文章信息
- 王暄, 秦鑫, 李红梅, 于家荣
- WANG Xuan, QIN Xin, LI Hongmei, YU Jiarong
- 植物寄生线虫效应子研究进展
- Advances in research of effectors secreted by plant-parasitic nematodes
- 南京农业大学学报, 2019, 42(6): 986-995
- Journal of Nanjing Agricultural University, 2019, 42(6): 986-995.
- http://dx.doi.org/10.7685/jnau.201906048
-
文章历史
- 收稿日期: 2019-06-24
植物寄生线虫(plant-parasitic nematodes, PPN)是农业生产上一类重要的病原生物, 每年给全世界造成约1 570亿美元的经济损失[1], 其中根结线虫(root-knot nematodes, RKN)和孢囊线虫(cyst nematodes, CN)是影响最大的2个类群。根结线虫寄主范围极其广泛, 能寄生3 000多种植物, 而孢囊线虫大多数种类具有寄主专化性, 只寄生在特定或有限的寄主植物上[2]。
根结线虫与孢囊线虫均为根系内固定寄生线虫, 其侵染性2龄幼虫从靠近根尖的部位侵入寄主, 待移动至根系维管束后选择一定数量的植物细胞, 通过干扰其正常的生理代谢从而建立线虫各自的取食位点——巨细胞(giant cells)或合胞体(syncytia), 以维持线虫的营养摄取及完成发育和繁殖[3]。在整个寄生过程中植物寄生线虫分泌的效应子(effector)发挥着举足轻重的作用。Hogenhout等[4]将效应子定义为病原物产生的能够改变寄主细胞结构和功能的蛋白或其他小分子物质。因此, 广义的植物寄生线虫效应子除了参与调控植物免疫的蛋白, 还包括参与降解和修饰植物细胞壁以及诱导取食位点形成的相关蛋白(表 1)。本文分别从不同方面介绍效应子参与线虫-植物亲和及非亲和互作的相关研究进展。
| 效应子 Effector |
线虫种类 Nematode species |
功能 Function |
文献 References |
| 细胞壁修饰Cell wall modification | |||
| Gr-ENG-1 | 马铃薯金线虫 Globodera rostochiensis |
纤维素酶, 降解植物细胞壁 Cellulose, degrading plant cell wall |
[5] |
| Mi-XYl1 | 南方根结线虫 Meloidogyne incognita |
木聚糖酶, 降解植物细胞壁 Xylanase, degrading plant cell wall |
[6] |
| Gr-PEL-1 | 马铃薯金线虫 G.rostochiensis |
果胶裂解酶, 降解植物细胞壁 Pectate lyases, degrading plant cell wall |
[7] |
| Gr-EXP1 | 马铃薯金线虫 G.rostochiensis |
扩展蛋白, 软化植物细胞壁 Expansin, loosening plant cell wall |
[8] |
| 促进取食位点建立Promoting feeding site formation | |||
| Hs19C07 | 甜菜孢囊线虫 Heterodera schachtii |
与激素转运蛋白互作调控合胞体发育 Manipulating syncytium development via interaction with auxin influx transporter |
[9] |
| Hs10A07 | 甜菜孢囊线虫 H.schachtii |
调控合胞体形成过程中的激素响应 Manipulating auxin response during syncytium formation |
[10] |
| Hs30D08 | 甜菜孢囊线虫 H.schachtii |
与寄主细胞辅助剪接体蛋白互作改变基因表达 Altering gene expression via interaction with host auxiliary spliceosomal protein |
[11] |
| HsIPT | 甜菜孢囊线虫 H.schachtii |
激活细胞分裂素信号控制细胞分化 Controlling cell division via activating cytokinin signaling |
[12] |
| Hs-Tyr | 甜菜孢囊线虫 H.schachtii |
调控植物激素的稳定状态 Modulating plant hormone homeostasis |
[13] |
| GpGS | 马铃薯白线虫 G.pallida |
调节植物细胞氧化还原平衡 Modulating redox homeostasis of plant cell |
[14] |
| MiPFN3 | 南方根结线虫 M.incognita |
扰乱植物肌动蛋白微丝 Disrupting plant actin filaments |
[15] |
| Mi8D05 | 南方根结线虫 M.incognita |
调节巨细胞中的水分运输 Regulating water transport within giant-cells |
[16] |
| Hg-SYV46(CLE) | 大豆孢囊线虫 H.glycines |
模仿植物CLE①肽调控寄主维管束干细胞通路 Manipulating plant vascular stem cell pathway via mimicking plant CLE① peptide |
[17] |
| MiIDL1 | 南方根结线虫 M.incognita |
编码类植物IDA②短肽 Encoding IDA②-like plant peptide |
[18] |
| 抑制植物防卫Plant defense suppression | |||
| Mi-CRT | 南方根结线虫 M.incognita |
钙网蛋白, 螯合质外体钙阻碍其转运 Calreticulin, chelating calcium in apoplasm and preventing calcium influx |
[19] |
| Mh265 | 北方根结线虫 M.hapla |
调控植物免疫反应 Modulating plant basal immune responses |
[20] |
| Mg01965 | 拟禾本科根结线虫 M.graminicola |
抑制植物免疫反应 Suppressing host innate immune responses |
[21] |
| MiSGCR1 | 南方根结线虫 M.incognita |
抑制植物免疫反应 Suppressing host innate immune responses |
[22] |
| MjTTL5 | 爪哇根结线虫 M.javanica |
激活活性氧清除抑制植物免疫反应 Suppressing host immune via activation of ROS③ scavenging |
[23] |
| MgGPP | 拟禾本科根结线虫 M.graminicola |
抑制植物免疫反应 Suppressing host immune |
[24] |
| RHA1B | 马铃薯白线虫 G.pallida |
利用泛素化途径抑制植物ETI④和PTI⑤反应 Suppressing ETI④ and PTI⑤ signaling via an E3-independent mechanism |
[25] |
| HsGLAND4 | 甜菜孢囊线虫 H.schachtii |
与LTP基因启动子结合抑制植物免疫 Suppressing host immune via binding to promoter of LTP genes |
[26] |
| Mj-CM-1 | 爪哇根结线虫 M.javanica |
干扰水杨酸的合成及其介导的防卫 Disrupting SA⑥ biosynthesis and SA-mediated defence |
[27] |
| Mj-FAR-1 | 爪哇根结线虫 M.javanica |
影响脂质相关的防卫 Manipulating host lipid-based defenses |
[28] |
| 10A06 | 甜菜孢囊线虫 H.schachtii |
清除活性氧抑制寄主免疫反应 Suppressing host immune via ROS scavenging |
[29] |
| Mg16820 | 拟禾本科根结线虫 M.graminicola |
抑制寄主免疫反应 Suppressing host innate immune responses |
[30] |
| 激活植物免疫Triggering plant immunity | |||
| MAP-1 | 南方根结线虫 M.incognita |
涉及线虫无毒性群体与抗性番茄的识别 Involving in recognition between resistant tomatoes and avirulent nematodes |
[31] |
| Cg-1 | 爪哇根结线虫 M.javanica |
涉及线虫无毒性群体与抗性番茄的识别 Involving in the recognition between resistant tomatoes and avirulent nematodes |
[32] |
| Gp-Rbp-1 | 马铃薯白线虫 G.pallida |
被NB-LRR蛋白识别激发防卫反应 Eliciting defense responses by recognition of by the NB-LRR protein Gpa2 |
[33] |
| Gr-VAP1 | 马铃薯金线虫G.rostochiensis | 激活Cf-2与Rcr3pim介导的番茄PCD Triggering the Cf-2- and Rcr3pim-dependent programmed cell death in tomato |
[34] |
| 注: ①CLE:CLAVATA3/Embryo surrounding region; ②IDA:Inflorescence deficient in abscission; ③ROS:活性氧Reactive oxygen species; ④ETI:效应子激发的免疫Effector triggered immunity; ⑤PTI:病原相关分子模式激发的免疫Pathogen associated molecular patterns(PAMP) triggered immunity; ⑥SA:水杨酸Salicylic acid. | |||
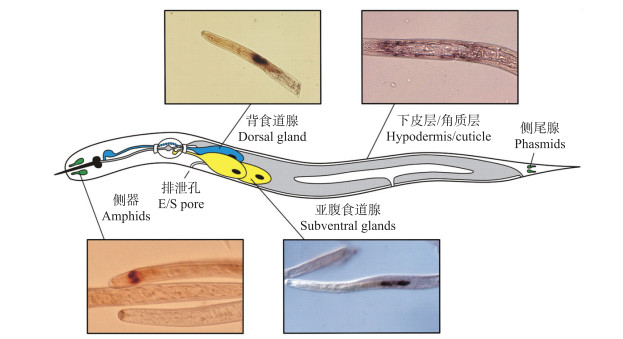
植物寄生线虫能够利用自身多个器官合成效应子并分泌至植物细胞内以促进寄生(图 1)[35], 其中2个亚腹食道腺细胞(subventral gland cells)和1个背食道腺细胞(dorsal gland cell)发挥着重要的作用。超微结构观察显示, 亚腹食道腺细胞在线虫侵入植物根系前活性较高, 而在固定寄生阶段萎缩变小; 与之相反, 背食道腺细胞则在固定寄生阶段逐渐膨大且活性增强, 因此前者被认为在线虫侵染早期发挥作用, 而后者主要与建立取食位点和维持寄生相关[36]。针对马铃薯白线虫(G.pallida)转录组数据的分析也证实, 在侵染前2龄幼虫特异性表达的基因主要定位于线虫亚腹食道腺细胞, 而多个在固定寄生阶段特异性表达的基因则定位于背食道腺细胞[37]。目前植物寄生线虫效应子的研究主要针对食道腺细胞分泌的蛋白, 然而事实上线虫其他器官分泌的蛋白同样在其寄生过程中发挥着重要的作用。例如, 马铃薯金线虫(G.rostochiensis)硫氧还蛋白过氧化物酶Gr-TpX和谷胱甘肽过氧化物酶Gr-GPX-1, 禾谷孢囊线虫(H.avenae)脂肪酸与视黄醇结合蛋白Ha-Far-1等, 均被证实定位于线虫表皮, 具有抗氧化及调控植物激素水平等功能[38-40]; 此外, 北方根结线虫(M.hapla)类甲状腺素运载蛋白MhTTL2、南方根结线虫(M. incognita)毒性变异相关蛋白MAP-1以及马铃薯白线虫HYP家族效应蛋白等, 则可能经由线虫头部侧器(amphids)分泌[20, 31, 41]。
2 降解和修饰植物细胞壁的效应子植物细胞壁是线虫侵入植物根系过程中必须逾越的障碍, 其主要成分包括纤维素、半纤维素、果胶以及木质素等[42]。尽管很早就发现线虫的匀浆具有纤维素酶活性, 但人们普遍认为动物并不产生细胞壁降解酶(cell wall degrading enzymes, CWDE), 上述活性可能来源于线虫体内的细菌。直至1998年从大豆孢囊线虫(H.glycines)体内发现了第1个动物源的纤维素酶(cellulase), 并且证实其能够水解β-1, 4-糖苷键从而降解纤维素, 首次确认了线虫可以利用CWDE软化植物细胞壁, 促进其侵入以及在根系中的移动[5]。进一步的研究证实线虫同时也分泌木聚糖酶(xylanases)[6]和果胶裂解酶(pectate lyases)[7]等效应子, 分别作用于植物的半纤维素和果胶层, 从而破坏植物细胞壁的完整性。不仅如此, 植物寄生线虫还能够利用自身分泌的扩展蛋白(expansins)破坏植物细胞壁不同组分间的非共价键结合, 帮助上述CWDE效应子更有效地结合各自底物[8]。
利用生物信息学技术分析不同线虫种类基因组数据的结果也显示, 植物寄生线虫均能编码CWDE, 而自由生活线虫及非致病的植物线虫中则未发现上述蛋白, 进一步证实了CWDE与线虫的寄生性密切相关[43]。而在已知的植物寄生线虫中, 南方根结线虫编码CWDE数量最多, 达到81个[1], 如此众多的CWDE是否与其寄主范围广泛具有相关性, 还有待进一步的证实。
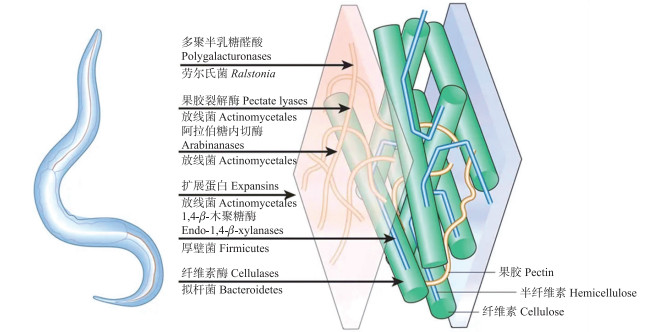
水平基因转移(horizontal gene transfer, HGT)是植物寄生线虫获得上述CWDE的主要途径。目前植物固定内寄生线虫已知的CWDE包括糖基水解酶家族(glycosyl hydrolase family)GH5、GH28、GH43, 多糖裂解酶家族(polysaccharide lyase family)PL3, 以及扩展蛋白等, 均能在放线菌类(actinomycete)、拟杆菌类(bacteroid)、梭菌(Clostridium spp.)以及劳尔氏菌(Ralstonia spp.)等原核生物中找到同源的基因, 因此原核生物被认为是植物固定内寄生线虫进化过程中CWDE的主要来源(图 2)[44-45]。此外, 植物迁移内寄生的滑刃线虫(Aphelenchoides spp.)和伞滑刃线虫(Bursaphelenchus spp.)CWDE则主要是糖基水解酶家族GH45, 系统进化分析则显示其可能来源于真菌[46]。
能否诱导形成取食位点, 是固定内寄生线虫成功寄生植物的关键, 甜菜孢囊线虫(H.schachtii)的多个效应子被证实在合胞体形成过程中发挥着重要作用。例如, Hs19C07通过与一类生长素转运蛋白(auxin influx transporter protein)LAX3互作调控取食位点的激素水平, 提高转运蛋白的活性, 从而影响合胞体的形成[9]; Hs10A07在植物细胞中被磷酸化修饰后进入细胞核, 与植物生长素响应因子(auxin response factors, ARF)的抑制子IAA16互作, 导致ARF表达上调, 一系列激素诱导的基因被激活, 从而促进合胞体的发育[10]; Hs30D08通过与植物细胞核中的辅助剪接体蛋白SMU2互作, 改变mRNA前体的剪接方式及取食位点相关基因的表达[11]; HsIPT和Hs-Tyr则被证实分别编码细胞分裂素合成异戊基转移酶(cytokinin-synthesizing isopentenyltransferase)和类酪氨酸酶蛋白(tyrosinase-like protein), 参与调控寄主细胞分裂和激素平衡[12-13]。此外, 甜菜孢囊线虫分泌的蛋白二硫键异构酶(protein disulfide isomerase)HsPDI和马铃薯白线虫分泌的谷胱甘肽合酶(glutathione synthetase)GpGS则能够扰乱植物细胞氧化还原平衡从而促进合胞体的形成[14, 47]。效应子同样影响根结线虫取食位点——巨细胞的形成, 南方根结线虫肌动蛋白抑制蛋白(profilin)MiPFN3, 通过与植物的肌动蛋白单体结合干扰其多聚化, 从而影响肌动蛋白微丝的组织和结构, 阻碍细胞的正常发育[15]; 同样来自南方根结线虫的效应子Mi8D05则能够与寄主植物的水通道蛋白(aquaporin tonoplast intrinsic protein 2, TIP2)互作, 可能参与调控巨细胞的水分运输[16]。
与此同时, 植物寄生线虫还能够利用分泌的效应子模拟植物多肽, 促进取食位点的形成。CLE(CLAVATA3/Embryo surrounding region)是迄今为止最大的植物多肽分子家族, 在调节植物分生组织的细胞分裂和分化中起着重要作用[48]。植物寄生线虫能够分泌一类含有植物CLE结构域的效应子, 其能够互补拟南芥clavata3-2缺失突变体的功能, 而异源表达则导致拟南芥的茎顶端分生组织生长提前终止及花蕊残缺, 产生类似于植物CLE过表达的表型[17]。此外, 线虫CLE能够与植物CLV1、CLV2、LRR-RK和BAM等多种受体互作, 沉默线虫CLE或者其植物互作靶标均能影响线虫取食位点的发育[49-51], 证实线虫可能通过模拟植物CLE的功能, 正向调控取食位点的发育。IDA(inflorescence deficient in abscission)也是植物中的1种短肽, 其能够与富含亮氨酸重复受体激酶HAE和HSL2互作, 激活MAPK级联反应及转录因子KNOX的表达, 进而调控植物花器的脱落和侧根形成[52-53]。南方根结线虫编码1种由47个氨基酸组成的类IDA短肽MiIDL1, 外施人工合成的MiIDL1或转基因表达均能够恢复拟南芥ida基因缺失突变体的表型, 且MiIDL1发挥功能需要上述受体激酶HAE/HSL2的参与, 揭示MiIDL1可能在线虫诱导巨细胞形成过程中发挥作用[18]。
4 抑制植物防卫反应的效应子线虫与植物的斗争同样遵循经典的“zigzag”模型, 即植物的模式识别受体(pattern recognition receptors, PRR)能够识别来自线虫的病原相关分子模式(pathogen associated molecular patterns, PAMP), 从而启动PAMP激发的免疫反应(PAMP triggered immunity, PTI); 与此同时, 线虫利用自身分泌的效应子抑制上述基础免疫反应从而造成寄主感病(effector-triggered susceptibility, ETS); 上述效应子进一步被植物NB-LRR类受体蛋白所识别, 从而诱发新一轮的防卫反应即效应子激发的免疫(effector triggered immunity, ETI), 线虫则进化出新的效应子以逃避识别或抑制ETI(图 3)[54-55]。

|
图 3 植物与线虫互作的“zigzag”模型[55] Fig. 3 The"zigzag"model of plant-nematode interactions[55] PRR:Pattern recognition receptors; PAMP(P):Pathogen associated molecular patterns; PTI:PAMP triggered immunity; HR:Hypersensitive response; ETS:Effector-triggered susceptibility; ETI:Effector triggered immunity; A, B, C, D:Immune receptors in plants; A, B, C, D in circle:Effectors secreted by nematodes. |
越来越多的线虫效应子已被证实能够抑制植物防卫反应, 如利用转基因拟南芥过表达南方根结线虫钙网蛋白Mi-CRT, 能够抑制elf18诱导的植物PTI相关基因的表达及胼胝质的积累, 促进南方根结线虫及寄生疫霉(Phytophthora parasitica)的侵染[19]。与之相类似, 来自北方根结线虫的一类线虫特有的效应蛋白Mh265抑制flg22诱导的胼胝质积累, 并且能够增加丁香假单胞菌(Pseudomonas syringae)的毒性[20]。质外体瞬时表达水稻拟禾本科根结线虫(M.graminicola)C型凝集素类效应蛋白Mg01965, 则抑制flg22激发的活性氧(ROS)爆发[21]。南方根结线虫分泌的一种富含甘氨酸和半胱氨酸的效应子MiSGCR1, 则能够抑制丁香假单胞菌无毒蛋白AvrPtob和寄生疫霉效应子NPP1引起的植物细胞死亡[22]。
不同线虫效应子与寄主植物的互作机制也正在被逐步阐明。爪哇根结线虫(M.javanica)分泌1种含有未知功能结构域的效应子MjTTL5, 其能够与拟南芥的1种还原酶亚基AtFTRc互作, 增强寄主清除活性氧的能力, 从而促进线虫的寄生[23]。水稻拟禾本科根结线虫在寄生过程中分泌1种效应子MgGPP至寄主细胞, MgGPP在内质网经N端糖基化与C端蛋白质水解等一系列修饰后进入植物细胞核发挥功能, 其中N端糖基化的MgGPP被证实能够抑制Gpa2/RBP-1激发的细胞死亡[24]。马铃薯白线虫效应子RHA1B具有E3泛素连接酶活性, RHA1B利用泛素化途径降解NB-LRR受体, 从而抑制多种NB-LRR自激突变体及激发子诱发的HR反应, 与此同时RHA1B也能以不依赖E3泛素连接酶活性的方式抑制flg22处理烟草叶片激发的Acre31和WRKY22等基因的表达[25]。甜菜孢囊线虫和大豆孢囊线虫的GLAND4效应子, 在进入植物细胞后被转运至细胞核, 与拟南芥脂质转运蛋白(lipid transfer protein genes, LTP)基因的启动子结合导致后者的表达下调, 降低了植物对丁香假单胞菌的抗性[26]。
植物寄生线虫除了利用多种不同的效应子抑制植物PTI或ETI反应, 还能够影响植物激素的合成及其介导的防卫反应。分支酸变位酶(chorismate mutase, CM)位于植物莽草酸代谢途径的末端, 为多种芳香族氨基酸的生物合成提供前体[56], 第一个动物源的CM来自爪哇根结线虫[57], 其过表达能够导致大豆的侧根发育异常, 而外源添加吲哚乙酸(IAA)则能够恢复正常表型[27]; 瞬时表达南方根结线虫Mi-cm-3能够影响烟草叶片中水杨酸(SA)的水平及PR1基因的表达[58], 揭示线虫CM可能通过竞争植物质体中的分支酸, 影响细胞中IAA和SA的正常合成, 从而影响水杨酸介导的防卫信号。脂肪酸与视黄醇结合蛋白(fatty acid- and retinol-binding protein, FAR)是线虫特有的一类蛋白, 被认为在线虫清除脂肪酸与视黄醇的生理代谢过程中发挥作用[59]。针对植物寄生线虫FAR蛋白的研究显示, 其能够影响根结的形成及雌虫的发育, 且能够调控与寄主植物JA相关的防卫信号通路, 从而促进线虫的寄生[28, 40, 60]。此外, 甜菜孢囊线虫效应子10A06能够与植物亚精胺合酶(spermidine synthase 2, SPDS2)互作, 通过调节ROS水平和SA信号通路影响植物免疫[29]。水稻拟禾本科根结线虫效应子Mg16820则能够与1种脱水胁迫诱导蛋白(dehydration stress-inducible protein 1, DIP1)互作, 而DIP1是1种脱落酸(ABA)应答基因, 两者间具体作用机制仍有待进一步明确[30]。
5 激活植物免疫的效应子在抑制植物防卫反应的同时, 部分线虫效应子已证实会被植物免疫系统识别, 从而导致非亲和互作。Map-1是最早报道的根结线虫无毒相关基因, 由于其仅在根结线虫无毒群体中表达, 而在克服番茄Mi基因抗性的毒性线虫群体中不表达, 因此该基因被认为对于抗性番茄识别南方根结线虫无毒群体具有关键作用[31]; 爪哇根结线虫中的Cg-1基因与之相类似, 沉默Cg-1能够使线虫无毒群体对含有Mi基因的抗性番茄产生毒性[32]。然而植物R基因如何识别线虫上述基因从而激活植物的抗性尚缺乏进一步的证实。
来自马铃薯白线虫的Gp-Rbp-1是第1个被证实的线虫无毒蛋白, Gp-Rbp-1属于SPRYSEC(SPRY结构域分泌蛋白)基因家族, 能够被NB-LRR蛋白Gpa2及其共受体RanGAP2(Ran GTPase-activating protein 2)识别, 从而激活植物防卫反应, 引起植物细胞程序化死亡(programmed cell death, PCD)[33]; 而来自同一基因家族的马铃薯金线虫SPRYSEC-19尽管同样能够与番茄CC-NBS-LRR蛋白SW5F互作, 但是却不能诱导PCD以及对马铃薯金线虫的抗性[61]; 相反SPRYSEC-19能够抑制GpRBP-1/Gpa2、PVX/Rx1以及CC-NBS-LRR蛋白SW5B自激突变体引起的PCD反应[62], 揭示这类蛋白在寄主抗性的选择压力下可能通过不断的进化和基因扩张, 从而逃避植物R蛋白的识别。
类毒液过敏原蛋白(venom allergen-like protein, VAP)是植物寄生线虫中普遍存在的一类效应子[63-65], 其中甜菜孢囊线虫及南方根结线虫的VAP被证实能够抑制植物的PTI及ETI反应, 同时促进多种病原菌的侵染[66]; 而来自马铃薯金线虫的Gr-VAP1则会被植物受体识别, 通过与胞外半胱氨酸蛋白酶Rcr3pim互作激活番茄叶霉菌(Cladosporium fulvum)抗性蛋白Cf-2介导的防卫反应, 引起PCD并增强植物对线虫的抗性[34]。此外, 马铃薯金线虫和禾谷孢囊线虫类扩展蛋白GrEXPB2和HaEXPB2, 均被证实在番茄或烟草上会引起植物细胞程序化死亡[67-68], 而针对禾谷孢囊线虫效应子的大规模筛选同样发现至少有7个不同的效应子能够在烟草上激发PCD, 但其各自对应的植物受体及激发植物免疫反应的机制仍不明确[68-69]。
6 问题与展望目前植物寄生线虫效应子的研究主要针对N端含有信号肽序列的一类蛋白, 其被认为能够经内质网-高尔基体蛋白分泌系统外泌[70], 再经线虫口针注射进植物细胞从而发挥功能。然而生物信息学分析显示, 南方根结线虫可编码近2万个蛋白, 其中仅约10%含有分泌信号肽[1]。事实上除了内质网-高尔基体途径外, 真核细胞中还存在分泌型溶酶体、直接跨膜、外来体释放和质膜出泡等其他多种不同分泌途径[71-73], 因此线虫中可能有大量蛋白通过非典型分泌途径外泌, 在线虫与植物互作过程中发挥功能。例如, 象耳豆根结线虫(M.enterolobii)不含信号肽的效应子MeTCTP在植物中表达能够促进线虫侵染, 并且抑制由促凋亡蛋白BAX激发的烟草叶片PCD[74]。因此, 拓展植物寄生线虫效应子研究范围, 明确大量不含N端分泌信号肽的效应子功能, 对于深入揭示线虫寄生植物的分子机制具有重要意义。
小RNA(microRNA, miRNA)是真核生物中广泛存在的一类21~25个核苷酸的非编码RNA分子, 在基因转录后的表达调控过程中发挥着重要的作用, 也能参与植物与病原物的亲和与非亲和互作。灰葡萄孢菌(Botrytis cinerea)和大丽花轮枝孢菌(Verticillium dahliae)能够利用miRNA作为一类新型效应子来跨界调控植物的免疫, 而植物同样也产生miRNA以抑制病原菌的毒性[75-77]。植物寄生线虫能否分泌miRNA进入寄主细胞目前尚未见相关报道, 然而已证实大豆孢囊线虫的侵染能够影响多个植物miRNA的表达[78-79]。此外甜菜孢囊线虫寄生过程中需要利用拟南芥mi396与生长调节类转录因子GRF1/GRF3的互作, 实现对根系发育的再编程[80]。明确植物寄生线虫能否利用miRNA作为效应子直接或间接调控植物的生理代谢, 从而促进其寄生, 有望成为今后线虫与植物互作机制研究的一个重要方向。
由于植物寄生线虫无法进行传统的遗传转化, 目前基因敲除主要利用RNA干扰(RNA interference, RNAi), 包括dsRNA浸泡, 病毒诱导的基因沉默(virus induced gene silencing, VIGS)以及转基因植物介导的基因沉默(host induced gene silencing, HIGS)。然而上述方法由于存在潜在的脱靶风险及沉默效率的差异[81], 在一定程度上影响了线虫效应子功能验证的可靠性。新兴的基因编辑技术(CRISPR-Cas), 已经在自由生活线虫和动物寄生线虫的遗传操作中取得了成功[82], 如果今后该技术可在植物寄生线虫学领域进行运用, 将对线虫效应子功能的研究起到极大的推动作用。
| [1] |
Abad P, Gouzy J, Aury J M, et al. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita[J]. Nature Biotechnology, 2008, 26(8): 909-915. DOI:10.1038/nbt.1482 |
| [2] |
Jones J T, Haegeman A, Danchin E G J, et al. Top 10 plant-parasitic nematodes in molecular plant pathology[J]. Molecular Plant Pathology, 2013, 14(9): 946-961. DOI:10.1111/mpp.12057 |
| [3] |
Kyndt T, Vieira P, Gheysen G, et al. Nematode feeding sites:unique organs in plant roots[J]. Planta, 2013, 238(5): 807-818. DOI:10.1007/s00425-013-1923-z |
| [4] |
Hogenhout S A, van der Hoorn R A L, Terauchi R, et al. Emerging concepts in effector biology of plant-associated organisms[J]. Molecular Plant-Microbe Interactions, 2009, 22(2): 115-122. DOI:10.1094/MPMI-22-2-0115 |
| [5] |
Smant G, Stokkermans J P W G, Yan Y T, et al. Endogenous cellulases in animals:isolation of β-1, 4-endoglucanase genes from two species of plant-parasitic cyst nematodes[J]. Proc Natl Acad Sci USA, 1998, 95(9): 4906-4911. DOI:10.1073/pnas.95.9.4906 |
| [6] |
Haegeman A, Vanholme B, Gheysen G. Characterization of a putative endoxylanase in the migratory plant-parasitic nematode Radopholus similis[J]. Molecular Plant Pathology, 2009, 10(3): 389-401. DOI:10.1111/j.1364-3703.2009.00539.x |
| [7] |
Popeijus H, Overmars H, Jones J, et al. Degradation of plant cell walls by a nematode[J]. Nature, 2000, 406(6791): 36-37. DOI:10.1038/35017641 |
| [8] |
Qin L, Kudla U, Roze E H, et al. Plant degradation:a nematode expansin acting on plants[J]. Nature, 2004, 427(6969): 30. DOI:10.1038/427030a |
| [9] |
Lee C, Chronis D, Kenning C, et al. The novel cyst nematode effector protein 19C07 interacts with the Arabidopsis auxin influx transporter LAX3 to control feeding site development[J]. Plant Physiology, 2011, 155(2): 866-880. DOI:10.1104/pp.110.167197 |
| [10] |
Hewezi T, Juvale P S, Piya S, et al. The cyst nematode effector protein 10A07 targets and recruits host posttranslational machinery to mediate its nuclear trafficking and to promote parasitism in Arabidopsis[J]. The Plant Cell, 2015, 27: 891-907. DOI:10.1105/tpc.114.135327 |
| [11] |
Verma A, Lee C, Morriss S, et al. The novel cyst nematode effector protein 30D08 targets host nuclear functions to alter gene expression in feeding sites[J]. New Phytologist, 2018, 219(2): 697-713. DOI:10.1111/nph.15179 |
| [12] |
Siddique S, Radakovic Z S, de la Torre C M, et al. A parasitic nematode releases cytokinin that controls cell division and orchestrates feeding site formation in host plants[J]. Proc Natl Acad Sci USA, 2015, 112(41): 12669-12674. DOI:10.1073/pnas.1503657112 |
| [13] |
Habash S S, Radakovic Z S, Vankova R, et al. Heterodera schachtii tyrosinase-like protein:a novel nematode effector modulating plant hormone homeostasis[J]. Scientific Reports, 2017, 7(1): 6874. DOI:10.1038/s41598-017-07269-7 |
| [14] |
Lilley C J, Maqbool A, Wu D Q, et al. Effector gene birth in plant parasitic nematodes:neofunctionalization of a housekeeping glutathione synthetase gene[J]. PLoS Genetics, 2018, 14(4): e1007310. DOI:10.1371/journal.pgen.1007310 |
| [15] |
Leelarasamee N, Zhang L, Gleason C. The root-knot nematode effector MiPFN3 disrupts plant actin filaments and promotes parasitism[J]. PLoS Pathogens, 2018, 14(3): e1006947. DOI:10.1371/journal.ppat.1006947 |
| [16] |
Xue B Y, Hamamouch N, Li C Y, et al. The 8D05 parasitism gene of Meloidogyne incognita is required for successful infection of host roots[J]. Phytopathology, 2013, 103(2): 175-181. DOI:10.1094/PHYTO-07-12-0173-R |
| [17] |
Wang X H, Mitchum M G, Gao B L, et al. A parasitism gene from a plant-parasitic nematode with function similar to CLAVATA3/ESR(CLE)of Arabidopsis thaliana[J]. Molecular Plant Pathology, 2005, 6(2): 187-191. DOI:10.1111/j.1364-3703.2005.00270.x |
| [18] |
Kim J, Yang R H, Chang C R, et al. The root-knot nematode Meloidogyne incognita produces a functional mimic of the Arabidopsis INFLORESCENCE DEFICIENT IN ABSCISSION signaling peptide[J]. Journal of Experimental Botany, 2018, 69(12): 3009-3021. DOI:10.1093/jxb/ery135 |
| [19] |
Jaouannet M, Magliano M, Arguel M J, et al. The root-knot nematode calreticulin Mi-CRT is a key effector in plant defense suppression[J]. Molecular Plant-Microbe Interactions, 2013, 26(1): 97-105. DOI:10.1094/MPMI-05-12-0130-R |
| [20] |
Gleason C, Polzin F, Habash S S, et al. Identification of two Meloidogyne hapla genes and an investigation of their roles in the plant-nematode interaction[J]. Molecular Plant-Microbe Interactions, 2017, 30(2): 101-112. DOI:10.1094/MPMI-06-16-0107-R |
| [21] |
Zhuo K, Naalden D, Nowak S, et al. A Meloidogyne graminicola C-type lectin, Mg01965, is secreted into the host apoplast to suppress plant defence and promote parasitism[J]. Molecular Plant Pathology, 2019, 20(3): 346-355. DOI:10.1111/mpp.12759 |
| [22] |
Nguyen C N, Perfus-Barbeoch L, Quentin M, et al. A root-knot nematode small glycine and cysteine-rich secreted effector, MiSGCR1, is involved in plant parasitism[J]. New Phytologist, 2018, 217: 687-699. DOI:10.1111/nph.14837 |
| [23] |
Lin B R, Zhuo K, Chen S Y, et al. A novel nematode effector suppresses plant immunity by activating host reactive oxygen species-scavenging system[J]. New Phytologist, 2016, 209: 1159-1173. DOI:10.1111/nph.13701 |
| [24] |
Chen J S, Lin B R, Huang Q L, et al. A novel Meloidogyne graminicola effector, MgGPP, is secreted into host cells and undergoes glycosylation in concert with proteolysis to suppress plant defenses and promote parasitism[J]. PLoS Pathogens, 2017, 13(4): e1006301. DOI:10.1371/journal.ppat.1006301 |
| [25] |
Kud J, Wang W J, Gross R, et al. The potato cyst nematode effector RHA1B is a ubiquitin ligase and uses two distinct mechanisms to suppress plant immune signaling[J]. PLoS Pathogens, 2019, 15(4): e1007720. DOI:10.1371/journal.ppat.1007720 |
| [26] |
Barnes S N, Wram C L, Mitchum M G, et al. The plant-parasitic cyst nematode effector GLAND4 is a DNA-binding protein[J]. Molecular Plant Pathology, 2018, 19(10): 2263-2276. DOI:10.1111/mpp.12697 |
| [27] |
Doyle E A, Lambert K N. Meloidogyne javanica chorismate mutase 1 alters plant cell development[J]. Molecular Plant-Microbe Interactions, 2003, 16(2): 123-131. DOI:10.1094/MPMI.2003.16.2.123 |
| [28] |
Iberkleid I, Vieira P, de Almeida Engler J, et al. Fatty acid- and retinol-binding protein Mj-FAR-1 induces tomato host susceptibility to root-knot nematodes[J]. PLoS One, 2013, 8(5): e64586. DOI:10.1371/journal.pone.0064586 |
| [29] |
Hewezi T, Howe P J, Maier T R, et al. Arabidopsis spermidine synthase is targeted by an effector protein of the cyst nematode Heterodera schachtii[J]. Plant Physiology, 2010, 152(2): 968-984. DOI:10.1104/pp.109.150557 |
| [30] |
Naalden D, Haegeman A, de Almeida-Engler J, et al. The Meloidogyne graminicola effector Mg16820 is secreted in the apoplast and cytoplasm to suppress plant host defense responses[J]. Molecular Plant Pathology, 2018, 19(11): 2416-2430. DOI:10.1111/mpp.12719 |
| [31] |
Semblat J P, Rosso M N, Hussey R S, et al. Molecular cloning of a cDNA encoding an amphid-secreted putative avirulence protein from the root-knot nematode Meloidogyne incognita[J]. Molecular Plant-Microbe Interactions, 2001, 14(1): 72-79. DOI:10.1094/MPMI.2001.14.1.72 |
| [32] |
Gleason C A, Liu Q L, Williamson V M. Silencing a candidate nematode effector gene corresponding to the tomato resistance gene Mi-1 leads to acquisition of virulence[J]. Molecular Plant-Microbe Interactions, 2008, 21(5): 576-585. DOI:10.1094/MPMI-21-5-0576 |
| [33] |
Sacco M A, Koropacka K, Grenier E, et al. The cyst nematode SPRYSEC protein RBP-1 elicits Gpa2- and RanGAP2-dependent plant cell death[J]. PLoS Pathogens, 2009, 5(8): e1000564. DOI:10.1371/journal.ppat.1000564 |
| [34] |
Lozano-Torres J L, Wilbers R H P, Gawronski P, et al. Dual disease resistance mediated by the immune receptor Cf-2 in tomato requires a common virulence target of a fungus and a nematode[J]. Proc Natl Acad Sci USA, 2012, 109(25): 10119-10124. DOI:10.1073/pnas.1202867109 |
| [35] |
Haegeman A, Mantelin S, Jones J T, et al. Functional roles of effectors of plant-parasitic nematodes[J]. Gene, 2012, 492(1): 19-31. DOI:10.1016/j.gene.2011.10.040 |
| [36] |
Hussey R S, Mims C W. Ultrastructure of esophageal glands and their secretory granules in the root-knot nematode Meloidogyne incognita[J]. Protoplasma, 1990, 156(1/2): 9-18. |
| [37] |
Thorpe P, Mantelin S, Cock P J, et al. Genomic characterisation of the effector complement of the potato cyst nematode Globodera pallida[J]. BMC Genomics, 2014, 15: 923. DOI:10.1186/1471-2164-15-923 |
| [38] |
Robertson L, Robertson W M, Sobczak M, et al. Cloning, expression and functional characterisation of a peroxiredoxin from the potato cyst nematode Globodera rostochiensis[J]. Molecular and Biochemical Parasitology, 2000, 111(1): 41-49. DOI:10.1016/S0166-6851(00)00295-4 |
| [39] |
Jones J T, Reavy B, Smant G, et al. Glutathione peroxidases of the potato cyst nematode Globodera rostochiensis[J]. Gene, 2004, 324: 47-54. DOI:10.1016/j.gene.2003.09.051 |
| [40] |
Le X H, Wang X, Guan T L, et al. Isolation and characterization of a fatty acid- and retinoid-binding protein from the cereal cyst nematode Heterodera avenae[J]. Experimental Parasitology, 2016, 167: 94-102. DOI:10.1016/j.exppara.2016.05.009 |
| [41] |
van den Akker S E, Lilley C J, Jones J T, et al. Identification and characterisation of a hyper variable apoplastic effector gene family of the potato cyst nematodes[J]. PLoS Pathogens, 2014, 10(9): e1004391. DOI:10.1371/journal.ppat.1004391 |
| [42] |
Gilbert H J. The biochemistry and structural biology of plant cell wall deconstruction[J]. Plant Physiology, 2010, 153(2): 444-455. DOI:10.1104/pp.110.156646 |
| [43] |
Ali M A, Azeem F, Li H J, et al. Smart parasitic nematodes use multifaceted strategies to parasitize plants[J]. Frontiers in Plant Science, 2017, 8: 1699. DOI:10.3389/fpls.2017.01699 |
| [44] |
Danchin E G J, Rosso M N, Vieira P, et al. Multiple lateral gene transfers and duplications have promoted plant parasitism ability in nematodes[J]. Proc Natl Acad Sci USA, 2010, 107(41): 17651-17656. DOI:10.1073/pnas.1008486107 |
| [45] |
Whiteman N K, Gloss A D. Parasitology:nematode debt to bacteria[J]. Nature, 2010, 468(7324): 641-642. DOI:10.1038/468641a |
| [46] |
Palomares-Rius J E, Hirooka Y, Tsai I J, et al. Distribution and evolution of glycoside hydrolase family 45 cellulases in nematodes and fungi[J]. BMC Evolutionary Biology, 2014, 14(1): 69. |
| [47] |
Habash S S, Sobczak M, Siddique S, et al. Identification and characterization of a putative protein disulfide isomerase(HsPDI)as an alleged effector of Heterodera schachtii[J]. Scientific Reports, 2017, 7: 13536. DOI:10.1038/s41598-017-13418-9 |
| [48] |
Cock J M, McCormick S. A large family of genes that share homology with CLAVATA3[J]. Plant Physiology, 2001, 126(3): 939-942. DOI:10.1104/pp.126.3.939 |
| [49] |
Replogle A, Wang J Y, Paolillo V, et al. Synergistic interaction of CLAVATA1, CLAVATA2, and RECEPTOR-LIKE PROTEIN KINASE 2 in cyst nematode parasitism of Arabidopsis[J]. Molecular Plant-Microbe Interactions, 2013, 26(1): 87-96. DOI:10.1094/MPMI-05-12-0118-FI |
| [50] |
Guo X L, Chronis D, de la Torre Cuba C M, et al. Enhanced resistance to soybean cyst nematode Heterodera glycines in transgenic soybean by silencing putative CLE receptors[J]. Plant Biotechnology Journal, 2015, 13: 801-810. DOI:10.1111/pbi.12313 |
| [51] |
Guo X L, Wang J Y, Gardner M, et al. Identification of cyst nematode B-type CLE peptides and modulation of the vascular stem cell pathway for feeding cell formation[J]. PLoS Pathogens, 2017, 13: e10006142. |
| [52] |
Cho S K, Larue C T, Chevalier D, et al. Regulation of floral organ abscission in Arabidopsis thaliana[J]. Proc Natl Acad Sci USA, 2008, 105(40): 15629-15634. DOI:10.1073/pnas.0805539105 |
| [53] |
Meng X Z, Zhou J G, Tang J, et al. Ligand-induced receptor-like kinase complex regulates floral organ abscission in Arabidopsis[J]. Cell Reports, 2016, 14: 1330-1338. DOI:10.1016/j.celrep.2016.01.023 |
| [54] |
Jones J D G, Dangl J L. The plant immune system[J]. Nature, 2006, 444(7117): 323-329. DOI:10.1038/nature05286 |
| [55] |
Smant G, Jones J. Suppression of plant defences by nematodes[M]//Jones J, Gheysen G, Fenoll C. Genomics and Molecular Genetics of Plant-Nematode Interactions. Heidelberg: Springer, 2011: 273-286.
|
| [56] |
Weaver L M, Herrmann K M. Dynamics of the shikimate pathway in plants[J]. Trends in Plant Science, 1997, 2(9): 346-351. DOI:10.1016/S1360-1385(97)84622-5 |
| [57] |
Lambert K N, Allen K D, Sussex I M. Cloning and characterization of an esophageal-gland-specific chorismate mutase from the phytoparasitic nematode Meloidogyne javanica[J]. Molecular Plant-Microbe Interactions, 1999, 12(4): 328-336. DOI:10.1094/MPMI.1999.12.4.328 |
| [58] |
Wang X, Xue B W, Dai J T, et al. A novel Meloidogyne incognita chorismate mutase effector suppresses plant immunity by manipulating the salicylic acid pathway and functions mainly during the early stages of nematode parasitism[J]. Plant Pathology, 2018, 67: 1436-1448. DOI:10.1111/ppa.12841 |
| [59] |
Garofalo A, Rowlinson M C, Amambua N A, et al. The FAR protein family of the nematode Caenorhabditis elegans. Differential lipid binding properties, structural characteristics, and developmental regulation[J]. Journal of Biological Chemistry, 2003, 278(10): 8065-8074. DOI:10.1074/jbc.M206278200 |
| [60] |
Iberkleid I, Sela N, Miyara S B. Meloidogyne javanica fatty acid- and retinol-binding protein(Mj-FAR-1)regulates expression of lipid-, cell wall-, stress- and phenylpropanoid-related genes during nematode infection of tomato[J]. BMC Genomics, 2015, 16: 272. DOI:10.1186/s12864-015-1426-3 |
| [61] |
Rehman S, Postma W, Tytgat T, et al. A secreted SPRY domain-containing protein(SPRYSEC)from the plant-parasitic nematode Globodera rostochiensis interacts with a CC-NB-LRR protein from a susceptible tomato[J]. Molecular Plant-Microbe Interactions, 2009, 22(3): 330-340. DOI:10.1094/MPMI-22-3-0330 |
| [62] |
Postma W J, Slootweg E J, Rehman S, et al. The effector SPRYSEC-19 of Globodera rostochiensis suppresses CC-NB-LRR-mediated disease resistance in plants[J]. Plant Physiology, 2012, 160: 944-954. DOI:10.1104/pp.112.200188 |
| [63] |
Wang X, Li H M, Hu Y J, et al. Molecular cloning and analysis of a new venom allergen-like protein gene from the root-knot nematode Meloidogyne incognita[J]. Experimental Parasitology, 2007, 117(2): 133-140. DOI:10.1016/j.exppara.2007.03.017 |
| [64] |
Lin S F, Jian H, Zhao H J, et al. Cloning and characterization of a venom allergen-like protein gene cluster from the pinewood nematode Bursaphelenchus xylophilus[J]. Experimental Parasitology, 2011, 127(2): 440-447. DOI:10.1016/j.exppara.2010.10.013 |
| [65] |
Luo S J, Liu S M, Kong L G, et al. Two venom allergen-like proteins, HaVAP1 and HaVAP2, are involved in the parasitism of Heterodera avenae[J]. Molecular Plant Pathology, 2019, 20(4): 471-484. DOI:10.1111/mpp.12768 |
| [66] |
Lozano-Torres J L, Wilbers R H P, Warmerdam S, et al. Apoplastic venom allergen-like proteins of cyst nematodes modulate the activation of basal plant innate immunity by cell surface receptors[J]. PLoS Pathogens, 2014, 10(12): e1004569. DOI:10.1371/journal.ppat.1004569 |
| [67] |
Ali S, Magne M, Chen S Y, et al. Analysis of putative apoplastic effectors from the nematode, Globodera rostochiensis, and identification of an expansin-like protein that can induce and suppress host defenses[J]. PLoS One, 2015, 10(1): e0115042. DOI:10.1371/journal.pone.0115042 |
| [68] |
Liu J, Peng H, Cui J K, et al. Molecular characterization of a novel effector expansin-like protein from Heterodera avenae that induces cell death in Nicotiana benthamiana[J]. Scientific Reports, 2016, 6: 35677. DOI:10.1038/srep35677 |
| [69] |
Chen C L, Chen Y P, Jian H, et al. Large-scale identification and characterization of Heterodera avenae putative effectors suppressing or inducing cell death in Nicotiana benthamiana[J]. Frontiers in Plant Science, 2018, 8: 2062. DOI:10.3389/fpls.2017.02062 |
| [70] |
Lee M C S, Miller E A, Goldberg J, et al. Bi-directional protein transport between the ER and Golgi[J]. Annual Review of Cell and Developmental Biology, 2004, 20(1): 87-123. DOI:10.1146/annurev.cellbio.20.010403.105307 |
| [71] |
Stinchcombe J, Bossi G, Griffiths G M. Linking albinism and immunity:the secrets of secretory lysosomes[J]. Science, 2004, 305(5680): 55-59. DOI:10.1126/science.1095291 |
| [72] |
Nickel W. Unconventional secretory routes:direct protein export across the plasma membrane of mammalian cells[J]. Traffic, 2005, 6(8): 607-614. DOI:10.1111/j.1600-0854.2005.00302.x |
| [73] |
Tournaviti S, Hannemann S, Terjung S, et al. SH4-domain-induced plasma membrane dynamization promotes bleb-associated cell motility[J]. Journal of Cell Science, 2007, 120(21): 3820-3829. DOI:10.1242/jcs.011130 |
| [74] |
Zhuo K, Chen J S, Lin B R, et al. A novel Meloidogyne enterolobii effector MeTCTP promotes parasitism by suppressing programmed cell death in host plants[J]. Molecular Plant Pathology, 2017, 18(1): 45-54. DOI:10.1111/mpp.12374 |
| [75] |
Weiberg A, Wang M, Lin F M, et al. Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways[J]. Science, 2013, 342(6154): 118-123. |
| [76] |
Jin Y, Zhao J H, Zhao P, et al. A fungal milRNA mediates epigenetic repression of a virulence gene in Verticillium dahliae[J]. Philosophical Transactions of the Royal Society B, 2019, 374(1767): 20180309. DOI:10.1098/rstb.2018.0309 |
| [77] |
Cai Q, Qiao L L, Wang M, et al. Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes[J]. Science, 2018, 360(6393): 1126-1129. DOI:10.1126/science.aar4142 |
| [78] |
Tian B, Wang S C, Todd T C, et al. Genome-wide identification of soybean microRNA responsive to soybean cyst nematodes infection by deep sequencing[J]. BMC Genomics, 2017, 18: 572. DOI:10.1186/s12864-017-3963-4 |
| [79] |
Li X Y, Wang X, Zhang S P, et al. Identification of soybean microRNAs involved in soybean cyst nematode infection by deep sequencing[J]. PLoS One, 2012, 7(6): e39650. DOI:10.1371/journal.pone.0039650 |
| [80] |
Hewezi T, Maier T R, Nettleton D, et al. The Arabidopsis microRNA396-GRF1/GRF3 regulatory module acts as a developmental regulator in the reprogramming of root cells during cyst nematode infection[J]. Plant Physiology, 2012, 159: 321-335. DOI:10.1104/pp.112.193649 |
| [81] |
Scacheri P C, Rozenblatt-Rosen O, Caplen N J, et al. Short interfering RNAs can induce unexpected and divergent changes in the levels of untargeted proteins in mammalian cells[J]. Proc Natl Acad Sci USA, 2004, 101(7): 1892-1897. DOI:10.1073/pnas.0308698100 |
| [82] |
Farboud B, Lo T. Targeted genome editing techniques in C.elegans and other nematode species[M]//Church G, Appasani K. Genome Editing and Engineering. Cambridge: Cambridge University Press, 2018: 3-21.
|