文章信息
- 张佳琦, 胡恒康, 徐川梅, 胡渊渊, 黄有军, 夏国华, 黄坚钦, 常英英, 叶磊, 娄和强, 张启香.
- Zhang Jiaqi, Hu Hengkang, Xu Chuanmei, Hu Yuanyuan, Huang Youjun, Xia Guohua, Huang Jianqin, Chang Yingying, Ye Lei, Lou Heqiang, Zhang Qixiang.
- 核桃JrGA2ox基因的克隆、亚细胞定位及功能验证
- Cloning, Subcellular Localization and Function Verification of Gibberellin 2-Oxidase Gene in Walnut (Juglans regia)
- 林业科学, 2019, 55(2): 50-60.
- Scientia Silvae Sinicae, 2019, 55(2): 50-60.
- DOI: 10.11707/j.1001-7488.20190206
-
文章历史
- 收稿日期:2018-03-12
- 修回日期:2018-05-10
-
作者相关文章
2. 林木遗传育种国家重点实验室 国家林业和草原局林木培育重点实验室 中国林业科学研究院林业研究所 北京 100091
2. State Key Laboratory of Tree Genetics and Breeding Key Laboratory of Tree Breeding and Cultivation of National Forestry and Grassland Administration Research Institute of Forestry, Chinese Academy of Forestry Beijing 100091
赤霉素(Gibberellin, GA)是一种四环二萜类化合物,是高等植物的天然产物,在植物株高等生长发育过程中起着重要的调控作用(Phinney et al., 1957;Yamaguchi, 2008)。研究发现,植株的高矮与GA的种类及其信号途径密切相关(Hedden et al., 2003)。由于赤霉素在植株的形态建成过程中起着十分重要的作用,因此,在果树矮化、短枝形成等机制的研究中,对赤霉素合成的关键酶的研究十分重要(Dong et al., 2014)。目前,大多数GAs生物合成代谢途径中关键酶基因的功能已被证实(Pearce et al., 2015)。赤霉素2氧化酶(GA2-oxidase,GA2ox)作为多基因家族编码的双加氧酶,是赤霉素代谢途径中的关键酶之一,它可将活性GA和其前体转变成不可逆的无活性的GA,与植株矮化密切相关。同时,GA2ox基因的表达具有明显的组织特异性,并在植株不同组织器官以及不同生长发育时期的表达量有显著差异(Yamaguchi, 2008; Chen et al., 2012)。
目前,已从拟南芥(Arabidopsis thaliana)等植物中克隆出GA2ox基因并进行了相关研究。拟南芥AtGA2ox基因过量表达会引起转基因植株中活性GAs含量降低,在不影响开花结实的情况下出现矮化表型(Lee et al., 2014);拟南芥AtGA2ox1在百喜草(Paspalum notatum)中异源表达导致植株内源活性GAs含量降低、植株矮化、分蘖数增加,并且提高了坪观质量(Shan et al., 2014);过量表达拟南芥AtGA2ox7或AtGA2ox8可导致本氏烟草(Nicotiana benthamiana)植株变矮(Schomburg et al., 2003)。水稻(Oryza sativa)OsGA2ox5过量表达使植株叶鞘部位细胞变小,茎节变短,株型变矮,同时,该基因在拟南芥中的异源表达也获得了相似的表型(Shan et al., 2014)。此外,果树中也有GA2ox基因的相关报道。白牡丹等(2012)以富士苹果(Malus domestica ‘Fuji’)茎尖为试验材料,对GA2ox基因DNA/cDNA序列进行了克隆、测序与序列比较分析,揭示了该基因的内含子和外显子结构特征。程飞飞(2012)成功从西洋梨‘中矮1号’(Pyrus communis ‘Zhongai 1’)矮化砧木中克隆得到GA2ox基因全长cDNA序列,通过研究发现,西洋梨‘中矮1号’的矮化与GA2ox基因在嫩叶及韧皮部组织不同时期的表达密切相关,并成功构建了GA2ox基因过量表达载体pCAMBIA1304-GA2ox。
然而,目前在果树中有关GA2ox基因克隆、同源转化的功能验证等相关研究仍鲜见报道。核桃(Juglans regia)为胡桃科(Juglandaceae)胡桃属(Juglans)优质油料坚果树种,经济价值高,生态效益好,是世界最重要的四大坚果树种之一,已成为主产区经济增长的重要引擎(吴国良等, 2009)。但核桃树体高大,大量分布于立地条件复杂的山坡、沟坎等处,采收管理困难、坚果商品率低,传统上树采收劳动强度大、事故时有发生、作业十分危险(Vahdati et al., 2009)。因此,获得矮化的核桃新品种是核桃产业发展亟待解决的难题。本研究首次克隆了核桃JrGA2ox基因,利用生物信息学方法对其进行分析并进行亚细胞定位,同时通过核桃体细胞胚同源转化对该基因进行了功能验证,以期为JrGA2ox基因功能研究和应用提供理论依据。
1 材料与方法 1.1 材料 1.1.1 植物材料省部共建亚热带森林培育国家重点实验室培育的核桃组培苗以及体细胞胚(简称“体胚”)。核桃组培苗以及体细胞胚均选用DKW(Tulecke et al., 1985)培养基。总RNA提取采用核桃子叶胚时期的体细胞胚以及植株幼嫩叶片,冻存于-80 ℃备用。瞬时表达试验选用本氏烟草。
1.1.2 酶及生化试剂各种限制性内切酶、rTaq DNA聚合酶、ExTaq酶、连接酶及DNA Marker购自TaKaRa公司和全式金公司,RNAprep pure Plant Kit购于天根生化科技(北京)有限公司,cDNA反转录试剂盒、凝胶回收试剂盒、质粒提取试剂盒均购自TaKaRa公司。引物和接头序列均由上海生工合成,其编号及序列见表 1。
|
|
亚细胞定位以及过表达载体由pCAMBIA1300改造而来(在多克隆位点前后分别加入花椰菜花叶病毒(CaMV)的35S启动子和绿色荧光蛋白基因sGFP,以下简称35S-sGFP)。大肠杆菌(Escherichia coli)DH5α与农杆菌(Agrobacterium)GV3101感受态细胞均购于TaKaRa。
1.2 方法 1.2.1 目的基因的克隆与载体构建植物总RNA的提取方法按照RNAprep pure Plant Kit(天根)及其说明书提供的方法进行。根据山核桃(Carya cathayensis)GA2ox基因的全长CDS序列和诺唯赞ClonExpress II One Step Cloning Kit试剂盒(Zhang et al., 2015),基于In-Fusion克隆技术,利用引物设计软件CE Design设计全长克隆引物(表 1)。反转录参照PrimeScriptTM RT Reagent Kit(Perfect Real Time)。根据Saito等(2013)的PCR扩增体系进行基因扩增,并将其PCR程序调整为:94 ℃预变性2 min;98 ℃变性10 s,60 ℃退火30 s,68 ℃延伸2 min,34个循环;68 ℃延伸7 min。PCR产物进行1.5%琼脂糖凝胶电泳分析。以此同时,将35S-sGFP质粒进行BamHⅠ和SalⅠ双酶切,分别回收PCR产物和双酶切产物,并利用诺唯赞One step cloning试剂盒连接。将35S∷JrGA2ox∷GFP融合表达载体转化大肠杆菌DH5α感受态细胞(Sambrook et al., 1995)。
PCR酶切筛选阳性克隆,并对阳性克隆进行测序验证,引物与扩增基因全长时所用一致(表 1)。将序列正确的大肠杆菌提取质粒并进行测序,测序成功的质粒则转化农杆菌(Hood et al., 1993)并在含有50 mg·L-1卡纳霉素的LB平板上筛选培养。选用Premix Taq(TaKaRa Taq Version 2.0),根据其说明书对单菌落进行PCR鉴定。PCR体系:菌液0.6 μL,正反向引物各0.6 μL,Premix Taq 7.5 μL,ddH2O 5.7 μL;PCR程序:94 ℃预变性2 min;94 ℃变性30 s,60 ℃退火30 s,72 ℃延伸2 min(34个循环);72 ℃再延伸5 min,4 ℃保存。最终确定正确的克隆以备下步试验所用。
1.2.2 JrGA2ox基因编码蛋白生物信息学分析将测序得到的核苷酸序列和对应的氨基酸序列分别在The Plant Genomics Resource(https://phytozome.jgi.doe.gov/pz/portal.html#)和NCBI数据库上用BLASTn和BLASTp进行序列相似性分析。利用MEGA构建系统发育树;利用Protparam(http://web.expasy.org/protparam/)对氨基酸序列的蛋白质分子量和等电点进行分析;应用ProtScale(http://www.expasy.ch/cgi-bin/protscale.pl)在线软件预测蛋白质的疏水性;应用TMHHMM Server V.2.0在线程序预测蛋白质的跨膜结构;利用Wolf psort(https://wolfpsort.hgc.jp/)进行蛋白亚细胞定位预测(屠煦童等, 2015)。
1.2.3 JrGA2ox基因编码蛋白的亚细胞定位本氏烟草在温度25 ℃、湿度60%、光照每天16 h的条件下,培养5~6周后,用于试验。
扩大培养含有35S∷JrGA2ox∷GFP过表达载体以及35S-sGFP空载体的农杆菌。将菌液于4 000 r·min-1 15 min条件下进行离心,收集菌体。以1:1的比例加入烟草转化液重悬农杆菌,调节菌液OD600为0.7~1.0之间,室温静置2 h。用同样的方法培养收集携带红色荧光核定位蛋白的农杆菌(此定位蛋白是由水稻ART1(Yamaji et al., 2009)和一个具有RFP信号的载体蛋白连接而来)和携带质膜定位蛋白(Nelson et al., 2007)的农杆菌。混合农杆菌重悬液注射本氏烟草叶片下表皮(Yamaguchi et al., 2005)。培养3~5天后通过激光共聚焦显微镜(蔡司LSM 710)进行观察。波长488 nm激发绿色荧光,波长514 nm时激发红色荧光。
1.2.4 核桃体细胞胚JrGA2ox基因同源转化及其表达将构建好的载体转入农杆菌GV3101中并扩大培养至菌液浓度OD600为1.0,5 000 r·min-1离心10 min收集菌体。加入含40 mg·L-1乙酰丁香酮的DKW液体培养基重悬菌液。将生长良好的核桃体细胞胚放入重悬好的菌液中侵染15 min后去除多余菌液,而后置于上述DKW固体培养基培养2周。再置于含有80 mg·L-1潮霉素和300 mg·L-1羧苄青霉素的DKW固体培养基中筛选阳性体细胞胚,具体步骤参考https://bio-protocol.org/e1258。利用体式荧光显微镜(Carl Zeiss Stereo D13covery V12,Axio Cam MRc system),在蓝光(488 nm)激发下,观察体胚荧光情况,并进行PCR验证(引物参照表 1中的JrGA2ox-F与GFP-R)。筛选出阳性体胚进行诱导萌发试验(Leslie et al., 2010)。利用实时荧光定量PCR(qPCR)(Guo et al., 2012)通过2-ΔΔCT法,测算JrGA2ox在核桃阳性植株中的相对表达量,引物参照表 1。同时,剪取生长条件较为一致且等长的核桃35S∷JrGA2ox∷GFP阳性再生植株的顶芽,将其培养10天后观察表型,并进行PCR验证,引物参照表 1。
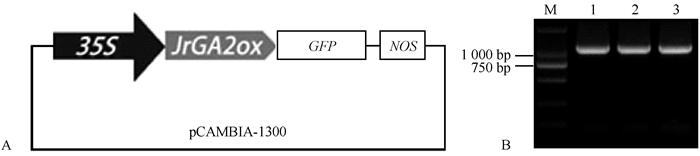
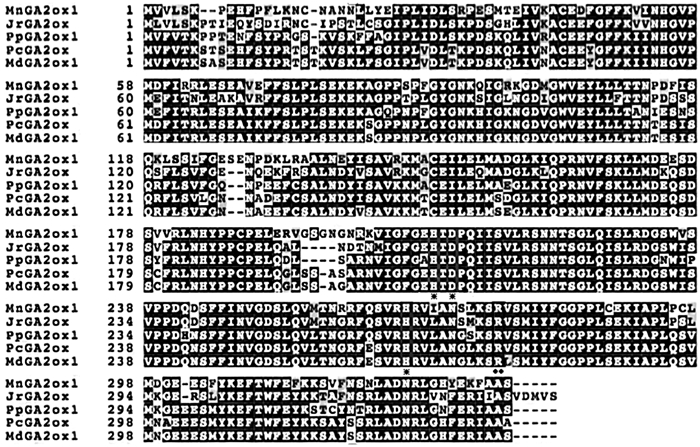
2 结果与分析 2.1 核桃JrGA2ox基因克隆与过表达载体构建根据设计的引物,扩增得到1条开放阅读框(ORF)全长为1 056 bp的条带,将此基因命名为JrGA2ox,且构建了35S∷JrGA2ox∷GFP的过表达载体(图 1A)。通过PCR验证和测序,结果发现电泳条带(1 056 bp)大小符合预期,表明JrGA2ox已插入载体(图 1B)。该基因编码351个氨基酸,分子量为39.25 kDa。利用NCBI BLAST进行检索,获得蛋白ID号为XP018824979.1。通过比对发现,核桃JrGA2ox蛋白序列与川桑(Morus notabilis)、西洋梨(Pyrus communis)、苹果(Malus domestica)、桃(Prunus persica)的相似度为72%~76%。此基因编码的蛋白序列含有保守20G-FeII-Oxy蛋白结构域、高度保守的2-酮戊二酸结合位点Arg-272,以及Fe2+结合位点His-205、Asp-207、His-262,具有GA2-氧化酶蛋白家族的共同结构特点,表明JrGA2ox属于GA2-氧化酶家族(图 2)。GA2ox基因家族聚为3簇:核桃GA2ox与毛白杨(Populus tomentosa)GA2ox(AFP58845.1)、桃GA2ox1(XP 007211571.1)、葡萄(Vitis vinifera)GA2ox(AFJ05044.1)、荔波连蕊茶(Camellia lipoensis) GA2ox2 (AHZ13201.1)、柿树(Diospyros kaki) GA2ox1(AID65070.1)、欧洲夹竹桃(Nerium oleander) GA2ox2(AAT92094.1)、矮牵牛(Petunia hybrida) GA2ox2(AFH56955.1)、烟草(Nicotiana tabacum) GA2ox1(NP 001312259.1)以及皱叶烟草(Nicotiana plumbaginifolia)GA2ox(CAR92132.1)聚为一个分支;且与柿树(Diospyros kaki)GA2ox2(AID65071.1)、可可(Theobroma cacao)GA2ox1(EOY13687.1)、裂叶牵牛(Ipomoea nil)GA2ox2(ADF42513.2)、川桑GA2ox1(XP 010110619.1)、豌豆(Pisum sativum)GA2ox(AAF08609.1)、赤豆(Vigna angularis)GA2ox1(NP 001316752.1)和野大豆(Glycine soja)GA2ox1(KHN47477.1)分为一簇。其次是蒺藜苜蓿(Medicago truncatula)GA2ox(XP 013464394.1)、矮牵牛(Petunia hybrid)GA2ox3(AFH56954.1)、陆地棉(Gossypium hirsutum)GA2ox2(AEK70418.1)、水稻(Oryza sativa)GA2ox3(AB092485)、麻疯树(Jatropha curcas)GA2ox(ACT87982.1)、西洋梨GA2ox(AEQ27855.1)、苹果GA2ox1(NP 001281028.1)和沙梨(Pyrus pyrifolia)GA2ox(BAU19310.1)聚为第2簇。水稻GA2ox1(AB059416)和GA2ox2(AB092484)、烟草GA2ox3(ABO70985.1)和GA2ox2(BAD17856.1)、中国李(Prunus salicina)GA2ox(AEA51242.1)、黄瓜(Cucumis sativus)GA2ox1(NP 001292640.1)、笋瓜(Cucurbita maxima)GA2ox(CAC85924.1)、番茄(Solanum lycopersicum)GA2ox2(NP001234338.1)、豌豆GA2ox2(AF100955)以及拟南芥GA2ox1(NP 177965.1)、GA2ox3(NP 181002.1)、GA2ox4(NP 175233.1)、GA2ox6(NP 171742.1)和GA2ox7(NP 175509.1)为第3簇(图 3)。
|
图 1 35S∷JrGA2ox∷GFP过表达载体的构建和大肠杆菌菌液PCR检测 Fig. 1 The construct of overexpression vector and PCR detection of Escherichia coli A: 35S∷JrGA2ox∷GFP过表达载体结构。pCAMBIA1300载体中,35S为载体中的强启动子,GFP为绿色荧光蛋白信号,NOS为终止子。B:泳道1-3为带有35S∷JrGA2ox∷GFP的单菌落PCR产物,M代表DNA marker。 A: The structure of 35S∷JrGA2ox∷GFP overexpression vector. In the vector of pCAMBIA1300, 35S indicates strong promoter in vector, GFP indicates green fluorescence protein signal, and NOS indicates the end codon. B: Lane 1-3 indicate three individual bacterial colonies with the 35S∷JrGA2ox∷GFP; M indicates DNA marker. |
|
图 2 JrGA2ox与同源蛋白的多序列对比 Fig. 2 Multiple sequence alignment of JrGA2ox and homological proteins Mn:川桑;Md:苹果;Pc:西洋梨;Pp:桃。*代表Fe2+结合位点,··代表 2-酮戊二酸结合位点。 Mn: Morus notabilis; Md: Malus domestica; Pc: Pyrus communis; Pp: Prunus persica. * indicates Fe2+ binding site, and ·· indicates 2-ketoglutaric acid binding site. |

|
图 3 JrGA2ox的系统发育树分析 Fig. 3 Phylogenetic tree analysis of JrGA2ox |
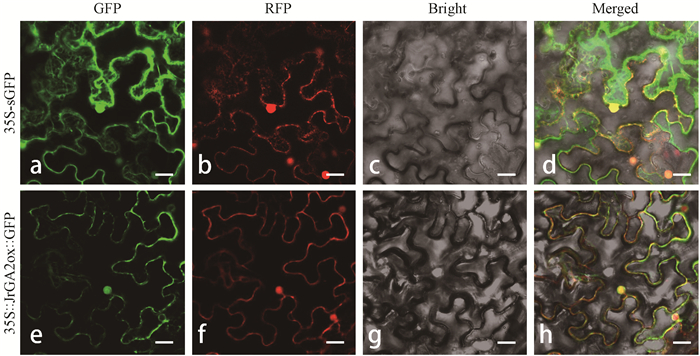
为了确定JrGA2ox蛋白的亚细胞定位,将JrGA2ox与35S-sGFP的增强型绿色荧光蛋白融合,激光共聚焦显微镜下观察JrGA2ox基因在本氏烟草叶片表皮细胞的瞬时表达情况。构建35S∷JrGA2ox∷GFP过表达载体,以35S- sGFP为对照,同时以核定位蛋白marker与膜定位蛋白marker对本氏烟草表皮细胞中的细胞膜和细胞核进行准确定位。试验表明,在注射35S-sGFP空载体及2个定位marker的本氏烟草叶片表皮细胞中,488 nm的波长激发下,细胞核、细胞膜以及细胞质等部位出现绿色荧光信号,但较模糊且弥散于整个细胞(图 4a);在516 nm波长激发下,出现红色荧光的部位即为细胞核与细胞膜(图 4b),表明35S-sGFP空载体以及2个定位蛋白marker均可正常表达。在注射含有35S∷JrGA2ox∷GFP过表达载体的农杆菌及定位蛋白marker农杆菌混合液的本氏烟草叶片表皮细胞中,488 nm的波长激发下,细胞核、细胞膜部位表达出强烈的绿色荧光信号,且轮廓清晰(图 4e);在516 nm波长激发下,核蛋白定位marker与膜蛋白定位marker正常表达,呈现清晰的红色荧光(图 4f);将GFP、RFP以及白光通道融合后,在细胞膜和细胞核中,35S∷JrGA2ox∷GFP所表达的绿色荧光和2个定位蛋白表达的红色荧光相重合,并呈现黄色荧光,这表明JrGA2ox蛋白在细胞中定位于细胞核与细胞膜中(图 4h)。
|
图 4 JrGA2ox蛋白在本氏烟草叶片中的亚细胞定位分析 Fig. 4 Subcellular localization analysis of JrGA2ox fusion protein in leaf epidermal cells of Nicotiana benthamiana a, e: GFP通道;b, f: RFP通道;c, g:白光通道;d, h:融合图像。35S-sGFP为空载体,35S∷JrGA2ox∷GFP为具有JrGA2ox的融合蛋白。标尺:50 μm。 a, e: GFP field; b, f: RFP field; c, g: Bright field; d, h: Merged pictures. 35S-sGFP indicates the empty vector, and 35S∷JrGA2ox∷GFP indicates the fusion protein with JrGA2ox. Bar: 50 μm. |
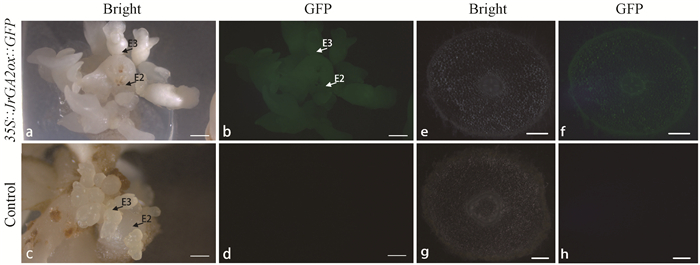
为了研究核桃JrGA2ox基因的生理功能,将35S∷JrGA2ox∷GFP过表达载体转入生长健壮的核桃体胚中。将筛选培养后E2代(浸染培养的体细胞胚为E0代)的体细胞胚在荧光体视显微镜下进行检测,发现阳性体细胞胚可在波长为488 nm的蓝光下发出绿色荧光,而对照则无荧光信号(图 5)。进一步将生长良好的阳性体细胞胚进行诱导萌发试验,待体胚生出胚根时,对其进行横切后置于荧光体视显微镜下检测,发现阳性体胚胚根横切面细胞可观察到激发产生的绿色荧光信号(图 5e,f),且维管束部位的GFP荧光表达的信号更多,而对照无荧光信号(图 5g,h)。同时,将阳性再生植株暗培养2周,茎横切面置于荧光体视显微镜下检测,同样可激发出绿色荧光,而对照无荧光信号(图 6)。为排除假阳性,将阳性体胚及再生植株进一步进行PCR检测(图 7),电泳条带大小接近1 800 bp左右为阳性体胚或再生植株,可用于进行下一步试验。
|
图 5 核桃阳性体细胞胚及再生植株根部荧光检测 Fig. 5 Fluorescence assay for the positive walnut somatic embryos and the root of regenerated plants E2:子二代体细胞胚;E3:子三代体细胞胚。a, b:白光和蓝光激发绿色荧光下的阳性过表达核桃体胚;c, d:白光和蓝光激发绿色荧光下的对照核桃体胚。e, f:白光和蓝光激发绿色荧光下阳性胚根横切面细胞形态;g, h:白光和蓝光激发绿色荧光下对照胚根横切面。标尺: 500 μm。 E2: The second-generation somatic embryo; E3: The third-generation somatic embryo. a, b: The positive overexpressed somatic embryos in bright and GFP field; c, d: Control somatic embryos in bright and GFP field; e, f: The somatic embryo's root transverse sections of overexpressed regenerated plants in bright and GFP field; g, h: The somatic embryo's root transverse sections of control regenerated plants in bright and GFP field. Bar: 500 μm. |

|
图 6 暗培养2周后核桃阳性再生植株的荧光表达情况 Fig. 6 The GFP fluorescence expression of positive regenerated plants in dark culture for 2 weeks A:核桃对照植株CK及35S∷JrGA2ox∷GFP阳性再生植株暗培养2周(标尺:10 mm); B:暗培养2周后再生植株茎横切面荧光检测(标尺:20 μm)。 A: The growth of walnut control and 35S∷JrGA2ox∷GFP regenerated plants after 2 weeks' dark culture (Bar:10 mm); B: Fluorescence detection of stem cross section of regenerated plants after 2 weeks' dark culture (Bar: 20 μm). |

|
图 7 核桃阳性体细胞胚及再生植株35S∷JrGA2ox∷GFP的PCR检测 Fig. 7 PCR assay of 35S∷JrGA2ox∷GFP in overexpression walnut somatic embryos and regenerated plants 1-3:核桃35S∷JrGA2ox∷GFP阳性体胚;4:对照核桃体胚;5-7:核桃35S∷JrGA2ox∷GFP阳性再生植株;8:对照核桃再生植株;M: DNA marker。 1-3: 35S∷JrGA2ox∷GFP somatic embryos; 4: Control somatic embryos; 5-7: 35S∷JrGA2ox∷GFP regenerated plants; 8: Control regenerated plants; M: DNA marker. |
为了明确JrGA2ox基因过量表达与核桃植株株高之间的相关性,选取3组阳性苗(分别由3个阳性体胚萌发得到),3个无性系分别命名为35S∷JrGA2ox∷GFP-1、35S∷JrGA2ox∷GFP-2和35S∷JrGA2ox∷GFP-3。本研究通过qPCR检测核桃35S∷JrGA2ox∷ GFP阳性再生植株中JrGA2ox基因的相对表达量,并测定其株高。结果表明,JrGA2ox的相对表达丰度越高,核桃苗的株高则越矮。35S∷JrGA2ox∷GFP-1中JrGA2ox的相对表达量约是对照植株的19倍,其平均株高为25.6 mm; 35S∷JrGA2ox∷GFP- 2中JrGA2ox的相对表达量约是对照植株的20.22倍,平均株高为23.4 mm; 35S∷JrGA2ox∷GFP-3中JrGA2ox的相对表达量约是对照植株的22.56倍,平均株高为22.5 mm(图 8A, B, C)。进一步观察植株茎段的直径,对照再生植株茎的直径平均约为2.08 mm,35S∷JrGA2ox∷GFP阳性再生植株茎的直径平均约为3.67 mm,较对照植株的直径更大,再生植株更健壮(图 8D)。

|
图 8 核桃阳性再生植株中JrGA2ox的相对表达量及植株表型 Fig. 8 The relative expression of JrGA2ox in positive regenerated walnut plants and the phenotype of plants CK:对照苗; 35S∷JrGA2ox∷GFP:阳性再生植株。A:核桃对照及阳性再生植株中JrGA2ox的相对表达量; B:核桃对照及阳性再生植株培养14天后的株高。利用单因素方差分析进行差异显著性分析,不同字母表示差异显著(P < 0.05)。C:对照及阳性再生植株的株高表型; D:对照及阳性再生植株的叶片表型。标尺: 10 mm。 CK: Control plant; 35S∷JrGA2ox∷GFP: Overexpression regenerated plants. A: The relative expression of JrGA2ox in control and positive regenerated plants; B: The height of the control and positive regenerated plants after 14 days' culture. Single factor analysis of variance is used to analyze the difference, and different letters indicate significant difference (P < 0.05). C: The plant stature of the control and positive regenerated plants; D: The leaf phenotype of the control and positive regenerated plants. Bar:10 mm. |
GA2-oxidase是在赤霉素合成过程中催化具生物活性赤霉素失活的关键酶。GA2-oxidase基因属于20G-Fe (Ⅱ)oxygenase 基因超家族(Han et al., 2011)。本研究基于前期研究中获得的山核桃的转录组数据,通过PCR扩增获得了1条核桃GA2ox基因,通过对该基因的生物信息学分析表明,JrGA2ox具有该蛋白家族共同的结构特点,含有保守的20G-FeⅡ-oxy蛋白结构域,这表明JrGA2ox属于GA2ox基因家族。将该基因编码的氨基酸序列与其他物种的氨基酸序列在NCBI中比对发现,其与栓皮栎(Quercus variabilis)QvGA2ox1同源性最高,达到80%;同时,构建氨基酸系统进化树分析表明,其与川桑MnGA2ox1(XP 010110619.1)的亲缘关系最近。GA的合成是在细胞的不同部位按阶段进行,亚细胞定位分析发现葡萄(Vitis vinifera)VvGA2ox2和VvGA2ox4均在细胞核表达(王西成等, 2012);苹果MdGA20ox1在细胞核和细胞膜中表达(姜志昂等, 2014);本研究中本氏烟草叶表皮亚细胞定位结果表明,JrGA2ox蛋白定位于细胞核和细胞膜中。分布于膜上的蛋白主要有与细胞活动相关的离子泵、通道蛋白和蛋白受体,以及载体蛋白等。定位于细胞核中的蛋白种类繁多,包括控制遗传信息复制与合成的酶等。因此核桃JrGA2ox蛋白可能既可调控GA合成也能维持细胞质膜的稳定性或对抑制蛋白转运有一定作用(邢浩然等, 2006)。
研究表明,GA2ox可以将有活性的GA1和GA4转化为无活性的GA8和GA34,导致GAs的活性降低,从而对植株的生长起负调控作用(Achard et al., 2009)。将拟南芥AtGA2ox7和AtGA2ox8基因分别在拟南芥和烟草中过量表达,可显著降低转基因植株中GAs的含量,并使植株出现矮化表型(Schomburg et al., 2003);同样,Zhou等(2012)将拟南芥AtGA2ox8在欧洲油菜(Brassica napus)中过量表达,转化植株出现了预期的矮化性状。将烟草35S∷GA2ox∷pCAMBIA1303过表达载体通过农杆菌转入长寿花(Kalanchoe blossfeldiana)及矮牵牛(Petunia hybrida)中,获得了比对照植株更紧凑的矮化株型(Gargul et al., 2013)。豌豆slender突变体中由于编码GA2ox的SLENDER基因缺失,导致GAs含量增加而使植株伸长,并伴随叶片变小、叶色变深等表型(Martin et al., 1999)。然而,在木本植物中,目前有关GA2ox基因功能的研究大多集中于基因克隆、时空表达分析(王西成等, 2012);GA2ox基因的异源遗传转化仅有少量报道。荔波连蕊茶ClGA2ox1-3基因在烟草中异源过量表达,可使植株矮化、生长减缓、推迟开花、叶片变小变圆以及叶色变深等(Xiao et al., 2016)。将李(Prunus salicina) PsGA2ox基因转入拟南芥中异源表达,发现PsGA2ox的过量表达也出现了植株矮化表型(Sharkawy et al., 2012)。本研究将核桃JrGA2ox基因在核桃中同源表达,得到了矮化再生植株,这与以上研究结果相似。进一步研究发现,核桃同源转化再生植株中JrGA2ox的表达丰度越高,其株高则会越矮。
目前,果树中关于GA2ox基因的报道较少。本研究从核桃中分离获得JrGA2ox基因,并对该基因序列进行了生物信息学分析;同时构建了35S∷GA2ox∷GFP过表达载体,开展了亚细胞定位及以核桃体细胞胚为受体的同源遗传转化。本研究结果为进一步明确该基因的功能特征及揭示其参与调控植株生长的分子机制提供了良好的理论依据。
4 结论核桃JrGA2ox蛋白与川桑MnGA2ox1、西洋梨PcGA2ox、桃PpGA2ox1、苹果MdGA2ox1蛋白同源性较高;核桃JrGA2ox蛋白亚细胞定位于细胞核与细胞膜;同源转化JrGA2ox基因,核桃再生植株中该基因的表达量升高,表现出明显的矮化特征。本研究结果为进一步分析该基因在核桃生长发育过程中的作用提供参考。
白牡丹, 王彩虹, 田义轲, 等. 2012. 苹果茎尖组织中GA2ox基因的结构特征及蛋白序列的生物信息学分析. 华北农学报, 27(5): 55-59. DOI:10.3969/j.issn.1000-7091.2012.05.012 |
Bai M D, Wang C H, Tian Y K, et al. 2012. Gene structure identification and protein sequence bioinformation analysis for GA2ox from apical tissue of apple shoots. Acta Agriculturae Boreali-Sinica, 27(5): 55-59. |
程飞飞. 2012.矮生梨'中矮1号'GA2-oxidase基因的克隆与功能分析.北京: 中国农业科学院硕士学位论文.
|
Cheng F F. 2012. Cloning and functional analysis of GA2-oxidase gene in the dwarf pears named 'Zhongai 1'. Beijing: MS thesis of Chinese Academy of Agricultural Sciences.[in Chinese]
|
姜志昂, 彭建营, 孙建设. 2014. 苹果MdKS、MdKOA1基因克隆与表达分析. 植物遗传资源学报, 15(2): 362-368. |
Jiang Z A, Peng J Y, Sun J S. 2014. Isolation and expression of MdKS and MdKOA1 gene in apple. Journal of Plant Genetic Resources, 15(2): 362-368. |
屠煦童, 张仕杰, 陈小云, 等. 2015. "南通小方柿"GA2ox基因的克隆、亚细胞定位及表达分析. 中国农业科学, 48(1): 197-206. |
Tu X T, Zhang S J, Chen X Y, et al. 2015. Cloning, subcellular localization and expression analysis of Gibberellin 2-Oxidase gene in Diospyros kaki Linn. cv. Nantongxiaofangshi. Scientia Agricultura Sinica, 48(1): 197-206. |
王西成, 任国慧, 房经贵, 等. 2012. 葡萄赤霉素合成相关基因克隆、亚细胞定位和表达分析. 中国农业科学, 45(11): 2224-2231. DOI:10.3864/j.issn.0578-1752.2012.11.011 |
Wang X C, Ren G H, Fang J G, et al. 2012. Cloning, subcellular localization and expression analysis of genes related to the synthesis of gibberellin from grapevine. Scientia Agricultura Sinica, 45(11): 2224-2231. |
吴国良, 刘群龙, 郑先波, 等. 2009. 核桃种质资源研究进展. 果树学报, 26(4): 539-545. |
Wu G L, Liu Q L, Zheng X B, et al. 2009. Advances in research on the worldwide walnut germplsam. Journal of Fruit Science, 26(4): 539-545. |
邢浩然, 刘丽娟, 刘国振. 2006. 植物蛋白质的亚细胞定位研究进展. 华北农学报, 21(s2): 1-6. |
Xing H R, Liu L J, Liu G Z. 2006. Advancement of protein sub-cellular localization in plants. Acta Agriculturae Boreali-Sinica, 21(s2): 1-6. |
Achard P, Genschik P. 2009. Releasing the brakes of plant growth:how GAs shutdown DELLA proteins. Journal of Experimental Botany, 60(4): 1085-1092. |
Chen Q, Ya H M, Li S, et al. 2012. Isolation and analysis of homoeologous genes encoding gibberellin 2-oxidase 3 isozymes in common wheat. Journal of Genetics, 91(3): 1-9. |
Chiu W, Niwa Y, Zeng W, et al. 1996. Engineered GFP as a vital reporter in plants. Current Biology, 6(3): 325-330. |
Dong H L, Lee I C, Kim K J, et al. 2014. Expression of gibberellin 2-oxidase 4 from Arabidopsis under the control of a senescence-associated promoter results in a dominant semi-dwarf plant with normal flowering. Journal of Plant Biology, 57(2): 106-116. |
Gargul J M, Mibus H, Serek M. 2013. Constitutive overexpression of Nicotiana GA2ox leads to compact phenotypes and delayed flowering in Kalanchoe blossfeldiana and Petunia hybrida. Plant Cell, Tissue and Organ Culture, 115: 407-418. |
Guo Y, Li W, Sun H, et al. 2012. Detection and quantification of Rhizoctonia cerealis in soil using real-time PCR. Journal of General Plant Pathology, 78(4): 247-254. |
Han F, Zhu B. 2011. Evolutionary analysis of three gibberellin oxidase genes in rice, Arabidopsis, and soybean. Gene, 473(1): 23-35. |
Hedden P, Phillips A L. 2003. Gibberellin metabolism:new insights revealed by the genes. Trends in Plant Science, 5: 523-530. |
Hood E E, Gelvin S B, Melchers L S, et al. 1993. New Agrobacterium helper plasmids for gene transfer to plants. Transgenic Research, 2(4): 208-218. DOI:10.1007/BF01977351 |
Lee D H, Lee I C, Kim K J, et al. 2014. Expression of gibberellin 2-oxidase 4 from Arabidopsis under the control of a senescence-associated promoter results in a dominant semi-dwarf plant with normal flowering. Journal of Plant Biology, 57(2): 106-116. |
Leslie C A, Hackett W P, McGranahan G H, et al. 2010. Improved rooting methods for walnut (Juglans) microshoots. Acta Horticulturae, 861: 365-372. |
Martin D N, Proebsting W M, Hedden P. 1999. The SLENDER gene of pea encodes a gibberellin 2-oxidase. Plant Physiology, 121(3): 775-781. |
Nelson B K, Cai X, Nebenführ A. 2007. A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. The Plant Journal, 51: 1126-1136. |
Pearce S, Huttly A K, Prosser I M, et al. 2015. Heterologous expression and transcript analysis of gibberellin biosynthetic genes of grasses reveals novel functionality in the GA3ox family. BMC Plant Biology, 15(1): 1-30. |
Phinney B O, West C A, Ritzel M, et al. 1957. Evidence for "gibberellin-like" substances from flowering plants. PNAS, 43: 398-404. |
Ross J J, Reid J B, Swain S M, et al. 1995. Genetic regulation of gibberellin deactivation in Pisum. The Plant Journal, 7: 513-523. |
Saito T, Bai S L, Ito A, et al. 2013. Expression and genomic structure of the dormancy-associated MADS box genes MADS13 in Japanese pears (Pyrus pyrifolia Nakai) that differ in their chilling requirement for endodormancy release. Tree Physiology, 33(6): 654-667. |
Sambrook J, Fritsch E F, Maniatis T. 1995. Molecular cloning:a laboratory manual. Beijing: Science Press, 96-99.
|
Schomburg F M, Bizzell C M, Lee D J, et al. 2003. Overexpression of a novel class of gibberellin 2-oxidases decreases gibberellin levels and creates dwarf plants. The Plant Cell, 15: 151-163. |
Shan C, Mei Z, Duan J, et al. 2014. OsGA2ox5, a gibberellin metabolism enzyme, is involved in plant growth, the root gravity response and salt stress. PLoS One, 9(1): e87110. |
Sharkawy I E, Kayal W E, Prasath D, et al. 2012. Identification and genetic characterization of a gibberellin 2-oxidase gene that controls tree stature and reproductive growth in plum. Journal of Experimental Botany, 63(3): 1225-1239. |
Tulecke W, Mcgranahan G. 1985. Somatic embryogenesis and plant regeneration from cotyledons of walnut, Juglans regia L. Plant Science, 40(1): 57-63. |
Vahdati K, Razaee R, Mirmasoomi M. 2009. Micropropagation of some dwarf and early mature walnut genotypes. Biotechnology, 8(1): 171-175. |
Xiao Z, Fu R, Li J, et al. 2016. Overexpression of the gibberellin 2-oxidase gene from Camellia lipoensis induces dwarfism and smaller flowers in Nicotiana tabacum. Plant Molecular Biology Reporter, 34(1): 182-191. |
Yamaguchi M, Sasaki T, Sivaguru M, et al. 2005. Evidence for the plasma membrane localization of Al-activated malate transporter (ALMT1). Plant and Cell Physiology, 46(5): 812-816. |
Yamaguchi S. 2008. Gibberelllin metabolism and its regulation. Annual Review of Plant Biology, 59(1): 225-251. |
Yamaji N, Huang C F, Nagao S, et al. 2009. A zinc finger transcription factor ART1 regulates multiple genes implicated in aluminum tolerance in rice. The Plant Cell, 21: 3339-3349. |
Zhang L, Li Y, Dai K, et al. 2015. Establishment of a successive markerless mutation system in Haemophilus parasuis through natural transformation. PLoS One, 10(1371): 1-12. |
Zhou B, Lin J, Peng W, et al. 2012. Dwarfism in Brassica napus L. induced by the over-expression of a gibberellin 2-oxidase gene from Arabidopsis thaliana. Molecular Breeding, 29(1): 115-127. |
 2019, Vol. 55
2019, Vol. 55

