文章信息
- 黄仁, 张韵, 张启香, 王正加, 夏国华, 黄坚钦, 胡渊渊.
- Huang Ren, Zhang Yun, Zhang Qixiang, Wang Zhengjia, Xia Guohua, Huang Jianqin, Hu Yuanyuan.
- 异源授粉山核桃果皮光合能力差异的转录组分析
- Transcriptome Analysis of Photosynthetic Capacity of Exocarp of Heterogeneously Pollinated Carya cathayensis
- 林业科学, 2019, 55(1): 128-137.
- Scientia Silvae Sinicae, 2019, 55(1): 128-137.
- DOI: 10.11707/j.1001-7488.20190115
-
文章历史
- 收稿日期:2018-04-09
- 修回日期:2018-10-10
-
作者相关文章
胡桃科(Juglandaceae)山核桃属(Carya)有2种普遍栽培的经济树种:山核桃(Carya cathayensis)与薄壳山核桃(C. illinoensis)。通过杂交授粉试验,发现山核桃具有明显的花粉直感现象(叶茂富等,1965;黎章矩等,1982),具体表现为果皮颜色变绿,平均单果质量提高39.1%(叶浩然等, 2013),性状与母本一致。洪丹丹(2007)、王正加等(2010)采用AFLP和SSR分子标记鉴定杂交后代,发现子代间、子代与母本间均无差异条带,推测山核桃可能存在无融合生殖。经证实,薄壳山核桃花粉授粉后53天,山核桃雌配子体珠心细胞解体,并竞争形成成熟胚,属于孢子体无融合生殖(张斌,2009)。综上所述,推测无融合生殖下山核桃果实的膨大应由薄壳山核桃花粉及其萌发过程引起。
果实的生长和发育所需的物质主要来源于绿色器官的光合作用。除叶片是植物主要的光合器官外,许多果实/种子等非叶器官也存在完整的叶绿体结构(Li et al., 2006; Hu et al., 2018),其光合产物是种实发育的一个重要补充来源(Aschan et al., 2003)。在蓝莓(Vaccinium)(Birkhold et al., 1992)、桃(Prunus persica)(Pavel et al., 2010)、荔枝(Litchi chinensis)(Hieke et al., 2002)、陆地棉(Gossypium hirsutum)(Hu et al., 2012)、山核桃属(Xu et al., 2016)和香榧(Torreya grandis)(Hu et al., 2018)的研究中,已明确果实光合作用对自身发育所需的碳骨架具有重要的贡献作用,基于此,徐沁怡等(2017)研究了不同授粉下山核桃果皮光合特性的差异,发现薄壳山核桃花粉授粉的山核桃果皮具有更高的光合速率和抗光抑制能力。作者研究发现,经光合抑制剂(DCMU)处理后(抑制率约为50%)的山核桃果实,单果质量分别降低了14.76%(薄壳山核桃授粉)和10.30%(山核桃授粉)(数据另文发表)。因此,推测薄壳山核桃花粉授粉后,山核桃子代果实膨大,很大程度上与果皮光合能力提高、抗光抑制能力增强有关,但其分子机理尚不清楚。
近年来,测序技术已广泛应用到逆境胁迫(Poong et al., 2018)、代谢途径分析(Lin et al., 2015; 马婧等, 2016)、基因挖掘(Huang et al., 2012; Li et al., 2015)等方面,能全面快速地揭示不同生物学特性的分子机制。基于山核桃果实的发育规律(解红恩等, 2008;Xu et al., 2016),本研究在山核桃果实快速膨大期内(授粉后65天),利用转录组测序技术分析薄壳山核桃和山核桃2种花粉授粉下山核桃果皮光合相关基因的差异表达,结合光合生理试验,试图从转录水平上揭示2种花粉授粉的山核桃果皮光合特性差异的分子机制。
1 材料与方法 1.1 试验材料试验点位于浙江农林大学山核桃试验林基地(119°43′11.63″E,30°14′10.68″N)。该基地地势平缓,2008年造林,采用“2+1”嫁接苗[砧木为2年生湖南山核桃(Carya hunanensis),接穗为山核桃,培育1年],2012年开始结果。每年4月份花期施少量复合肥及9月果实采收后施有机肥,田间栽培技术措施基本相同。
选取2株长势一致的山核桃母树,1株作为1个生物学重复。于4月下旬套袋,4月底5月初进行授粉,将每一株母树中同一高度、长势基本一致的相邻2个树枝作为1个试验组合,每个组合分别设置2种处理:山核桃花粉处理(2株母树分别记作hp1、hp2)和薄壳山核桃(‘Pawnee’)花粉处理(2株母树分别记作pp1、pp2)。授粉5~7天后除去纸袋,挂牌标记。在果实快速膨大期内——授粉后65天(65DAP),测定果皮光合能力及形态指标;采集不同处理的山核桃果实各5个,均匀切取相同部位的果皮(约1/5大小),对应混合后在-80 ℃冰箱保存,用于叶绿素、可溶性蛋白、光合关键酶的测定和转录组测序。在果实成熟时,测定不同处理的山核桃果实品质。
1.2 测定方法1) 果实表型的测定果实成熟后每个重复每个处理随机选取50个果实,3个重复共计150个果实,采用数显式游标卡尺测量果长(L,mm)、果横径(W,mm)、果纵径(T,mm)。用电子天平称量果实鲜质量,精确到0.001 g;用陶瓷刀片将果皮及种仁进行分离,置于105 ℃烘箱中杀青40 min,再在65 ℃烘箱烘至恒重(24 h以上),冷却后用电子天平对果皮及种仁干质量进行称量,精确到0.001 g。
2) 可溶性总糖、含油率的测定可溶性总糖测定参考崔喜艳(2008)和孔祥生等(2008)的方法,并稍加改进。称取干样样品(约1 g),加20 mL 80%的酒精磨样,于80 ℃水浴锅中水浴30 min~1 h,然后4 000 r ·min-1离心10 min,取上清液,再加入20 mL蒸馏水4 000 r ·min-1离心5 min,合并2次上清液,并定容到100 mL。0.5 mL提取液+0.5 mL水+5 mL蒽酮溶液(90 mL浓硫酸+10 mL水+0.4 g蒽酮溶解),水浴100 ℃,10 min;冷却后,分光光度计620 nm下比色,在葡萄糖标准曲线下计算可溶性糖含量。含油率的测定使用索式提取法,具体操作参考郝再彬等(2004)。
3) 光合速率的测定选择晴天光强稳定在1 200~1 400 μmol ·m-2s-1(北京时间8:00—11:00)时,采用Li6400-22簇状叶室测定不同花粉授粉的山核桃果实的气体交换参数,以18-RGB为光源。每株树作为1个重复,4个重复,每个重复3个果实。气体交换测定时,设定叶室内气体流速为500 μmol ·s-1,温度为28 ℃,CO2浓度为400 μmol ·mol-1,光强为1 300 μmol ·m-2s-1。果实的光合速率为光下的光合速率和黑暗下的呼吸速率之和。山核桃果皮单位面积的光合速率通过代入山核桃果实的表面积/2进行校准重新计算。
4) 叶绿素含量、Rubisco酶、PEPC酶的测定用自制陶瓷双刀片(厚度为3 mm)切取山核桃外果皮,去除外果皮基部与顶部后,取中间部分果皮2~3片(每片果皮面积约为1 cm×0.5 cm),称鲜质量后快速切碎后放入装有8 mL 95%乙醇的离心管中,置于暗处浸提24 h,定时振荡。同时取相同果实上的中部果皮,称其鲜质量后,再在方格纸上记下面积。用UV-2550型分光光度计(日本岛津),于665、649、470 nm波长下测定OD值,叶绿素含量计算方法参照Lichtenthaler(1987)。Rubisco酶活性的测定方法、计算方法参考Chen等(2003)。磷酸烯醇式丙酮酸羧化酶(PEPC)酶活性的测定参照施教耐等(1979)方法。
5) RNA文库构建与测序采用多糖多酚植物总RNA提取试剂盒(北京天根生化科技有限公司)分别提取65DAP 2种花粉授粉的山核桃果皮总RNA。NanoDrop检测RNA浓度,用Agilent 2100检测28S/18S以及RIN值,同时使用1%琼脂糖凝胶电泳检测提取RNA的质量以及完整性。用RNAClean XP Kit和RNase-Free DNase Set试剂盒进行纯化。最后进行PCR扩增,构建好的文库用Qubit® 2.0 Fluorometer和Agilent 2100进行质量检测,文库质量合格后委托天津诺禾致源测序公司使用Illumina HiSeq 2000进行测序,采用双端测序方式,测序长度125 bp。
6) 测序数据的处理根据已完成测序的山核桃基因组进行有参转录组分析。质控后使用TopHat2对过滤后的reads进行参考基因组的比对分析。用Cufflinks(v2.2.1)构建4个RNA测序数据。将得到的scaffold序列利用Blast+(ncbi-blast-2.2.28+)与UniProt数据库和Nr数据库进行Blastx比对(E < 1×e-5),应用rpstblastn程序将scaffold与CDD数据库进行比对(E≤1×e-5),进行KOG功能分类预测;利用HMMER3.0软件的hmmscane程序与Pfam结构数据库进行比对(E=0.01),利用EMBOSS(6.4.0)-getorf对所有scaffold进行开放阅读框(ORF)预测,选择最长且长度大于300 bp的ORF作为scaffold的ORF。利用hmmsearch程序对所有的ORF通过HMM模型与PlantTFDB的转录因子结构域进行比对,对ORF进行转录因子预测。差异基因的筛选先以P < 0.05为标准,在此基础上筛选出差异倍数在1.5倍以上的基因作为差异基因。
7) GO分析和KEGG代谢通路分析使用Blast对scaffold进行NT、NR、COG、KEGG以及SwissProt注释,使用Blast2GO以及NR注释结果进行GO注释,使用InterProScan5进行InterPro注释。在NT数据库中比对分析非冗余核酸序列,利用NR、SwissProt数据库分析蛋白序列,然后利用COG数据库根据直系同源家族蛋白进行功能分类以及通过InterPro数据库根据蛋白结构域进行家族分类以提供功能注释。对所有scaffold基因在分子功能、细胞组分和生物过程进行GO功能分析以及在KEGG数据库中进行代谢途径的分析。然后使用RSEM计算各个样品的基因表达水平。使用R(v3.5.1)中的princomp包进行PCA分析。
1.3 数据分析试验数据采用Excel 2016计算和分析,其独立样本t检验和方差分析处理均采用SPSS18.0版统计软件进行统计学分析处理,利用R语言pheatmap、ggplot2包等进行作图。
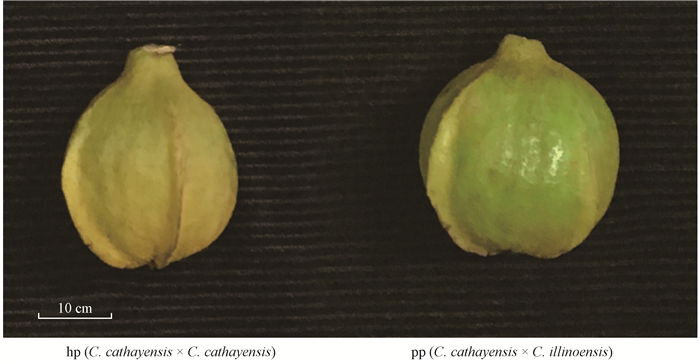
2 结果与分析 2.1 2种花粉授粉的山核桃果实的形态指标及品质的变化在山核桃果实快速膨大期(65DAP),山核桃果实的直感现象已非常明显,果皮颜色及形态差异显著,授薄壳山核桃花粉(pp)的山核桃果实的横径、纵径分别增加了12.18%、13.36%(图 1)。由表 1可知,pp授粉的山核桃果实鲜质量、干质量、胚质量分别为8.35、2.14、0.78 g,较山核桃花粉(hp)授粉的果实提高了34.90%、25.15%、30.00%。hp与pp授粉的山核桃果仁可溶性糖之间并无显著差异,但pp授粉的山核桃果仁的含油率比hp授粉的高1.9%(P=0.002)。
|
图 1 授粉后65天不同花粉授粉的山核桃果实外形的变化 Fig. 1 Changes in appearance of Carya cathayensis fruits pollinated with two different pollens at 65 days after pollination |
|
|
由表 2可知,65DAP时2种花粉授粉的山核桃果皮可溶性蛋白含量无显著差异。pp授粉的山核桃果皮叶绿素含量、光合速率、Rubisco酶活性、PEPC酶活性均显著高于hp授粉的山核桃果皮,叶绿素含量增加了31%(P=0.047),光合速率增高了12.4%(P=0.000 43),Rubisco活性增强了64.6%(P=0.036),PEPC活性增强了22.8%(P=0.001 3)。
|
|
在山核桃果实发育快速膨大期(65DAP),对不同授粉的山核桃果皮(每个处理2个生物学重复)进行转录组测序(表 3),分别获得95 453 610个、106 467 510个、106 440 170个、99 918 970个clean reads,均占总reads的93%以上,Q20、Q30比例均大于90%,GC含量约为48%。利用TopHat软件将测序数据与山核桃基因组进行比对,比对率均大于80%(表 3),基因分布情况及相关性分析(图 2)表明样品测序质量较好,相关系数均大于0.8,重复间表达模式相似度较高。
|
|

|
图 2 样品间差异基因分布情况及相关性分析 Fig. 2 The distribution and the correlation analysis of all samples A.样品间基因的分布情况;B.样品间的相关系数 A. The genes distribution pattern of all samples; B. The Pearson's correlation analysis of all samples. |
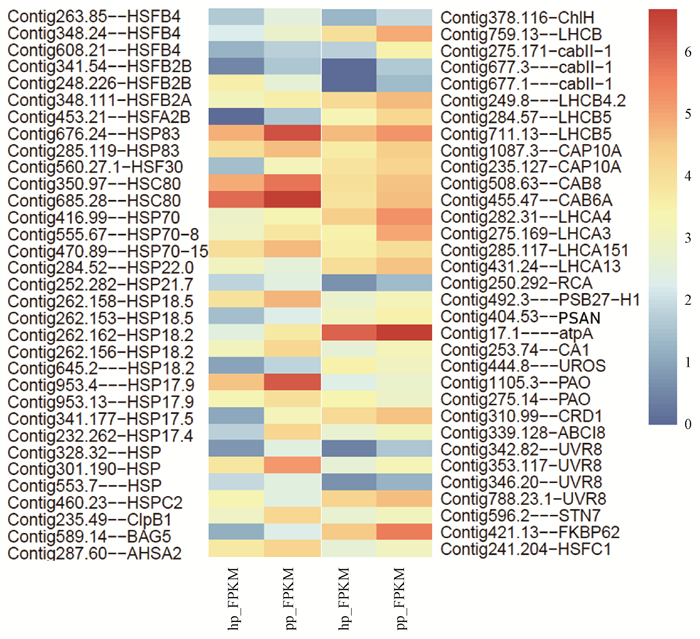
对转录组数据进行差异分析,共筛选出1 984个差异表达的基因(P < 0.05,foldchange>1.5)。结合GO、KEGG注释结果,共筛选出与光合相关的基因66个(图 3),主要富集在卟啉环及叶绿素代谢通路中(图 4)及光合固碳通路中(图 5),其中42个基因与光保护相关。
|
图 3 光合相关差异基因(DEGs)表达热图 Fig. 3 Heat map diagram of the expression levels of differentially expressed genes which are involved in plant photoprotection 表达量为lg(FPKM+1)。 Gene expression data were normalized to lg(FPKM+1). |
|
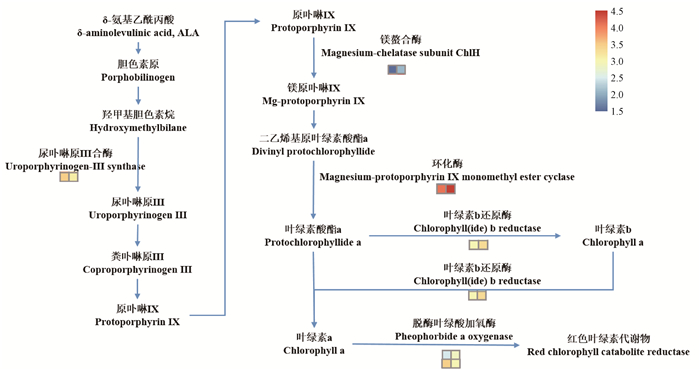
图 4 卟啉及叶绿素合成通路 Fig. 4 KEGG pathway of porphyrin and chlorophyll metabolism 左侧方格为山核桃花粉(hp)授粉的山核桃果皮表达,右侧方格为薄壳山核桃花粉(pp)授粉的山核桃果皮表达。 The left row indicates differentially expressed genes in hp(Carya cathayensis ×C. cathayensis), the right row indicates those in pp(C. cathayensis ×C. illinoensis). |
|
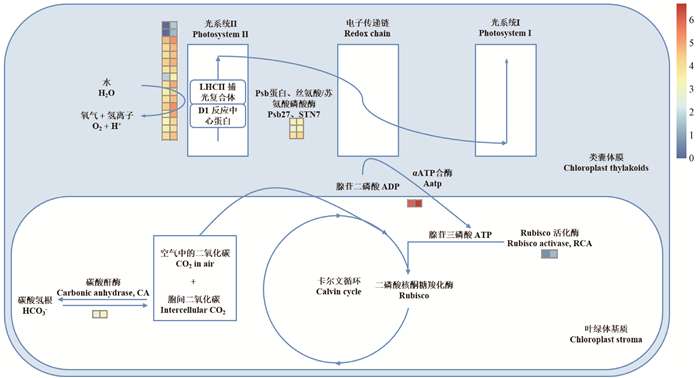
图 5 山核桃果皮光合固碳通路差异基因富集 Fig. 5 Photosynthetic carbon fixation related differentially expressed genes involved in exocarp of Carya cathayensis fruit 左列为hp授粉山核桃果皮表达量,右列为pp授粉山核桃果皮表达量。 The left and right row indicate the expression of related genes in fruits with hp (Carya cathayensis ×C. cathayensis) and pp (C. cathayensis ×C. illinoensis), repectively. |
叶绿素(Chlorophyll, Chl)合成过程复杂,从谷氨酰到Chl a再到Chl b的合成,共由20多个基因编码的16种酶参与完成(Beale, 2005;Ito et al., 2014)。研究表明,可以通过编码番茄(Lycopersicon esculentum)果皮叶绿体发育相关的基因调控叶绿素的合成,改变其果实的光合作用,进而影响其内在品质(Cocaliadis et al., 2014; Meng et al., 2018)。玉米(Zea mays)中镁螯合酶基因(ZmCHLI)突变后该突变体叶呈黄绿色(Sawers et al., 2006);敲除镁-原卟啉单甲酯环化酶基因(CRD)的拟南芥(Arabidopsis thaliana),植株生长缓慢,颜色褪绿(Bang et al., 2008);水稻(Oryza sativa)中叶绿素合酶活性降低导致苗期叶片呈黄绿色,且LHC蛋白显著降低(Wu et al., 2007)。将对比获得的差异基因进行统计分析发现,不同花粉授粉处理之间涉及叶绿素代谢途径的相关基因表现出显著差异,其中薄壳山核桃花粉(pp)授粉处理下叶绿素合成的关键酶编码基因——CHLH、CRD、叶绿素b还原酶编码基因(NYC)以及捕光复合蛋白编码基因(LHC)均显著上调,以及叶绿素降解过程中的关键限速酶脱酶叶绿酸加氧酶编码基因(PAO)低表达(图 4),表明pp授粉后山核桃果皮能合成更多的Chl很可能是叶绿素生物合成和代谢途径中的关键限制基因表达的差异造成的。不同花粉授粉的山核桃果皮光合速率与其叶绿素含量呈极显著相关(徐沁怡,2017),本研究显示,pp授粉增强了山核桃果皮叶绿素合成能力。
与山核桃花粉(hp)授粉相比,pp授粉的山核桃果实具有更高的PSⅡ光化学量子产率,更高的光化学荧光猝灭系数以及更强的光合能力(徐沁怡等,2017)。根据测序结果构建山核桃果皮光合固碳调控途径(图 5),发现除上述LHC,还存在与电子传递速率、光化学荧光猝灭显著相关基因——LHCB、PSAN,均显著上调。LHCB除了捕获和传递能量外,还具有消耗过剩光能,调节光系统Ⅱ状态转换速率和结构的功能,使LHC不易受到强光破坏(张亚杰, 2006; Damkjaer et al., 2009; Adamiec et al., 2015)。PSAN基因缺失的拟南芥突变体叶片明显褪绿,其叶片光合速率、PSⅡ光化学量子产率及光化学荧光猝灭均显著降低(Haldrup et al., 2010; Ihalainen et al., 2002)。由此可知,pp授粉后上述基因表达的变化,很可能与其果皮光合能力提高有关。
LHCⅡ磷酸激酶(STN7)参与植物对光的短期响应与长期适应(Pesaresi et al., 2009),在强光下能加速LHCⅡ蛋白的磷酸化和状态转换过程,耗散过剩光能,避免PSⅡ被破坏(Depège et al., 2003; Bonardi et al., 2005)。当热耗散等保护机制不能有效地保护光合机构时,反应中心蛋白尤其是D1蛋白的修复可被看作是防御光破坏的最后一道防线。研究表明,PSB蛋白参与PSⅡ修复(Nowaczyk et al., 2006),能有效提高PSⅡ活性的恢复速率。本研究结果显示,pp授粉的山核桃果皮中STN7、PSB27及UVR 8 表达显著上调(图 3)。当拟南芥植株处于UV-B水平较高时,细胞核内积累的UVR 8 (UV-B特异光受体)能促进其叶片的光合作用效率(Brown et al., 2005)。另外,热休克蛋白(HSP)是植物产生耐热性所必需的,起着“伸展酶”的作用(Waters, 1995),在高温胁迫下起维持和保护细胞环境稳态的作用。研究表明,大豆(Glycine max)叶绿体中的HSPs可保护PSⅡ的反应中心在热激时免受损伤(Schuster et al., 1988)。本研究结果显示,pp授粉的山核桃果实中有38个HSP基因(包括HSP 17-30、HSP70、HSP80)均显著上调。在果实发育后期(授粉后103~120天),hp授粉的山核桃果实的Y(NO)(PSⅡ中荧光和不依赖光的基础热耗散量子效率,是光损伤的重要指标)显著增加,pp授粉的果实Y(NO)无明显变化,表明hp授粉的果实较易受到光损伤,pp授粉的果实可能具有较强的抗光抑制能力(徐沁怡等,2017)。因此,pp授粉下山核桃果实中与光保护机制相关的基因——STN 7、PSB27、UVR8及HSP等的显著高表达可能使其具有更强的光耐受性及应对不可逆破坏的修复能力。
核酮糖-1, 5-二磷酸羧化酶/加氧酶(Ribulose-1, 5-bisphosphate carboxylase/oxygenase, Rubisco)是卡尔文循环的关键限速酶,约占植物可溶性蛋白的50%左右(Makino et al., 1983),但必须通过Rubisco活化酶(Rubisco activase,RCA)活化(Portis, 1995; Salvucci et al., 1996)。尹吴等(2017)在毛果杨(Populus trichocarpa)的研究中发现转RCA基因的植株能够充分利用强光进行光合作用,其光饱和点较对照组提高了12.5%~37.5%,并表现出更高的耐光氧化能力。与hp授粉相比,pp授粉的山核桃具有明显较高的光饱和点(徐沁怡等,2017)。本研究结果显示,不同花粉授粉的山核桃果皮之间的Rubisco表达量、可溶性蛋白含量无明显差异(表 2),但pp授粉下其RCA显著上调(图 5),Rubisco酶活性亦显著增强,表明pp授粉后的山核桃果皮光合固碳能力、光饱和点的提高很可能与其RCA高表达有关。碳酸酐酶(CA)在加速植物光合碳同化过程中起着十分重要的作用,负责HCO3-与CO2之间的可逆转换。研究表明,CA能促进叶片Rubisco的羧化作用,由于CA加速了HCO3-向CO2的转换,为Rubisco的羧化作用提供底物(王玮蔚,2014)。此外,果实重新固定其呼吸释放的CO2与其较强的磷酸烯醇式丙酮酸羧化酶(PEPC)活性有关(Hiratsuka et al., 2012)。小麦(Triticum aestivum ‘Xiaoyan54’)非叶光合器官穗具有较高的CA活性,为PEPC提供更多的HCO3-,促进其PEPC活性的提高,是其较高光合速率的原因(Li et al., 2004)。综上所述,pp授粉的山核桃果实RCA、CA的高表达和较强的PEPC活性为其较高碳同化能力提供了有力保障。
4 结论转录组分析表明,异源花粉授粉后,与山核桃果皮光合相关的差异基因多达66个,主要集中在叶绿素合成、PSⅡ捕光蛋白、PSⅡ光修复相关蛋白和碳固定关键酶上。薄壳山核桃花粉授粉的山核桃果皮显绿、光合速率增强可能与其叶绿素合成相关基因、光系统Ⅰ反应中心N亚基(PSAN)、碳酸酐酶编码基因(CA)及Rubisco活化酶编码基因(RCA)表达量的上调有关;而其抗光抑制能力的增强则与较高表达量的光系统Ⅱ修复蛋白(PSB 27 )、LHCⅡ磷酸激酶(STN 7 )/紫外线B受体(UVR 8 )及其余38个热激蛋白编码基因(HSP)有关。
崔喜艳. 2008. 基础生物化学实验方法和技术. 北京: 中国林业出版社. (Cui X Y. 2008. Basic biochemical experiment methods and techniques. Beijing: China Forestry Publishing House. [in Chinese]) |
郝再彬, 苍晶, 徐仲. 2004. 植物生理实验. 哈尔滨: 哈尔滨工业大学出版社. (Hao Z B, Cang J, Xu Z. 2004. Plant physiology experiment. Harbin: Harbin Institute of Technology Press. [in Chinese]) |
洪丹丹. 2007.山核桃×薄壳山核桃授粉子代AFLP、SSR分析.杭州: 浙江林学院硕士学位论文. (Hong D D. 2007. AFLP and SSR Analysis of fruit resulting from C. cathayensis×C.cathayensis and C. cathayensis×C. illinoensis. Hangzhou: MS thesis of Zhejiang A & F University.[in Chinese]) http://cdmd.cnki.com.cn/article/cdmd-10341-2007215571.htm |
孔祥生, 易现峰. 2008. 植物生理学实验技术. .北京: 中国农业出版社.
|
Kong X S, Yi X F. 2018. Experimental techniques for plant physiology. Beijing: China Agriculture Press.
|
黎章矩, 夏逍鸿, 施拱生. 1982. 山核桃种间杂交试验研究. 浙江林学院科技通讯, (1). (Li Z J, Xia X H, Shi G S. 1982. Hickory interspecific hybridization experiment research. Science and Technology Communication of Zhejiang Forestry College, (1). [in Chinese]) |
马婧, 邓楠, 褚建民, 等. 2016. 泡泡刺高通量转录组鉴定及其黄酮类代谢途径初步分析. 林业科学研究, 29(1): 61-66. (Ma J, Deng N, Chu J M, et al. 2016. High-throughput transcriptome identification and flavonoids metabolic pathways in Nitraria sphaerocarpa.. Forest Research, 29(1): 61-66. DOI:10.3969/j.issn.1001-1498.2016.01.009 [in Chinese]) |
施教耐, 吴敏贤, 查静娟. 1979. 植物磷酸烯醇式丙酮酸羧化酶的研究Ⅰ:PEP羧化酶同功酶的分离和变构特性的比较. 植物生理学报, (3): 44-54. (Shi J N, Wu M X, Zhai J J. 1979. Study on the phosphate enzymatic acetone carboxylation of plant phosphate-I.Comparison of isolation and allosteric properties of PEP carboxylation. Journal of Plant Physiology, (3): 44-54. [in Chinese]) |
王玮蔚. 2014.蛋白核小球藻碳酸酐酶基因筛选及环境因素对其调控的研究.宁波: 宁波大学硕士学位论文. (Wang W W. 2014. Screening of carbonic anhydrase gene from Chlorella pyrenoidosa and study on its regulation by environmental factors. Ningbo: MS thesis of Ningbo University.[in Chinese]) http://cdmd.cnki.com.cn/Article/CDMD-11646-1014086017.htm |
王正加, 张斌, 夏国华, 等. 2010. 山核桃×薄壳山核桃花粉直感效应与后代分析. 果树学报, 27(6): 908-913. (Wang Z J, Zhang B, Xia G H, et al. 2010. Analysis of the progeny of Carya cathayensis×C. illinoensis and the xenia effect. Journal of Fruit Science, 27(6): 908-913. [in Chinese]) |
解红恩, 黄有军, 薛霞铭, 等. 2008. 山核桃果实生长发育规律. 浙江农林大学学报, 25(4): 527-531. (Xie H E, Huang Y J, Xue X M, et al. 2008. Growth and development of pecan fruit. Journal of Zhejiang A & F University, 25(4): 527-531. DOI:10.3969/j.issn.2095-0756.2008.04.024 [in Chinese]) |
徐沁怡, 王标, 赵建文, 等. 2017. 2种花粉授粉山核桃果皮光合特性的差异比较. 林业科学, 53(1): 38-46. (Xu Q Y, Wang B, Zhao J W, et al. 2017. Variation in photosynthetic characteristics of exocarp of Carya cathayensis fruits pollinated with different pollens. Scientia Silvae Sinicae, 53(1): 38-46. [in Chinese]) |
叶浩然, 邵慰忠, 常君, 等. 2013. 山核桃与薄壳山核桃的杂交优势在山核桃生产中的应用试验. 浙江林业科技, 33(4): 83-85. (Ye H R, Shao W Z, Chang J, et al. 2013. Experiment on artificial pollination of Carya cathayensis by C. illinoensis.. Journal of Zhejiang Forestry Science & Technology, 33(4): 83-85. DOI:10.3969/j.issn.1001-3776.2013.04.018 [in Chinese]) |
叶茂富, 吴厚钧. 1965. 山核桃与薄壳山核桃杂交的研究. 林业科学, 10(1): 50-56. (Ye M F, Wu H J. 1965. Study of artificial pollination of Carya cathayensis by C. illinoensis.. Scientia Silvae Sinicae, 10(1): 50-56. [in Chinese]) |
尹吴, 孙伟博, 周燕, 等. 2017. 毛果杨Rubisco活化酶基因的克隆与功能分析. 林业科学, 53(4): 83-95. (Yi W, Sun W B, Zhou Y, et al. 2017. Cloning and functional analysis of Rubisco activase gene from Populus euphratica.. Scientia Silvae Sinicae, 53(4): 83-95. [in Chinese]) |
张斌. 2009.山核桃雌配子体发育及其生殖过程研究.杭州: 浙江林学院硕士学位论文. (Zhang B. 2009. Female gametophyte development and reproductive process in gickory(Carya cathayensis). Hangzhou: MS thesis of Zhejiang A & F University.[in Chinese]) |
张亚杰. 2006.高等植物光系统Ⅱ大量捕光色素蛋白复合体的稳定性研究.北京: 中国科学院植物研究所博士学位论文. (Zhang Y J. 2006. Stability of the major light-harvesting complex of photosystem Ⅱ of higher plant. Beijing: PhD thesis of Institute of Botany, The Chinese Academy of Sciences.[in Chinese]) http://cdmd.cnki.com.cn/Article/CDMD-80105-2006189610.htm |
Adamiec M, Gibasiewicz K, Luciński R, et al. 2015. Excitation energy transfer and charge separation are affected in Arabidopsis thaliana mutants lacking light-harvesting chlorophyll a/b binding protein Lhcb3. Journal of Photochemistry & Photobiology B:Biology, 153: 423-428. |
Aschan G, Pfanz H. 2003. Non-foliar photosynthesis-a strategy of additional carbon acquisition. Flora, 198: 81-97. DOI:10.1078/0367-2530-00080 |
Bang W Y, Jeong I S, Kim D W, et al. 2008. Role of Arabidopsis CHL27 protein for photosynthesis, chloroplast development and gene expression profiling. Plant & Cell Physiology, 49(9): 1350-136. |
Beale S I. 2005. Green genes gleaned. Trends in Plant Science, 10(7): 309-312. DOI:10.1016/j.tplants.2005.05.005 |
Birkhold K T, Koch K E, Darnell R L, et al. 1992. Carbon and nitrogen economy of developing rabbiteye blueberry fruit. Journal of the American Society for Horticulturalence, 117(1): 139-145. |
Bonardi V, Pesaresi P, Becker T, et al. 2005. Photosystem Ⅱ core phosphorylation and photosynthetic acclimation require two different protein kinases. Nature, 437(7062): 1179-1182. DOI:10.1038/nature04016 |
Brown B A, Cloix C, Jiang G H, et al. 2005. A UV-B-specific signaling component orchestrates plant UV protection. Proceedings of the National Academy of Sciences of the United States of America, 102(50): 18225-18230. DOI:10.1073/pnas.0507187102 |
Chen L S, Cheng L. 2003. Carbon assimilation and carbohydrate metabolism of 'concord' grape (Vitis labrusca L.) leaves in response to nitrogen supply. Journal of the American Society for Horticultural Science, 128(5): 754-760. |
Cocaliadis M F, Fernándezmuñoz R, Pons C, et al. 2014. Increasing tomato fruit quality by enhancing fruit chloroplast function. A double-edged sword?. Journal of Experimental Botany, 65(16): 4589-4598. |
Damkjaer J T, Kereïche S, Johnson M P, et al. 2009. The photosystem Ⅱ light-harvesting protein Lhcb3 affects the macrostructure of photosystem Ⅱ and the rate of state transitions in Arabidopsis.. The Plant Cell, 21(10): 3245-3256. DOI:10.1105/tpc.108.064006 |
Depége N, Bellafiore S, Rochaix J D. 2003. Role of chloroplast protein kinase Stt7 in LHCⅡ phosphorylation and state transition in Chlamydomonas.. Science, 299(5612): 1572-1575. DOI:10.1126/science.1081397 |
Haldrup A, Naver H, Scheller H V. 2010. The interaction between plastocyanin and photosystem I is inefficient in transgenic Arabidopsis plants lacking the PSI-N subunit of photosystem. Plant Journal, 17(6): 689-698. |
Hieke S, Menzel C M, Ludders P. 2002. Effects of leaf, shoot and fruit development on photosynthesis of Lychee trees (Litchi chinensis). Tree Physiol, 22(13): 955-961. DOI:10.1093/treephys/22.13.955 |
Hiratsuka S, Yokoyama Y, Nishimura H, et al. 2012. Fruit photosynthesis and phosphoenolpyruvate carboxylase activity as affected by lightproof fruit bagging in satsuma mandarin. American Society for Horticultural Science, 137(4): 215-220. |
Hu Y Y, Zhang Y L, Luo H H, et al. 2012. Important photosynthetic contribution from the non-foliar green organs in cotton at the late growth stage. Planta, 235(2): 325-336. DOI:10.1007/s00425-011-1511-z |
Hu Y Y, Zhang Y L, Yu W W, et al. 2018. Novel insights into the influence of seed sarcotesta photosynthesis on accumulation of seed dry matter and oil content in Torreya grandis cv. 'Merrillii'. Frontiers in Plant Sicence, 8: 2179. DOI:10.3389/fpls.2017.02179 |
Huang H H, Xu L L, Tong Z K, et al. 2012. De novo, characterization of the Chinese fir (Cunninghamia lanceolata) transcriptome and analysis of candidate genes involved in cellulose and lignin biosynthesis. BMC Genomics, 13(1): 648. DOI:10.1186/1471-2164-13-648 |
Ihalainen J A, Jensen P E, Haldrup A, et al. 2002. Pigment organization and energy transfer dynamics in isolated photosystemⅠ(PSⅠ) complexes from Arabidopsis thaliana depleted of the PSⅠ-G, PSⅠ-K, PSⅠ-L, or PSⅠ-N subunit. Biophysical Journal, 83(4): 2190-2201. DOI:10.1016/S0006-3495(02)73979-9 |
Ito H, Tanaka A. 2014. Evolution of a new chlorophyll metabolic pathway driven by the dynamic changes in enzyme promiscuous activity. Plant & Cell Physiology, 55(3): 593-603. |
Li S W, Shi R F, Leng Y. 2015. De novo characterization of the mung bean transcriptome and transcriptomic analysis of adventitious rooting in seedlings using RNA-Seq. PLoS One, 10(7): e0132969. DOI:10.1371/journal.pone.0132969 |
Li X, Hou J, Bai K, et al. 2004. Activity and distribution of carbonic anhydrase in leaf and ear parts of wheat (Triticum aestivum L.). Plant Science, 166(3): 627-632. DOI:10.1016/j.plantsci.2003.10.031 |
Li X J, Wang H G, Li H B, et al. 2006. Awns play a dominant role in carbohydrate production during the grain-filling stages in wheat (Triticum aestivum). Physiologia Plantarum, 127: 701-709. DOI:10.1111/ppl.2006.127.issue-4 |
Lichtenthaler H K. 1987. Chlorophylls and carotenoids:pigments of photosynthetic biomembranes. Methods Enzymol, 148(1): 350-382. |
Lin Q, Wang C, Dong W, et al. 2015. Transcriptome and metabolome analyses of sugar and organic acid metabolism in Ponkan (Citrus reticulata) fruit during fruit maturation. Gene, 554(1): 64-74. DOI:10.1016/j.gene.2014.10.025 |
Makino A, Mae T, Ohira K. 1983. Photosynthesis and ribulose 1, 5-bisphosphate carboxylase in rice leaves:changes in photosynthesis and enzymes involved in carbon assimilation from leaf development through senescence. Plant Physiology, 73(4): 1002-1007. DOI:10.1104/pp.73.4.1002 |
Meng L, Fan Z, Zhang Q, et al. 2018. BEL1-LIKE HOMEODOMAIN 11 regulates chloroplast development and chlorophyll synthesis in tomato fruit. Plant Journal for Cell & Molecular Biology, 94(6): 1126-1140. |
Nowaczyk M M, Hebeler R, Schlodder E, et al. 2006. Psb27, a cyanobacterial lipoprotein, is involved in the repair cycle of photosystem Ⅱ. Plant Cell, 18(11): 3121-3131. DOI:10.1105/tpc.106.042671 |
Pavel E W, Dejong T M. 2010. Estimating the photosynthetic contribution of developing peach (Prunus persica) fruits to their growth and maintenance carbohydrate requirements. Physiologia Plantarum, 88(2): 331-338. |
Pesaresi P, Hertle A, Pribil M, et al. 2009. Arabidopsis STN7 kinase provides a link between short-and long-term photosynthetic acclimation. Plant Cell, 21(8): 2402-2423. DOI:10.1105/tpc.108.064964 |
Poong S W, Lee K K, Lim P E, et al. 2018. RNA-Seq-mediated transcriptomic analysis of heat stress response in a polar Chlorella sp. (Trebouxiophyceae, Chlorophyta). Journal of Applied Phycology. DOI:10.1007/s10811-018-1455-9 |
Portis R. 1995. The regulation of Rubisco by Rubisco activase. Journal of Experimental Botany, 46: 1285-1291. DOI:10.1093/jxb/46.special_issue.1285 |
Salvucci M E, Ogren W L. 1996. The mechanism of Rubisco activase:Insights from studies of the properties and structure of the enzyme. Photosynthesis Research, 47(1): 1-11. |
Sawers R J, Viney J, Farmer P R, et al. 2006. The maize oil yellow1 (Oy1) gene encodes the I subunit of magnesium chelatase. Plant Mol Biol, 60(1): 95-106. DOI:10.1007/s11103-005-2880-0 |
Schuster G, Timberg R, Ohad I. 1988. Turnover of thylakoid photosystem Ⅱ proteins during photoinhibition of Chlamydomonas reinhardtii.. Febs Journal, 177(2): 403-410. |
Waters E R. 1995. The molecular evolution of the small heat-shock proteins in plants. Genetics, 141(2): 785-795. |
Wu Z, Zhang X, He B, et al. 2007. A chlorophyll-deficient rice mutant with impaired chlorophyllide esterification in chlorophyll biosynthesis. Plant Physiology, 145(1): 29-40. DOI:10.1104/pp.107.100321 |
Xu Q, Wu J, Cao Y, et al. 2016. Photosynthetic characteristics of leaves and fruits of Hickory (Carya cathayensis Sarg.) and Pecan (Carya illinoensis K. Koch) during fruit development stages. Trees, 30(5): 1-12. |
 2019, Vol. 55
2019, Vol. 55

