSERK1基因的表达分析 Fig. 8 BcSERK1 expression analysis of different tissues in non-heading Chinese cabbage |
由图 9可见:BcSERK1在‘二桩白’新鲜游离的小孢子(0 d)中的表达量最高; 热激1 d后, 其表达量显著下降至原来的一半; 培养3和5 d后其表达量相差不大; 培养7 d后基因表达量下降至最低。在‘四九菜心’新鲜游离的小孢子和热激1 d后的小孢子中BcSERK1表达量最高, 培养3 d后基因表达量开始下降, 之后一直维持在较低水平。此外, BcSERK1基因在‘四九菜心’不同培养时间的表达量均低于‘二桩白’。

|
图 9 不结球白菜小孢子不同培养时间BcSERK1基因的表达分析 Fig. 9 Expression analysis of BcSERK1 gene of microspores at different culture time in non-heading Chinese cabbage |
胚胎发生是一个复杂的过程, 涉及各种生命活动。SERK在植物生长, 发育和防御反应中具有广泛作用[10]。研究表明SERK与BRI1(BRASSINOSTEROID-INSENSITIVE 1)互作, 在芸薹素(BR)信号传导途径中发挥重要作用[23], 而BR对调节植物细胞伸长和分裂具有重要作用[24-26], 且有研究表明向诱导培养基中添加油菜素内酯可以使不出胚的品种出少量的胚[27]。本研究对不结球白菜BcSERK1基因的克隆及分析结果表明, 该基因ORF为1 878 bp, 编码625个氨基酸; 蛋白相对分子质量为155.588×103, 含有2个跨膜结构域, 含有信号肽。氨基酸序列比对和进化分析显示, BcSERK1基因与其他物种中的同源基因具有很高的相似性, 说明该基因在进化上具有较高的保守性。亚细胞定位研究表明, 不结球白菜BcSERK1定位于细胞膜上, 这与大豆[28]、大麦[29]等其他物种的定位结果相一致。
本研究发现BcSERK1基因在‘二桩白’和‘四九菜心’的根、茎、叶、蕾以及花中均有表达。研究表明, 在甘蓝型油菜再生植株的芽和根中均检测到BnSERK1基因的表达[30]; 在中粒咖啡的根、茎、叶、芽顶端分生组织、幼花蕾和老花蕾中均检测到CcSERK1基因的表达[15]; 在大麦的根、茎和叶中也可以检测到HvSERK1基因的表达[29]。结合本研究的结果分析发现SERK1在非胚性组织中也有表达。此外, 本研究发现BcSERK1基因在不出胚的‘四九菜心’各个组织中表达量均较低。
Ahmadi等[30]研究表明BnSERK1基因在甘蓝型油菜小孢子胚胎发育1~5 d时显著上调, 而在球型-心形胚和鱼雷型胚期间表达量显著下调[30]; HvSERK1基因在大麦小孢子培养3 d后上调, 并在之后保持高表达[29]。研究表明, 培养前7 d是小孢子胚胎发育的关键时期[27], 本研究发现BcSERK1在不结球白菜‘二桩白’新鲜游离的小孢子(0 d)中表达量最高, 之后在热激1 d后显著下降, 在培养3和5 d后其表达量相差不大, 在培养7 d后其表达量下降至最低; ‘四九菜心’新鲜游离小孢子和培养1 d后其表达量相差不大, 之后下降并维持在较低的水平。这与Malik等[27]的研究结果一致。SERK1在小孢子胚胎发生过程中表达趋势不一致可能是由于培养条件不同[27, 29-30], SERK1表达量下降说明其作用可能主要在胚胎发生早期, 因此被认为可能是胚胎发育的标记基因[11]。结合本研究中BcSERK1基因在不出胚材料‘四九菜心’中的表达量远低于易出胚材料, 进一步表明SERK1基因对胚胎发生具有重要作用。
综上, 本研究中BcSERK1基因在易出胚材料除根外的组织及小孢子胚胎发育早期的表达量均高于不出胚材料, 且在胚胎发育早期其表达量呈下降趋势。该结论对研究BcSERK1基因在胚胎发生机制中的作用具有重要意义。
| [1] |
Liu C, Wang J L, Huang T D, et al. A missense mutation in the VHYNP motif of a DELLA protein causes a semi-dwarf mutant phenotype in Brassica napus[J]. Theoretical and Applied Genetics, 2010, 121(2): 249-258. DOI:10.1007/s00122-010-1306-9 |
| [2] |
Lu Y, Dai S Y, Gu A X, et al. Microspore induced doubled haploids production from ethyl methanesulfonate(EMS)soaked flower buds is an efficient strategy for mutagenesis in Chinese cabbage[J]. Frontiers in Plant Science, 2016, 7: 1780. |
| [3] |
李菲, 张淑江, 章时蕃, 等. 基因枪法介导大白菜小孢子转基因技术研究初报[J]. 园艺学报, 2017, 44(1): 62-68. Li F, Zhang S J, Zhang S F, et al. Transformation of Chinese cabbage microspores by particle bombardment[J]. Acta Horticulturae Sinica, 2017, 44(1): 62-68 (in Chinese with English abstract). |
| [4] |
孟茜.细胞穿透肽介导的大白菜小孢子遗传转化体系的构建[D].沈阳: 沈阳农业大学, 2014. Meng Q. Establishment of system for genetic transformation mediated by cell penetrating peptides under isolated microspore culture in Chinese cabbage[D]. Shenyang: Shenyang Agricultural University, 2014(in Chinese with English abstract). http://cdmd.cnki.com.cn/Article/CDMD-10157-1016188260.htm |
| [5] |
Brew-Appiah R A T, Ankrah N, Liu W, et al. Generation of doubled haploid transgenic wheat lines by microspore transformation[J]. PLoS One, 2013, 8(11): e80155. DOI:10.1371/journal.pone.0080155 |
| [6] |
Kitashiba H, Taguchi K, Kaneko I, et al. Identification of loci associated with embryo yield in microspore culture of Brassica rapa by segregation distortion analysis[J]. Plant Cell Reports, 2016, 35(10): 2197-2204. DOI:10.1007/s00299-016-2029-4 |
| [7] |
Liu X, Han F Q, Kong C C, et al. Rapid introgression of the Fusarium wilt resistance gene into an elite cabbage line through the combined application of a microspore culture, genome background analysis, and disease resistance-specific marker assisted foreground selection[J]. Frontiers in Plant Science, 2017, 8: 354. |
| [8] |
Valdés A, Clemens R, Möllers C. Mapping of quantitative trait loci for microspore embryogenesis-related traits in the oilseed rape doubled haploid population DH4069×Express 617[J]. Molecular Breeding, 2018, 38(5): 65. DOI:10.1007/s11032-018-0822-1 |
| [9] |
den Toorn M A, Albrecht C, de Vries S. On the origin of SERKs:bioinformatics analysis of the somatic embryogenesis receptor kinases[J]. Molecular Plant, 2015, 8(5): 762-782. DOI:10.1016/j.molp.2015.03.015 |
| [10] |
Li J. Multi-tasking of somatic embryogenesis receptor-like protein kinases[J]. Current Opinion in Plant Biology, 2010, 13(5): 509-514. DOI:10.1016/j.pbi.2010.09.004 |
| [11] |
Schmidt E D, Guzzo F, Toonen M A J, et al. A leucine-rich repeat containing receptor-like kinase marks somatic plant cells competent to form embryos[J]. Development, 1997, 124: 2049-2062. |
| [12] |
Hecht V, Vielle-Calzada J P, Hartog M V, et al. The Arabidopsis somatic embryogenesis receptor kinase 1 gene is expressed in developing ovules and embryos and enhances embryogenic competence in culture[J]. Plant Physiology, 2001, 127(3): 803-816. DOI:10.1104/pp.010324 |
| [13] |
Baudino S, Hansen S, Brettshneider R, et al. Molecular characterisation of two novel maize LRR receptor-like kinases, which belong to the SERK gene family[J]. Planta, 2001, 213(1): 1-10. DOI:10.1007/s004250000471 |
| [14] |
Santos M O, Romano E, Vieira L S, et al. Suppression of SERK gene expression affects fungus tolerance and somatic embryogenesis in transgenic lettuce[J]. Plant Biology, 2009, 11(1): 83-89. DOI:10.1111/j.1438-8677.2008.00103.x |
| [15] |
Pérez-Pascual D, Jiménez-Guillen D, Villanueva-Alonzo H, et al. Ectopic expression of the Coffea canephora SERK1 homolog-induced differential transcription of genes involved in auxin metabolism and in the developmental control of embryogenesis[J]. Physiologia Plantarum, 2018, 163(4): 530-551. DOI:10.1111/ppl.12709 |
| [16] |
Hu H, Xiong L, Yang Y. Rice SERK1 gene positively regulates somatic embryogenesis of cultured cell and host defense response against fungal infection[J]. Planta, 2005, 222(1): 107-117. DOI:10.1007/s00425-005-1534-4 |
| [17] |
Singla B, Khurana J P, Khurana P. Characterization of three somatic embryogenesis receptor kinase genes from wheat, Triticum aestivum[J]. Plant Cell Reports, 2008, 27(5): 833-843. DOI:10.1007/s00299-008-0505-1 |
| [18] |
Pandey D K, Singh A K, Chaudhary B. Boron-mediated plant somatic embryogenesis:a provocative model[J]. Journal of Botany, 2012, 2012: 1-9. |
| [19] |
侯喜林, 宋小明. 不结球白菜种质资源的研究与利用[J]. 南京农业大学学报, 2012, 35(5): 35-42. Hou X L, Song X M. Research and utilization of Brassica campestris ssp. chinensis Makino(non-heading Chinese cabbage)germplasm resources[J]. Journal of Nanjing Agricultural University, 2012, 35(5): 35-42 (in Chinese with English abstract). DOI:10.7685/j.issn.1000-2030.2012.05.005 |
| [20] |
申浩冉, 肖栋, 侯喜林. 不结球白菜调控开花时间候选基因BcVIL1的克隆与表达分析[J]. 南京农业大学学报, 2018, 41(5): 825-831. Shen H R, Xiao D, Hou X L. Cloning and expression analysis of flowering time candidate gene BcVIL1 in non-heading Chinese cabbage[J]. Journal of Nanjing Agricultural University, 2018, 41(5): 825-831 (in Chinese with English abstract). DOI:10.7685/jnau.201711011 |
| [21] |
黄天虹, 张娅, 梁超凡, 等. 不结球白菜游离小孢子培养及植株再生研究[J]. 核农学报, 2019, 33(2): 240-247. Huang T H, Zhang Y, Liang C F, et al. Isolated microspore culture and microspore-derived plant regeneration in non-heading Chinese cabbage[J]. Acta Agriculturae Nucleatae Sinica, 2019, 33(2): 240-247 (in Chinese with English abstract). |
| [22] |
Livak K J, Schmittgen T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method[J]. Methods, 2001, 25(4): 402-408. DOI:10.1006/meth.2001.1262 |
| [23] |
Karlova R, de Vries S C. Advances in understanding brassinosteroid signaling[J]. Science's STKE, 2006, 2006(354): pe36. |
| [24] |
Wang Z Y, Bai M Y, Oh E, et al. Brassinosteroid signaling network and regulation of photomorphogenesis[J]. Annual Review of Genetics, 2012, 46(1): 701-724. DOI:10.1146/annurev-genet-102209-163450 |
| [25] |
Gou X P, Yin H J, He K, et al. Genetic evidence for an indispensable role of somatic embryogenesis receptor kinases in brassinosteroid signaling[J]. PLoS Genetics, 2012, 8(1): e1002452. DOI:10.1371/journal.pgen.1002452 |
| [26] |
Wu W Z, Wu Y J, Gao Y, et al. Somatic embryogenesis receptor-like kinase 5 in the ecotype Landsberg erecta of Arabidopsis is a functional RD LRR-RLK in regulating brassinosteroid signaling and cell death control[J]. Frontiers in Plant Science, 2015, 6: 852. |
| [27] |
Malik M R, Wang F, Dirpaul J M, et al. Isolation of an embryogenic line from non-embryogenic Brassica napus cv. Westar through microspore embryogenesis[J]. Journal of Experimental Botany, 2008, 59(10): 2857-2873. DOI:10.1093/jxb/ern149 |
| [28] |
Yang C, Zhao T J, Yu D Y, et al. Isolation and functional characterization of a SERK gene from soybean(Glycine max(L.)Merr.)[J]. Plant Molecular Biology Reporter, 2011, 29(2): 334-344. DOI:10.1007/s11105-010-0235-8 |
| [29] |
Li Y B, Liu C H, Guo G M, et al. Expression analysis of three SERK-like genes in barley under abiotic and biotic stresses[J]. Journal of Plant Interactions, 2017, 12(1): 279-285. DOI:10.1080/17429145.2017.1339836 |
| [30] |
Ahmadi B, Masoomi-Aladizgeh F, Shariatpanahi M E, et al. Molecular characterization and expression analysis of SERK1 and SERK2 in Brassica napus L.:implication for microspore embryogenesis and plant regeneration[J]. Plant Cell Reports, 2016, 35(1): 185-193. DOI:10.1007/s00299-015-1878-6 |




