文章信息
- 王思文, 卫星, 李虹谕, 韦庆钰.
- Wang Siwen, Wei Xing, Li Hongyu, Wei Qingyu.
- 水曲柳轻基质容器苗菌根化生长效应
- Effect of Mycorrhizae on the Growth of Fraxinus mandshurica Container Seedlings in Light Culture Media
- 林业科学, 2019, 55(2): 173-181.
- Scientia Silvae Sinicae, 2019, 55(2): 173-181.
- DOI: 10.11707/j.1001-7488.20190218
-
文章历史
- 收稿日期:2018-08-18
- 修回日期:2018-12-08
-
作者相关文章
水曲柳(Fraxinus mandshurica)为木犀科(Oleaceae)白蜡属(Fraxinus)落叶乔木,属国家Ⅱ级重点保护植物。水曲柳是我国东北林区珍贵的阔叶用材树种。构建优质高效的育苗、造林和抚育技术体系有助于合理开发和利用水曲柳资源(孙玥,2007;汤园园,2015)。目前水曲柳造林主要以裸根苗为主,苗木出圃成活率较高,但因起苗时容易伤到其发达盘结的根系,造林后缓苗时间较长,影响造林质量(王玉和等,2011)。轻基质容器苗具有容器轻、提高苗木质量、造林成活率高等优点,目前已在农业、果蔬及花卉培育中广泛应用(刘建福等,2005; 龙良鲲等,2009; 任禛等,2015)。早期对水曲柳轻基质容器苗基质类型、基质配比的研究表明以木耳废弃菌渣为主要成分的轻基质可显著促进苗木生长(汤园园,2015;卫星等,2016),但轻基质网袋育苗中基质孔隙度大、保水性较差、苗木易受到干旱胁迫的问题也显示出来。解决水曲柳轻基质网袋苗木保水抗旱性成为目前研究的首要问题。
菌根(Mycorrhiza)是在长期演化过程中土壤真菌与植物根系形成的互利共生体(刘润进等,2007;Smith et al., 2008; Denison et al., 2011; Cheng et al., 2013)。菌丝外延可增加根系的吸收面积,促进植物生长(Neumann et al., 2005)。同时,菌根也可以影响根系的生理功能以及寿命等(Guo et al.,2008;孙玥,2007),提高寄主植物根系抗病性(龙良鲲等,2009)、抗旱性(赵平娟等,2007;王玉和等,2011)、抗盐性(赵平娟,2004;王立等,2010;宋圆圆等,2018)。为提高苗木抗旱性,菌根化育苗技术已经在国内外苗木集约化培育上广泛应用(Smith et al., 2011; Shi et al., 2016)。我国近年来的研究发现,接菌方式(陈宝玲等,2014)、剂量(洪文君等,2017)、菌种类型(刘建福等,2005)等因素均显著影响苗木生长及抗性表现。因此,目前东北地区主要速生用材树种,如赤松(Pinus densiflora)、油松(Pinus tabulaeformis)、北美蓝云杉(Picea pungens)、樟子松(Pinus sylvestris var. mongolica)等外生菌根型苗木菌根化培育技术的研究已陆续开展起来(王殿东等,2007;李滢等,2010;杜蕊等,2012;张文泉,2013),但对于水曲柳等内生菌根型苗木菌根化育苗技术的研究还不完善。因此,本研究以水曲柳1年生轻基质容器苗为对象,以2种内生菌根真菌,3种水曲柳林地菌源为材料,探索单一菌种和混合菌种对水曲柳轻基质容器苗地上形态及地下根系发育的影响,筛选出水曲柳轻基质苗木生长的适宜菌源,为水曲柳菌根化苗木培育提供理论和技术支撑。
1 材料与方法 1.1 供试材料种子来源及处理:种子来源于东北林业大学帽儿山实验林场。2016年秋季采种后低温沙藏处理4~5个月。2017年4月在温室无菌育苗盘(基质为沙子和草炭土)中育苗。当苗木出现两片真叶时移栽到混有菌源的轻基质中,于温室内正常培养,每天早晚浇水。试验菌种:摩西球囊霉菌(Glomus mosseae)和幼套球囊霉菌(Glomus etunicatum),由新疆石河子大学提供。混合菌种分别为取自水曲柳纯林下、落叶松(Larix gmelinii)纯林下及水曲柳落叶松混交林下腐殖质,腐殖质粉碎成0.2~0.5 mm大小备用。基质处理:木耳废弃菌渣高温灭菌后按体积比为2:1与蛭石混合,用5%的高锰酸钾对基质进行消毒处理,晾干后备用。试验时按基质:菌源=5:2的体积比接菌。对照组为灭菌草炭土。容器为无纺布袋(10 cm×10 cm)。
1.2 试验设计本试验共设计7个处理:摩西球囊霉菌单独接种(T1)、幼套球囊霉菌单独接种(T2)、摩+幼(摩西球囊霉菌和幼套球囊霉菌)混合接种(T3)、水曲柳纯林下腐殖质(T4)、落叶松纯林下腐殖质(T5)、水落混交林林下腐殖质(T6)及无菌对照组CK。试验采用完全区组试验设计,共3个区组,每个处理培养36株苗木,共756株苗。
1.3 试验方法移栽缓苗后每10天进行观察测量。用游标卡尺测量地径,常规直尺、米尺测量苗高,用手持激光叶面积仪(美国CID公司,CI-203)测量叶面积。最后一次每处理取样10株测量根生物量、茎生物量及叶生物量。
在苗木生长旺盛期(8月13日),每处理分别选取生长均匀的5株苗木进行根系发育和解剖结构的观察。根系洗净后用Epson数字化扫描仪(Expression 10000XL1.0)扫描,用winRHIZO(pro2004b)软件(RegentInstruments Canada)测量根系直径、根表面积、根尖数量及根系体积。采用锥虫蓝染色法测菌根侵染率,菌根侵染率=(侵染根数/总观察根数)×100%。采用石蜡切片技术观察根系解剖结构。制作根尖后1 cm成熟区横切片,在OLYMPUS BX-51生物显微镜观察,用Motic3000软件测量维管束直径、皮层薄壁细胞层数及侵染层数,用Motic 3000 CCD数码成像系统拍照保存。
1.4 数据处理采用Excel 2007软件整理数据,SPSS 13.0软件对处理后各指标均值进行单因素方差分析,采用LSD法进行多重比较。
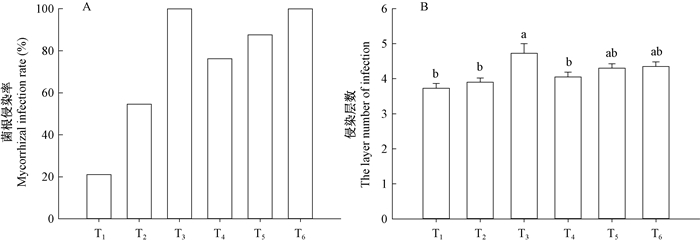
2 结果与分析 2.1 接菌对水曲柳菌根侵染的影响不同接菌类型对苗木菌根侵染率及皮层侵染层数均产生了显著的影响。摩西球囊霉菌和幼套球囊霉菌混合接种(T3)及水落混交林腐殖质(T6)处理下水曲柳苗木菌根侵染率最高,均为100%(图 1A)。单一接菌下苗木菌根侵染率显著低于混合接菌处理,不同菌种作用效果也明显不同。幼套球囊霉菌(T2)处理下菌根侵染率约为摩西球囊霉菌(T1)处理的2.57倍。不同接菌类型下,菌丝在皮层内伸展的层数也不同(图 2)。摩+幼混合接种(T3)处理下菌丝深入到约第5层皮层薄壁细胞内(图 1B),菌丝深入层数显著高于单一接菌(T1、T2)。摩西球囊霉菌(T1)处理下菌丝侵染层数最少。
|
图 1 接菌类型对水曲柳菌根侵染的影响 Fig. 1 Effects of inoculations on root infection of Fraxinus mandshurica Rupr. Seedlings T1:摩西球囊霉菌; T2:幼套球囊霉菌; T3:摩西球囊霉菌和幼套球囊霉菌混合接菌; T4:水曲柳纯林下腐殖质; T5:落叶松纯林下腐殖质; T6:水落混交林下腐殖质.不同小写字母表示处理间差异显著(P < 0.05)。下同。 T1. Glomus mosseae; T2. Glomus etunicatum; T3. Glomus mosseae and Glomus etunicatum; T4.Humus from F. mandshurica stands; T5. Humus from larch stands; T6.Humus from the mixed stands. Lowercase letters indicate significant difference between treatments (P < 0.05). The same below. |

|
图 2 接菌对水曲柳菌根解剖结构的影响 Fig. 2 Effects of AMF inoculation on root anatomical structures of Fraxinus mandshurica seedlings A.摩西球囊霉菌(T1);B.幼套球囊霉菌(T2);C.摩西球囊霉菌和幼套球囊霉菌混合接菌(T3);D.水曲柳纯林下腐殖质(T4);E.落叶松纯林下腐殖质(T5);F.水落混交林下腐殖质(T6)。Ⅰ表皮;Ⅱ外皮层;Ⅲ皮层薄壁细胞;Ⅳ维管束。 A. Glomus mosseae(T1); B. Glomus etunicatum(T2); C. Glomus mosseae and Glomus etunicatum(T3); D. Humus from Fraxinus mandshurica stands(T4); E. Humus from larch stands(T5); F. Humus from the ash and larch mixed stands(T6); Ⅰ. Epidermis; Ⅱ. Exodermis; Ⅲ. Parenchymal cell; Ⅳ. Vascular bundle. |
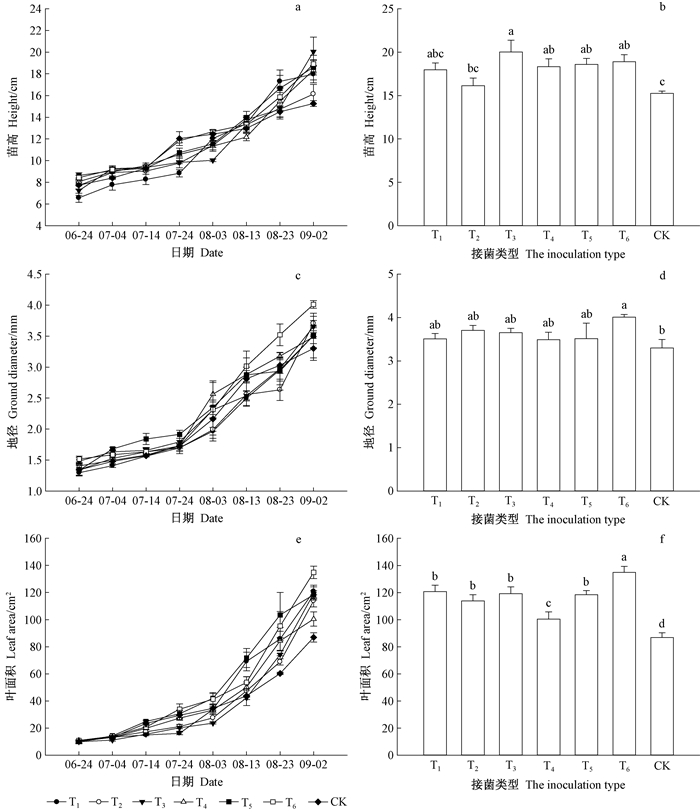
接菌后苗木苗高、地径、叶面积与对照生长趋势一致(图 3A、C、E),生长前期各处理间差异不大,生长后期摩+幼混合接菌处理(T3)和水落混交林下腐殖质处理(T6)的苗木后期增长速率加快(图 3B、D、F)。8月中旬起水落混交林下腐殖质处理(T6)的水曲柳苗木地径大于其他6种接菌方式,生长结束时是对照的1.21倍(P < 0.05)。8月下旬摩+幼混合接菌(T3)苗高增长最快,生长后期约为对照的1.31倍(P < 0.05)。其中水落混交林下腐殖质处理(T6)的苗木叶面积增长最快,生长结束时是对照叶面积的1.55倍(P < 0.05)。
|
图 3 接菌类型对水曲柳苗高、地径、叶面积生长的影响 Fig. 3 Effects of inoculations on seedling height, ground diameter and leaf area of Fraxinus mandshurica T1:摩西球囊霉菌Glomus mosseae;T2:幼套球囊霉菌Glomus etunicatum;T3:摩西球囊霉菌和幼套球囊霉菌混合接菌Glomus mosseae and Glomus etunicatum;T4:水曲柳纯林下腐殖质Humus from F. mandshurica stands;T5:落叶松纯林下腐殖质Humus from larch stands;T6:水落混交林下腐殖质Humus from the ash and larch mixed stands.下同。The same below. |
接菌对水曲柳苗木根生物量、茎生物量、叶生物量产生显著影响(表 1)。摩+幼混合接菌处理(T3)下苗木根、茎、叶生物量最大,分别比对照高出65.18%(P < 0.05)、55.26%(P < 0.05)、66.66%(P < 0.05)。水落混交林下腐殖质处理(T6)苗木的根、茎、叶生物量也分别比对照高出47.19%(P < 0.05)、40.40%(P < 0.05)、56.49%(P < 0.05)。单一接菌下苗木根生物量、茎生物量虽然低于各混合接菌处理,但差异不显著(P>0.05)。摩西球囊霉菌(T1)处理下苗木总生物量显著高于幼套球囊霉菌(T2)。
|
|
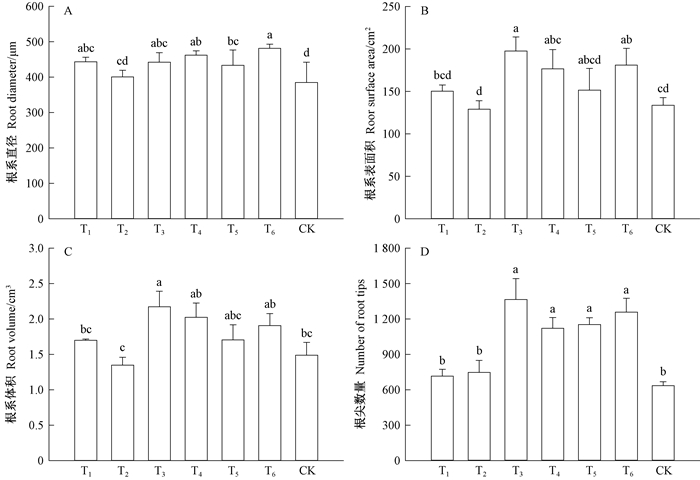
接菌对根系直径、根系表面积、根系体积和根尖数量产生了不同的影响。接菌后苗木根系平均直径比对照高15.51%(P < 0.05)(图 4A)。接菌处理根系平均直径由大到小依次为混交林下腐殖质(T6)>水曲柳纯林下腐殖质(T4)>摩+幼混合菌(T3)>摩西球囊霉菌(T1)>落叶松纯林下腐殖质(T5)>幼套球囊霉菌(T2)。混合接菌可显著的提高根系表面积,分别比单一和对照高27.6%和32.10%(图 4B)。根系体积在混合接菌后也显著高于对照和单一接菌(P < 0.05)(图 4C),其中摩+幼混合接菌处理(T3)苗木根系体积最大。单一接菌处理下水曲柳苗木根系体积与对照无显著差异(P>0.05)。另外,混合接菌后根尖数量的变化趋势也与根系表面积、根系体积一致,均为混合接菌>单一接菌>对照。其中,摩+幼混合接菌(T3)下苗木根尖数量最多(图 4D),是对照的2.14倍(P < 0.05)。单一接菌与对照差异不显著(P>0.05)。
|
图 4 接菌类型对水曲柳根系形态的影响 Fig. 4 Effects of inoculations on the morphology of Fraxinus mandshurica seedlings A.根系直径; B.根系表面积; C.根系体积; D.根尖数量 A. Root diameter; B. Root surface area; C. Root volume; D. Number of root tips |
接菌促进了根系薄壁细胞直径、薄壁细胞层数和维管束直径生长。薄壁细胞直径由大到小依次为混合接菌>单一接菌>对照。摩+幼混合接菌(T3)、水曲柳纯林下腐殖质(T4)、落叶松纯林下腐殖质(T5)和混交林下腐殖质(T6)处理下薄壁细胞直径分别比对照显著增加17.95%、25.12%、13.91%和18.22%(P < 0.05)。单一接菌下薄壁细胞直径虽大于对照,但变化不显著(P>0.05)。接菌对薄壁细胞层数影响不一,除摩+幼混合接菌(T3)和混交林下腐殖质(T6)处理下薄壁细胞层显著高于对照外(P < 0.05),其他处理与对照没有显著差异(P>0.05)。接菌对维管束直径的影响也不一致。混合接菌处理苗木维管束平均直径比对照高出24.15%。摩+幼混合处理(T3)时苗木维管束平均直径最大(表 2)。单一接菌处理的苗木维管束平均直径比对照高20.82%,摩西球囊霉菌处理(T1)下维管束直径比幼套球囊霉菌(T2)高33.05%。
|
|
陆地上80%左右的植物借助与菌根真菌共生以调节自身的生长(Smith et al., 2008)。菌根可促进共生植物的生长和物质积累(郭渊,2007;陈丹明等,2010)。本研究发现与对照相比,接菌后水曲柳苗高增加12.33%~81.63%,总生物量增加17.91%~64.18%。植物的高生长、生物量积累与光合能力密切相关(Aidar et al., 2014),光合作用叶片的面积、叶片生物量及光合效率等直接影响光合产物的合成(赵昕等,2006;Shen et al., 2015)。本研究发现接菌后水曲柳苗木叶面积比对照增加9.05%~50.16%,叶生物量比对照增加19.78%~66.67%。接菌后植物光合作用器官增加的原因,一是接菌后植株在生产自身能量代谢产物的同时也要为共生真菌提供一定的物质保障,构建更多的光合器官可满足共生体系的需求;二是菌根侵染后,菌丝为植物拓展了更广泛的地下养分空间,为地上营养器官的生长提供了物质保障。根尖是菌根共生的直接部位,其形态及结构变化直接决定根系吸收功能的变化(Guo et al., 2008; Wang et al., 2016; Zou et al., 2017)。根系直径变化在很大程度上影响根系菌根真菌的侵染(Mark et al., 2002), 本研究发现,接菌后根系直径显著高于对照。根系皮层薄壁细胞细胞大小和皮层厚度决定菌丝生长和物质交换的空间,本研究发现接菌后薄壁细胞直径和皮层厚度分别比对照增加18.55%,34.11%,可见接菌后根系内部结构的变化有助于菌丝在根内的扩展和生存,有助于增大菌丝与根系细胞的接触面积,为菌与根之间的物质交换提供保障。
菌种不同,其吸收土壤养分的能力也不同,而且单一接菌与混合接菌对植物生长的影响也不同(Hodge et al., 2010;Cheng et al., 2013)。菌丝侵染率的高低与其调节植物生长的能力直接相关(范继红,2006;田蜜等,2013;Jayne et al., 2014)。本研究发现水曲柳苗木在几种混合接菌(摩+幼混合接菌、水曲柳纯林下腐殖质、落叶松纯林下腐殖质、混交林下腐殖质)下,高生长、地径生长、生物量、根尖数量、根系表面积等均优于单一接菌(摩西球囊霉、幼套球囊霉)和不接菌。这与之前在木薯(Manihot esculenta)(黄华成等,2005)和连翘(Forsythia suspensa)(赵平娟等,2007)幼苗上接菌处理的试验结果一致。同时,本研究也发现混合接菌的侵染率均高于单一接菌,摩+幼混合接菌(T3)及混交林下腐殖质(T6)处理菌根侵染率均为100%。这与在水稻(Oryza sativa)(马放等,2013)和菊花(Dendranthema morifolium)(Singh et al., 2008)上的研究也一致。混合接菌优于单一接菌的原因可能是由于混合菌剂中不同菌种在根系侵染空间上互补,充分利用根系资源,形成更加完善的菌根结构(黄华成等,2005;赵平娟等,2007;任禛等,2015)。虽然大多数情况下菌根真菌混合接种表现为正效应,但也有相反结论。在短命植物番茄(Lycopersicon esculentum)(龙良鲲等,2009)和白三叶草(Trifolium repens)(王立等,2012)上的研究发现混合接菌时菌根侵染率小于单一接菌,这可能是由多种菌剂与植物建立共生关系的复杂性所致。混合共生真菌为更好的完成生活史,彼此在接菌势、菌丝发展、菌根分泌物、根系生理变化及养分吸收等方面发生竞争。不合理的菌种搭配可能产生抑制或排斥作用,从而影响根系发育及植物生长。由此可见,混合菌剂是否提高侵染效率和作用效果取决于菌种关系协调作用(任禛等,2015)。本研究的4种混合接菌下苗木生长均优于对照,表明4种菌源内菌种之间没有明显的抑制作用。摩西与幼套混合球囊霉菌及混交林下腐殖质处理下苗木生长更优,建议可作为水曲柳轻基质袋苗菌根化培育的主要菌源。
4 结论本研究通过形态观察及解剖学方法揭示了接菌对水曲柳苗木生长的影响。根系解剖结构、植物生物量变化及生长情况均表明:几种菌源接菌后均促进水曲柳苗木生长及物质积累;接菌后的根系结构更有助于菌丝的生长和物质交换;混合接菌作用效果更佳;单一接菌因菌种不同,作用效果不同。本研究认为明确菌源差异及菌种作用效果是影响水曲柳菌根化育苗成效的关键。
陈丹明, 郭娜, 郭绍霞. 2010. 丛枝菌根真菌对牡丹生长及相关生理指标的影响. 西北植物学报, 30(1): 131-135. (Chen D M, Guo N, Guo S X. 2010. Effects of arbuscular mycorrhizal fungi on growth and some physiological indices of Paeonia suffruticosa. Acta Botanica Boreali-Occidentalia Sinica, 30(1): 131-135. [in Chinese]) |
陈宝玲, 王华新, 陈尔, 等. 2014. 铁皮石斛生根组培苗污染后的处理和利用. 广西林业科学, 43(4): 435-437. (Chen B L, Wang H X, Chen E, et al. 2014. Treatment and utilization for polluted tissue-cultured plants of Dendrobium officinale. Guangxi Forestry Science, 43(4): 435-437. DOI:10.3969/j.issn.1006-1126.2014.04.018 [in Chinese]) |
杜蕊, 郑红娟, 贾桂霞. 2012. 北美蓝云杉菌根化育苗技术研究. 北京林业大学学报, 34(1): 70-74. (Du R, Zheng H J, Jia G X. 2012. Seedling cultivation of Picea pungens with mycorrhizalfung. Journal of Beijing Forestry University, 34(1): 70-74. [in Chinese]) |
范继红. 2006.黄檗丛枝菌根生理生态学研究.哈尔滨: 东北林业大学博士学位论文. (Fan J H. 2006. Ecological and physiological research on VA mycorrhizas of Amur Cork-tree. Harbin: PhD thesis of Northeast Forestry University.[in Chinese]) |
郭渊. 2007.菌根化苗木造林试验研究.杨凌: 西北农林科技大学硕士学位论文. (Guo Y. 2007. Afforestation mycorrhizal seedlings of the pilot study. Yangling: MS thesis of Northwest A&F University.[in Chinese]) http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=Y1232359 |
洪文君, 莫惠芝, 方素琴, 等. 2017. 接种光合菌和菌根菌对圆叶乌桕幼苗生长的影响. 西南农业学报, 30(1): 129-133. (Hong W J, Mo H Z, Fang S Q, et al. 2017. Effects of inoculation with photosynthetic bacteria and AMF on growth of Triadica rotundifolia. Southwest China Journal of Agricultural Sciences, 30(1): 129-133. [in Chinese]) |
黄华成, 唐光大, 罗晓莹, 等. 2005. 三种球囊霉属真菌对盆栽术薯生长影响. 华南农业大学学报, 26(4): 44-47. (Huang H C, Tang G D, Luo X Y, et al. 2005. The effects of three Glomus fungi on growth of Manihot esculenta. Journal of South China Agricultural University, 26(4): 44-47. [in Chinese]) |
李滢, 张宝琴. 2010. 油松菌根容器苗培育及造林技术. 内蒙古林业, (3): 22. (Li Y, Zhang B Q. 2010. Cultivation and afforestation techniques of Pinus koraiensis root container seedlings. Inner Mongolia Forestry, (3): 22. [in Chinese]) |
刘建福, 张勇, 谢丽源, 等. 2005. 丛枝菌根真菌对澳洲坚果幼苗的生长效应. 热带作物学报, 26(3): 16-19. (Liu J F, Zhang Y, Xie L Y, et al. 2005. The growth effect of arbuscular mycorrhizal fungi on macadamia seedlings. Chinese Journal of Tropical Crops, 26(3): 16-19. DOI:10.3969/j.issn.1000-2561.2005.03.004 [in Chinese]) |
刘润进, 陈应龙. 2007. 菌根学. 北京: 科学出版社. (Liu R J, Chen Y L. 2007. Mycorrhizal. Beijing: Science Press. [in Chinese]) |
龙良鲲, 黎志坤, 姚青, 等. 2009. 番茄菌根化育苗及对青枯病的防治试验. 中国蔬菜, 1(4): 52-55. (Long L K, Li Z K, Yao Q, et al. 2009. Tomato mycorrhizal seedling and its control experiment on bacterial wilt. China Vegetables, 1(4): 52-55. [in Chinese]) |
马放, 李哲, 王立, 等. 2013. 丛枝菌根真菌对农药三环唑的残留减量研究. 哈尔滨工业大学学报, 45(4): 58-63. (Ma F, Li Z, Wang L, et al. 2013. Research of residue reduction of AMF to tricyclazole. Journal of Harbin Institute of Technology, 45(4): 58-63. [in Chinese]) |
任禛, 韩丽, 张永福, 等. 2015. 不同丛枝菌根真菌对玉米生长生理的影响. 江苏农业科学, 43(5): 63-66. (Ren Z, Han L, Zhang Y F, et al. 2015. Effects of different arbuscular mycorrhizal fungi on growth physiology of maize. Jiangsu Agricultural Sciences, 43(5): 63-66. [in Chinese]) |
宋圆圆, 夏明, 林熠斌, 等. 2018. 丛枝菌根真菌摩西管柄囊霉侵染增强番茄对机械损伤的响应. 应用生态学报, 29(11): 3811-3818. (Song Y Y, Xia M, Lin Y, et al. 2018. Colonization with arbuscular mycorrhizal fungus Funneliformis mosseae enhanced the responses of tomato plants to mechanical wounding. Chinese Journal of Applied Ecology, 29(11): 3811-3818. [in Chinese]) |
孙玥. 2007.菌根和施肥对水曲柳和落叶松人工林一级细根形态的影响.哈尔滨: 东北林业大学硕士学位论文. (Sun Y. 2007. Effects of mycorrhiza and fertilization on the morphology of fine roots in ash and larch plantations. Harbin: MS thesis of Northeast Forestry University.[in Chinese]) http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=Y1145824 |
汤园园. 2015.水曲柳轻基质容器苗基质筛选及养分管理.哈尔滨: 东北林业大学硕士学位论文.
|
Tang Y Y, 2015. Matrix screening and nutrient management of ash. Harbin: MS thesis of Northeast Forestry University.[in Chinese]
|
田蜜, 陈应龙, 李敏, 等. 2013. 丛枝菌根结构与功能研究进展. 应用生态学报, 24(8): 2369-2376. (Tian M, Chen Y L, Li M, et al. 2013. Structure and function of arbuscular mycorrhiza. Chinese Journal of Applied Ecology, 24(8): 2369-2376. [in Chinese]) |
王殿东, 潘丽梅, 姜贵全. 2007. 赤松菌根化育苗技术的研究. 北方园艺, (7): 198-200. (Wang D D, Pan L M, Jiang G Q. 2007. Study on the technique of rooting and seedling raising of Pinus koraiensis. Northern Horticulture, (7): 198-200. DOI:10.3969/j.issn.1001-0009.2007.07.095 [in Chinese]) |
王立, 贾文奇, 马放, 等. 2010. 菌根技术在环境修复领域中的应用及展望. 生态环境学报, 19(2): 487-493. (Wang L, Jia W Q, Ma F, et al. 2010. Application and prospect of mycorrhizal technology in the field of environmental restoration. Ecology and Environmental Sciences, 19(2): 487-493. DOI:10.3969/j.issn.1674-5906.2010.02.043 [in Chinese]) |
王玉和, 温琼文, 赵宝荣, 等. 2011. 不同水分下菌根育苗对大叶相思苗木造林成效的影响. 西北林学院学报, 26(5): 100-104. (Wang Y H, Wen Q W, Zhao B R, et al. 2011. Effect of mycorrhizal seedling raising on afforestation of Acacia grandis seedlings under different water conditions. Journal of Northwest Forestry University, 26(5): 100-104. [in Chinese]) |
王立, 徐亚男, 马放, 等. 2012. 不同AMF菌剂对白三叶草坪的扶壮作用. 哈尔滨工业大学学报, 44(10): 43-47. (Wang L, Xu Y N, Ma F, et al. 2012. Effect of different AMF microbial inoculum on the growth of Trifoliumrepens lawn. Journal of Harbin Institute of Technology, 44(10): 43-47. DOI:10.11918/j.issn.0367-6234.2012.10.009 [in Chinese]) |
卫星, 吕琳, 李贵雨, 等. 2016. 空气修根对水曲柳无纺布袋容器苗生长及根系发育的影响. 林业科学, 52(9): 133-138. (Wei X, Lü L, Li G Y, et al. 2016. Effects of air-cutting on seedling growth and root development of Fraxinus mandshurica in non-waven bags. Scientia Silvae Sinicae, 52(9): 133-138. [in Chinese]) |
张文泉. 2013.樟子松外生菌根真菌多样性及菌根生物技术研究.呼和浩特: 内蒙古农业大学博士学位论文. (Zhang W Q. 2013. Study on the diversity of mycorrhizal fungi and the biotechnology of mycorrhizal fungi of Pinus sylvestris. Inner Mongolia: PhD thesis of Inner Mongolia Agricultural University.[in Chinese]) http://cdmd.cnki.com.cn/Article/CDMD-10129-1013249789.htm |
赵平娟. 2004.菌根真菌提高植物抗逆性的研究.杨凌: 西北农林科技大学硕士学位论文. (Zhao P J. 2004. Studied on effect of Mycorrhizal fungi on promoting the resistance to adversity. Yangling: MS thesis of Northwest A&F University.[in Chinese]) http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=Y641520 |
赵平娟, 安锋, 唐明. 2007. 丛枝菌根真菌对连翘幼苗抗旱性的影响. 西北植物学报, 27(2): 396-399. (Zhao P J, An F, Tang M. 2007. Mechanism of plant salt tolerance enhanced by arbuscular mycorrhizal fungi. Acta Botanica Boreali-Occidentalia Sinica, 27(2): 396-399. DOI:10.3321/j.issn:1000-4025.2007.02.031 [in Chinese]) |
赵昕, 阎秀峰. 2006. 丛枝菌根对喜树幼苗生长和氮、磷吸收的影响. 植物生态学报, 30(6): 947-953. (Zhao X, Yan X F. 2006. Effects of Arbuscular Mycorrhizal fungi on th growth and absorption of nitrogen and phosphorus in gamptotheca acuminate seedlings. Journal of Plant Ecology, 30(6): 947-953. DOI:10.3321/j.issn:1005-264X.2006.06.009 [in Chinese]) |
Aidar S T, Meirelles S T, Oliveira R F, et al. 2014. Photosynthetic response of poikilochlorophyllous desiccation-tolerant Pleurostima purpurea (Velloziaceae) to dehydration and rehydration. Photosynthetica, 52: 124-133. DOI:10.1007/s11099-014-0014-0 |
Cheng L, Cord-Ruwisch R. 2013. Selective enrichment and production of highly urease active bacteria by non-sterile (open) chemostat culture. Journal of Industrial Microbiology & Biotechnology, 40(10): 1095-1104. |
Denison R F, Kiers E T. 2011. Life histories of symbiotic rhizobia and mycorrhizal fungi. Current Biology, 21(18): 775-785. DOI:10.1016/j.cub.2011.06.018 |
Guo D L, Xia M X, Wei X, et al. 2008. Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytologist, 180(3): 673-683. DOI:10.1111/nph.2008.180.issue-3 |
Hodge A, Fitter A H. 2010. Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proc Natl Acad Sci, 107(31): 13754-13759. DOI:10.1073/pnas.1005874107 |
Jayne B, Quigley M. 2014. Influence of arbuscular mycorrhiza on growth and reproductive response of plants under water deficit:a meta-analysis. Mycorrhiza, 24(2): 109-119. DOI:10.1007/s00572-013-0515-x |
Mark C. Brundrett. 2002. Coevolution of roots and mycorrhizas of land plants. New Phytologist, 154(2): 275-304. DOI:10.1046/j.1469-8137.2002.00397.x |
Neumann E, George E. 2005. Does the presence of arbuscular mycorrhizal fungi influence growth and nutrient uptake of a wild-type tomato cultivar and a mycorrhiza-defective mutant, cultivated with roots sharing the same soil volume. New Phytologist, 166(2): 601-609. DOI:10.1111/nph.2005.166.issue-2 |
Smith S E, Read D J. 2008. Mycorrhizal Symbiosis(3rd ed). London: Academic press.
|
Shen X F, Dong Z X, Chen Y. 2015. Drought and UV-B radiation effect on photosynthesis and antioxidant parameters in soybean and maize. Acta Physiologiae Plantarum, 37: 25. DOI:10.1007/s11738-015-1778-y |
Shi S M, Chen K, Gao Y, et al. 2016. Arbuscular mycorrhizal fungus species dependency governs better plant physiological characteristics and leaf quality of mulberry(Murus alba L.)seedlings. Frontiers in Microbiology, 7: 1030. |
Smith S.E., Smith F.A. 2011. Roles of arbuscular mycorrhizas in plant nutrition and growth, new paradigms from cellular to ecosystem scales. Annul Rev Plant Biol, 62(1): 227-250. DOI:10.1146/annurev-arplant-042110-103846 |
Singh K P, Kumar K R, Prasad K V, et al. 2008. influence of VAM inoculation on root colonization, survival, physiological and biochemical characteristics of Chrysanthemum plantlets. Indian Journal of Horticulture, 65(4): 974-0112. |
Wang P, Wu S H, Wen M X, et al. 2016. Effects of combined inoculation with Rhizophagus intraradices and Paenibacillus mucilaginosus on plant growth, root morphology, and physiological status of trifoliate orange (Poncirus trifoliate L. Raf.) seedlings under different levels of phosphorus. Scientia Horticulturae, 205: 97-105. DOI:10.1016/j.scienta.2016.04.023 |
Zou Y N, Wang P, Liu C Y, et al. 2017. Mycorrhizal trifoliate orange has greater root adaptation of morphology and phytohormones in response to drought stress. Scientific Reports, 7: 41134. DOI:10.1038/srep41134 |
 2019, Vol. 55
2019, Vol. 55

