文章信息
- 董慧杰, 韩克, 侯喜林, 张志硕, 胡春梅
- DONG Huijie, HAN Ke, HOU Xilin, ZHANG Zhishuo, HU Chunmei
- 6种不结球白菜材料叶片抗氧化活性的比较及花青苷合成相关基因的表达分析
- Comparison of anthocyanins antioxidant activities and the expression analysis of genes related to anthocyanins biosynthesis in the leaves of six non-heading Chinese cabbage materials
- 南京农业大学学报, 2018, 41(3): 422-428
- Journal of Nanjing Agricultural University, 2018, 41(3): 422-428.
- http://dx.doi.org/10.7685/jnau.201706044
-
文章历史
- 收稿日期: 2017-06-29
不结球白菜(Brassica rapa ssp. chinensis)因其适应性广、营养丰富、栽培方便和生长周期短, 而受到人们的青睐。紫色不结球白菜叶片上、下表皮具有较大的颜色差异, 通常上表皮呈深紫色或亮紫色, 下表皮紫色分布不均匀, 富含花青苷[1-2]。花青苷主要存在于植物液泡中, 使植物花、叶片、果实、种子等呈现不同色彩[3]。研究表明, 花青苷是最理想的抗自由基物质, 具有很强的抗氧化性[4]和抗癌及预防心血管疾病等作用[5-6]。花青苷的抗氧化性与活性酚羟基有关, 酚羟基具有很强的抗氧化活性, 使花青苷具有清除各种活性氧自由基的能力[7-8]。
植物花青苷生物合成途径属于类黄酮生物合成途径分支之一, 其生物合成过程不仅受结构基因的作用, 还受相关调节基因的影响[9-10]。在植物花青苷合成途径中, 结构基因直接参与花青苷和中间代谢产物的合成与贮藏, 主要包括查尔酮合酶基因(CHS)、查尔酮异构酶基因(CHI)、黄烷酮3-羟化酶基因(F3H)和类黄酮3′-羟化酶基因(F3′H)、NADPH依赖性二氢黄酮醇还原酶基因(DFR)、无色花青苷双加氧酶/花青苷合成酶基因(LDOX/ANS)和类黄酮-3-O-葡糖基转移酶基因(UF3GT)。与植物花青苷合成有直接关系的转录因子主要包括R2R3-MYB、MYC(bHLH)和WD40三类, 通过其单体或者复合体来调控结构基因的表达, 进而影响花色素的积累。
目前, 植物中花青苷的研究多集中在其组分研究上。本研究以6种不同着色程度的不结球白菜为试验材料, 比较其总花青苷含量, 采用DPPH自由基清除能力法(DPPH法)和铁离子还原能力法(FRAP法)来研究叶色差异的不结球白菜叶片的抗氧化活性, 并利用实时定量和半定量方法测定花青苷合成相关结构基因和调节基因的表达水平, 为进一步研究不结球白菜中花青苷的特性及生物合成机制提供理论基础。
1 材料与方法 1.1 材料选用6种不结球白菜材料(图 1), 分别为深紫色的NJZX1-4(常规紫色可育系), 紫色的NJZX1-1(常规紫色不育系), 绿色的'苏州青'和NJZX1-0(由NJZX1-4经化学诱变后挑选出的可稳定遗传的绿色突变单株), 深紫色单株(NJZX2-2)和浅紫色单株(NJZX2-1)由NJZX1-4(母本)和NJZX1-0(父本)杂交后F1代经自交后所得F2代。

|
图 1 6种不结球白菜材料叶色的比较 Figure 1 Comparison of leaf color in six non-heading Chinese cabbage materials a.'苏州青''Suzhouqing'; b. NJZX1-0;c. NJZX2-1;d. NJZX1-1;e. NJZX2-2;f. NJZX1-4. |
将种子用蒸馏水冲洗干净, 室温催芽3 d, 播种至穴盘。幼苗长到2叶1心时移至大棚, 待材料长至7叶期时取样, 并将所有样品置于-80 ℃保存备用。
1.2 花青苷的提取与测定参照王小青等[11]的方法并略有改动。取新鲜功能叶片0.1 g用液氮研磨后, 加入1.5 mL酸化甲醇(含体积分数3%的HCl), 适当摇晃条件下室温浸提10 h; 常温10 000 r · min-1离心5 min, 然后取上清液经0.22 μm滤膜过滤, 备用。采用pH值示差法测定总花青苷含量, 具体步骤参照许玉超等[12]的方法。
1.3 不结球白菜叶片花青苷抗氧化活性测定DPPH法:参照殷丽琴等[13]的方法测定DPPH自由基清除率, 生物学重复3次。
FRAP法:参照Benzie等[14]的方法测定FRAP值并略有改动。将FRAP工作液预热至37 ℃, 提取的甲醇粗液150 μL, 加入2 850 μL FRAP工作液, 暗室反应30 min后测定593 nm波长下的吸光值。以没食子酸溶液作为标准样品绘制标准曲线y=0.006 6 x+0.112 7(R2=0.969 6)。单位为μg · g-1。重复3次。
1.4 总RNA的提取与单链cDNA的合成总RNA的提取参照RNA提取试剂盒使用说明书(TaKaRa)。单链cDNA的合成参照PrimeScript RT reagent Kit with gDNA Eraser反转录试剂盒(TaKaRa)。
1.5 实时荧光定量PCR利用Beacon Designer v 7.9软件设计引物, 引物序列如表 1所示。Actin基因作为内参[15], 并通过普通PCR验证引物的特异性。RT-qPCR反应采用SYBR Premix Ex Taq试剂盒, 以稀释10倍的单链cDNA作为模板。体系共20 μL, 含SYBR Premix Ex Taq 10 μL, ddH2O 7.2 μL, 模板2 μL, 正、反引物各0.4 μL。生物学重复3次, 相对定量测定参照ΔΔCT法[16]。
| 引物Primer | 序列Sequence(5′→3′) | 用途Usage |
| BrcCHI-F/R | TATTAAGGTGACGATGAAG/GAGAGAGCAAAGAGAATC | RT-qPCR |
| BrcCHS-F/R | ATCCAGACTACTACTTCC/GACTTCAACAACCACAAT | RT-qPCR |
| BrcF3H-F/R | CGAGCAGACTATCAATAG/ATATCTCTACTCATCTTTCTC | RT-qPCR |
| BrcDFR-F/R | CATAAGACAAGGACAGTAT/CGGAGATAGTAAGAATCG | RT-qPCR |
| BrcANS-F/R | ACCTTCATTCTACACAAC/AATCCTTACCTTCTCCTT | RT-qPCR |
| BrcUFGT-F/R | GTAATGTATCCGTGGTTAG/GGTAGAGGTTAAGAGGTT | RT-qPCR |
| BrcGL3-F/R | TCTATCTCTGCTTCTCAA/CTTCCTCGTCTTAATGTC | RT-PCR |
| BrcEGL3.1-F/R | GTGAAGAACTCAATAACTG/GTGAAGAACTCAATAACTG | RT-PCR |
| BrcEGL3.2-F/R | AGAGTGATATAGGAGAAGAT/TCGTATCATCAAGAATCG | RT-PCR |
| BrcTT8-F/R | CTGGAATCTACTCATCAC/GCATCTCATCTCTAACAA | RT-PCR |
| BrcTTG1-F/R | CACTCCACCATCATCTAC/GTCAAGAATCACAACCTTAT | RT-PCR |
| BrcMYB90-F/R | ACCCATTTGAGTAAGAAA/GGTATAAGAGCAAGGAAT | RT-PCR |
| BrcMYB75-F/R | GCTGTAATACCAAGATGA/AAGGAATAGAGGAATAACG | RT-PCR |
| Actin-F/R | GTTGCTATCCAGGCTGTTCT/AGCGTGAGGAAGAGCATAAC | RT-PCR or RT-qPCR |
以单链cDNA作为模板, 体系20 μL:2×HiQPCR Mix 10 μL, 引物各1 μL, 模板1 μL, ddH2O 7 μL。反应程序为:96 ℃ 2 min; 96 ℃ 30 s, 57 ℃ 30 s, 72 ℃ 30 s, 28个循环; 72 ℃ 10 min。
1.7 数据处理采用Microsoft Excel 2007和SPSS 20.0软件对数据进行统计分析, 以x±SD表示。
2 结果与分析 2.1 不结球白菜叶片的总花青苷含量由图 2可以看出:不结球白菜深紫色材料NJZX2-2和NJZX1-4叶中总花青苷含量较高, 分别为(693.0±50.6)和(801.5±57.4)μg · g-1, 极显著高于浅紫色材料NJZX1-1(P < 0.01);NJZX2-1叶中总花青苷含量为(279.7±56.5)μg · g-1, 且极显著低于NJZX1-1;绿色材料NJZX1-0和'苏州青'叶中总花青苷含量基本上为0。

|
图 2 6种不结球白菜材料叶片中总花青苷含量的比较 Figure 2 Comparison of total anthocyanin content of 6 materials in non-heading Chinese cabbage leaves 不同大写字母表示在0.01水平差异显著。下同。 Different capital letters indicate significant difference at 0.01 level. The same as follows. |
由图 3可见:6种不结球白菜材料叶片提取液对DPPH自由基均具有良好的清除能力, DPPH自由基清除率为(49.31±3.91)%~(84.94±6.20)%, 变异系数为20.7%。6种材料中叶片提取液的DPPH自由基清除率由大到小依次为NJZX1-1、NJZX1-4、NJZX2-2、NJZX2-1、NJZX1-0、'苏州青', 且紫色材料叶片的DPPH自由基清除率极显著高于绿色材料叶片。叶片提取液的铁离子还原能力整体上是紫色叶片提取液高于绿色叶片(P < 0.05)。6种材料叶片提取液的FRAP值为(256.8±136.3)~(1 279.5±202.0)μg · g-1。其中, NJZX1-1叶片提取液的铁离子还原能力最强, 而NJZX2-1与NJZX1-0差异不显著。相关性分析表明, 不同叶色白菜叶片中总花青苷含量与铁离子还原能力极显著相关, 相关系数为0.646, 与DPPH自由基清除率也极显著相关, 相关系数为0.829。

|
图 3 不结球白菜叶片提取液的DPPH自由基清除率和FRAP值 Figure 3 DPPH free radical-scavenging rates and the values of FRAP of the extracts from non-heading Chinese cabbage leaves 不同小写字母表示在0.05水平差异显著。 Different small letters indicate significant difference at 0.05 level. |
由图 4可见:花青苷合成途径中结构基因BrcF 3 H、BrcDFR和BrcANS的表达水平在紫色叶片中极显著高于绿色叶片, 且在6种材料中相对表达量与叶片总花青苷含量呈正相关。除BrcUFGT基因外其他5个结构基因在深紫色材料NJZX1-4和NJZX2-2中均有较高的表达量; BrcCHI基因在绿色的'苏州青'中的表达量显著高于紫色材料NJZX1-1, BrcCHS基因在'苏州青'中的表达量显著高于浅紫色材料NJZX2-1, 而BrcUFGT基因在所有材料中的表达均处于较低水平。
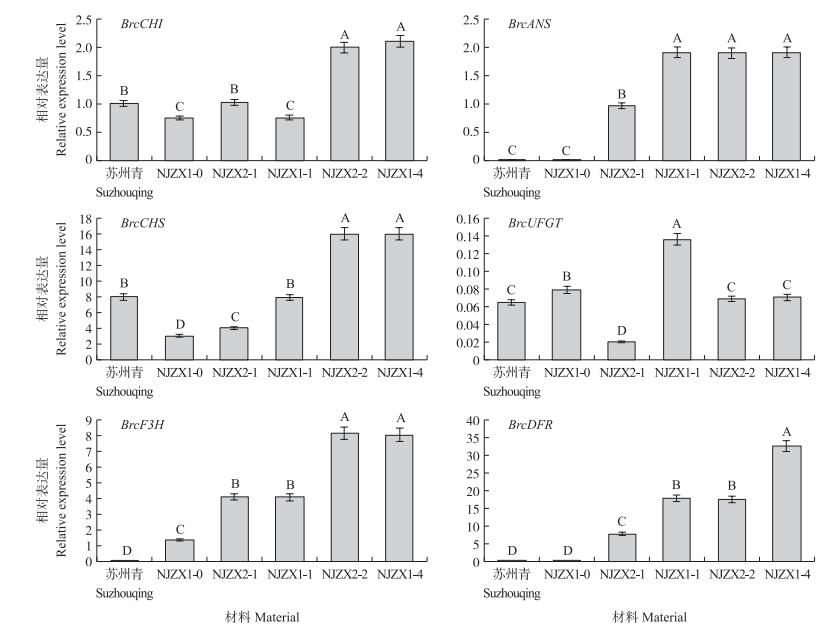
|
图 4 不结球白菜花青苷合成途径中结构基因的相对表达水平 Figure 4 Relative expression levels of the structural genes of the anthocyanin biosynthesis in non-heading Chinese cabbage |
7个调控花青苷的调节基因在6种材料叶片中的表达结果(图 5)显示:调节基因表达具有特异性。其中, BrcGL 3和BrcEGL3.1 基因表现出相似的表达模式, 即在6种材料叶片中均有表达且在材料间差异不明显。此外, BrcMYB 75基因和BrcTTG1 基因在深紫色材料NJZX1-4叶片中有较高表达, 而BrcEGL 3.2基因和BrcMYB90基因在所有材料的叶片中均不表达; BrcTT8 基因在所有材料中表现不同的表达水平, 其表达量由大到小依次为NJZX2-2、NJZX1-4、NJZX1-1、NJZX2-1、'苏州青'、NJZX1-0, 与花青苷含量测定结果一致。

|
图 5 不结球白菜花青苷合成途径中调节基因的表达量 Figure 5 Expression level of the regulatory genes of the anthocyanin biosynthesis in non-heading Chinese cabbage |
紫色白菜类叶片中所含色素大多为花青苷类化合物[17]。红叶芥菜、紫红色大白菜、红菜薹、紫色小白菜和紫结球甘蓝5种芸薹属新鲜叶片花青苷含量为130.0~719.0 μg · g-1[18]。本研究中, 紫色不结球白菜叶片中花青苷含量为0~801.5 μg · g-1, 绿色叶片基本不含花青苷; 其中, 紫色不结球白菜NJZX1-4叶片中花青苷含量明显高于红叶芥菜(719.0 μg · g-1)。紫色白菜叶片中DPPH自由基清除率和铁离子还原能力(FARP值)均显著高于绿色叶片, 且叶片花青苷含量与DPPH自由基清除率和铁离子还原能力基本呈正相关, 这与殷丽琴等[13]研究结果一致。
植物中花青苷生物合成受结构基因的作用。Gong等[19]研究发现F3H基因在红色紫苏叶片中表达, 而在绿叶材料中不表达, Sparvoli等[20]在葡萄中也发现类似结果。过量表达DFR1 基因, 可以增加转基因植株中花青苷的含量[21-22]。紫色羽衣甘蓝中DFR和ANS的表达明显高于绿色材料[23]。在本研究中, 结构基因BrcF3H、BrcDFR和BrcANS在紫色不结球白菜叶片中的表达极显著高于绿色叶片, 且其表达水平与不结球白菜叶片紫色着色程度呈正相关, 即叶片花青苷含量越高, 基因的表达量越高, 因此推测基因BrcF3H、BrcDFR和BrcANS为影响不结球白菜花青苷生物合成的关键基因。
双子叶植物花青苷的生物合成不但受结构基因的影响, 还受转录因子的调控作用[24]。植物花青苷生物合成调控因子主要有三大类, 包括MYB蛋白、bHLH蛋白和WD40蛋白, 3种蛋白之间均可发生相互作用, 进而形成二元复合物或者相互作用成MYB-bHLH-WD40三元复合物共同调控基因表达[25-26]。在拟南芥中, WD-repeat蛋白TTG1通过结合bHLH转录因子(GL3、TT8或EGL3)和R2R3-MYB转录因子(MYB75/PAP1、MYB90/PAP2、MYB113或MYB114)形成WD-repeat/Mybs/bHLH复合物来上调花青苷合成后期基因(DFR、ANS或UF3GT)的表达[27-28]。本研究中, 我们发现在验证的7个调节基因中, BrcGL3、BrcEGL3.1和BrcTT8基因在绿色和紫色材料中均有表达, 其中BrcTT8基因在不同材料中的表达水平与其叶片着色程度相关, 即花青苷含量越多, BrcTT8表达量越高。另外, BrcMYB75和BrcTTG1在深紫色材料NJZX1-4叶片中有较高表达。本试验结果表明这些转录因子在很大程度上参与了叶片中花青苷的生物合成调控, 但其是如何通过调节结构基因进而调控花青苷的积累, 有待进一步研究。
| [1] |
李长新. 紫色小白菜花青素理化性质研究[D]. 杨凌: 西北农林科技大学, 2011.
Li C X. The study of physicochemical property of anthocyanidin in purple pakchoi[D]. Yangling: Northwest A&F University, 2011(in Chinese with English abstract). |
| [2] |
徐学玲, 赵岫云, 王丹, 等. HPLC-ESI-MS分析紫色小白菜中花青苷组成分析[J].
食品工业科技, 2014, 35(11): 278-281.
Xu X L, Zhao X Y, Wang D, et al. HPLC-ESI-MS analysis of the structure and content of anthocyanins in purple pakchoi[J]. Science and Technology of Food Industry, 2014, 35(11): 278-277. (in Chinese with English abstract) |
| [3] | Tanaka Y, Sasaki N, Ohmiya A. Biosynthesis of plant pigments:anthocyanins, betalains and carotenoids[J]. Plant Journal for Cell and Molecular Biology, 2008, 54(4): 733-749. DOI: 10.1111/j.1365-313X.2008.03447.x |
| [4] | Pascual-Teresa S D, Sanchez-Ballesta M T. Anthocyanins:from plant to health[J]. Phytochemistry Reviews, 2008, 7(2): 281-299. DOI: 10.1007/s11101-007-9074-0 |
| [5] | Lam T K, Gallicchio L, Boyd K, et al. Cruciferous vegetable consumption and lung cancer risk:a systematic review[J]. Cancer Epidemiology Biomarkers & Prevention, 2009, 18(1): 184-195. |
| [6] | Poppel G V, Verhoeven D T H, Verhagen H, et al. Brassica vegetables and cancer prevention[J]. Advances in Experimental Medicine & Biology, 1999, 472(472): 159-168. |
| [7] |
王荣姣, 李昭华, 黄红林, 等. 北陆花青素的抗氧化作用[J].
安徽农业科学, 2011, 39(12): 7061-7063.
Wang R J, Li Z H, Huang H L, et al. Antioxidative activity of anthocyanins from Northland[J]. Journal of Anhui Agricultural Sciences, 2011, 39(12): 7061-7063. DOI: 10.3969/j.issn.0517-6611.2011.12.052 (in Chinese with English abstract) |
| [8] |
倪勤学, 霍艳荣, 陆国权. 花青苷保健功能的研究进展[J].
安徽农业科学, 2010, 38(35): 20025-20028.
Ni Q X, Huo Y R, Lu G Q. Advances in health functions of anthocyanins[J]. Journal of Anhui Agricultural Sciences, 2010, 38(35): 20025-20028. DOI: 10.3969/j.issn.0517-6611.2010.35.057 (in Chinese with English abstract) |
| [9] | Holton T A, Cornish E C. Genetics and biochemistry of anthocyanin biosynthesis[J]. Plant Cell, 1995, 7(7): 1071-1083. DOI: 10.1105/tpc.7.7.1071 |
| [10] | Grotewold E. The genetics and biochemistry of floral pigments[J]. Annual Review of Plant Biology, 2006, 57(1): 761-780. DOI: 10.1146/annurev.arplant.57.032905.105248 |
| [11] |
王小青, 韩键, 文杨, 等. 呈色机制不同的桃叶片花色素苷积累及合成相关基因表达的季节性差异[J].
南京农业大学学报, 2016, 39(6): 924-931.
Wang X Q, Han J, Wen Y, et al. Seasonal difference in the expression pattern of genes related to anthocyanin accumulation and biosynthesis in leaves of peach with different coloration modes[J]. Journal of Nanjing Agricultural University, 2016, 39(6): 924-931. DOI: 10.7685/jnau.201602027 (in Chinese with English abstract) |
| [12] |
许玉超, 侯喜林, 徐玮玮, 等. 紫色不结球白菜花青苷合酶基因BrcANS的克隆与表达分析[J].
作物学报, 2016, 42(6): 850-859.
Xu Y C, Hou X L, Xu W W, et al. Cloning and expression analysis of anthocyanidin synthase gene BrcANS from purple non-heading Chinese cabbage[J]. Acta Agronomica Sinica, 2016, 42(6): 850-859. DOI: 10.7505/j.issn.1007-9084.2016.06.021 (in Chinese with English abstract) |
| [13] |
殷丽琴, 韦献雅, 钟成, 等. 不同品种彩色马铃薯总花青苷含量与总抗氧化活性[J].
食品科学, 2014, 35(5): 96-100.
Yin L Q, Wei X Y, Zhong C, et al. Total anthocyanin content and total antioxidant activities in different varieties of pigmented potato(Solanum tuberosum L.)[J]. Food Science, 2014, 35(5): 96-100. DOI: 10.7506/spkx1002-6630-201405019 (in Chinese with English abstract) |
| [14] | Benzie I F, Strain J J. The ferric reducing ability of plasma(FRAP)as a measure of 'antioxidant power':the FRAP assay[J]. Analytical Biochemistry, 1996, 239: 70-76. DOI: 10.1006/abio.1996.0292 |
| [15] |
李妍, 王雪花, 陈忠文, 等. 不结球白菜抗坏血酸合成基因BcGME的同源克隆及胁迫下的表达分析[J].
南京农业大学学报, 2016, 39(2): 205-212.
Li Y, Wang X H, Chen Z W, et al. Homologous cloning and expression analysis of ascorbic acid biosynthesis gene BcGME under stress from non-heading Chinese cabbage[J]. Journal of Nanjing Agricultural University, 2016, 39(2): 205-212. DOI: 10.7685/jnau.201507026 (in Chinese with English abstract) |
| [16] |
谭国飞, 王枫, 贾晓玲, 等. 芹菜甘露醇脱氢酶基因的分离与表达分析[J].
园艺学报, 2013, 40(11): 2189-2198.
Tan G F, Wang F, Jia X L, et al. Isolation and expression of mannitol dehydrogenase gene in celery[J]. Acta Horticulturae Sinica, 2013, 40(11): 2189-2198. DOI: 10.3969/j.issn.0513-353X.2013.11.010 (in Chinese with English abstract) |
| [17] | Podsedek A. Natural antioxidants and antioxidant capacity of Brassica vegetables:a review[J]. LWT, 2007, 40(1): 1-11. DOI: 10.1016/j.lwt.2005.07.023 |
| [18] |
张淑江, 马越, 徐学玲, 等. 芸薹属5种紫红色蔬菜花青素苷含量及组分分析[J].
园艺学报, 2014, 41(7): 1451-1460.
Zhang S J, Ma Y, Xu X L, et al. Components and amounts of anthocyanins in several Brassica vegetables[J]. Acta Horticulturae Sinica, 2014, 41(7): 1451-1460. (in Chinese with English abstract) |
| [19] | Gong Z Z, Yamazaki M, Sugiyama M, et al. Cloning and molecular analysis of struetural genes involved in anthocyanin biosynthesis and expressed in a forma-specific manner in Perilla frutescens[J]. Plant Molecular Biology, 1997, 35(6): 915-927. DOI: 10.1023/A:1005959203396 |
| [20] | Sparvoli F, Martin C, Scienza A, et al. Cloning and molecular analysis of structural genes involved in flavonoid and stilbene biosynthesis in grape(Vitis vinifera L.)[J]. Plant Molecular Biology, 1994, 24(5): 743. DOI: 10.1007/BF00029856 |
| [21] | Xie D Y, Jackson L A, Cooper J D, et al. Molecular and biochemical analysis of two cDNA clones encoding dihydroflavonol-4-reductase from Medicago truncatula[J]. Plant Physiology, 2004, 134(3): 979-994. DOI: 10.1104/pp.103.030221 |
| [22] | Huang Y, Gou J Q, Jia Z, et al. Molecular cloning and characterization of two genes encoding dihydroflavonol-4-reductase from Populus trichocarpa[J]. PLoS One, 2012, 7(2): e30364. DOI: 10.1371/journal.pone.0030364 |
| [23] | Zhang B, Hu Z, Zhang Y, et al. A putative functional MYB transcription factor induced by low temperature regulates anthocyanin biosynthesis in purple kale(Brassica oleracea var. acephala f. tricolor)[J]. Plant Cell Reports, 2012, 31(2): 281-289. DOI: 10.1007/s00299-011-1162-3 |
| [24] | Albert N W, Davies K M, Lewis D H, et al. A conserved network of transcriptional activators and repressors regulates anthocyanin pigmentation in eudicots[J]. The Plant Cell, 2014, 26(3): 962-980. DOI: 10.1105/tpc.113.122069 |
| [25] | Baudry A, Caboche M, Lepiniec L. TT8 controls its own expression in a feedback regulation involving TTG1 and homologous MYB and bHLH factors, allowing a strong and cell-specific accumulation of flavonoids in Arabidopsis thaliana[J]. The Plant Journal, 2006, 46(5): 768-779. DOI: 10.1111/tpj.2006.46.issue-5 |
| [26] |
刘晓芬, 李方, 殷学仁, 等. 花青苷生物合成转录调控研究进展[J].
园艺学报, 2013, 40(11): 2295-2306.
Liu X F, Li F, Yin X R, et al. Recent advances in the transcriptional regulation of anthocyanin biosynthesis[J]. Acta Horticulturae Sinica, 2013, 40(11): 2295-2306. DOI: 10.3969/j.issn.0513-353X.2013.11.023 (in Chinese with English abstract) |
| [27] | Gonzalez A, Zhao M, Leavitt J M, et al. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings[J]. Plant Journal, 2008, 53(5): 814-827. DOI: 10.1111/tpj.2008.53.issue-5 |
| [28] | Zhang F, Gonzalez A M, Payne C T, et al. A network of redundant bHLH proteins functions in all TTG1-dependent pathways of Arabidopsis[J]. Development, 2003, 130(20): 4859-4869. DOI: 10.1242/dev.00681 |




