文章信息
- 刘媛, 魏虹, 马文超, 张雯, 曾成城, 周翠, 王婷
- Liu Yuan, Wei Hong, Ma Wenchao, Zhang Wen, Zeng Chengcheng, Zhou Cui, Wang Ting
- 秋华柳对镉的积累及其亚细胞分布特征
- Accumulation and Subcellular Distribution of Cadmium in Salix variegate
- 林业科学, 2018, 54(8): 48-55.
- Scientia Silvae Sinicae, 2018, 54(8): 48-55.
- DOI: 10.11707/j.1001-7488.20180806
-
文章历史
- 收稿日期:2017-05-08
- 修回日期:2017-07-20
-
作者相关文章
受工业生产排污,农业耕作施肥等人类活动的影响,我国土壤镉(Cd)污染十分严峻(潘芳慧等,2018), 其点位超标率高达7.0%(环境保护部等,2014)。而镉(Cadmium)对植物的毒性是其他重金属的2~10倍(Kabata-Pendias et al., 1984)。植物对Cd的过量积累会降低对其他营养元素的吸收,限制植物的光合和呼吸代谢,影响植物正常的生长发育(Shi et al., 2010)。植物可通过控制Cd在不同器官和亚细胞结构的分布来避免过量的Cd进入敏感性器官和功能性细胞器,从而减小其对自身的毒害(董萌等, 2013;韩超等, 2014),如根系对Cd有显著的滞留效应,限制Cd进入敏感的地上部分;植物可以通过细胞壁金属结合位点,固定大量的Cd,阻止其进入细胞原生质(Lai, 2015)或通过主动运输将其排出细胞(Meharg, 2006; Tong et al., 2004)。激活细胞内区室化机制,将进入细胞的Cd离子从细胞溶质或代谢活跃的场所转运至液泡累积起来(Giovanni et al., 2010; Mohammad et al., 2012),或通过螯合作用将其转化成不具生物活性的解毒形态(Hall, 2002)。
植物细胞壁对Cd的固定和液泡区室化在维持植物正常的生理功能中起着重要的作用(He et al., 2008)。苎麻(Boehmeria nivea)根细胞中80%的Cd被固定在细胞壁(Zhu et al., 2013);白羽扇豆(Lupinus albus)叶、茎、根中细胞壁累积的Cd含量分别可达到该部分细胞Cd总含量的47%、51%、42%(Vázquez et al., 2006);矮菜豆(Phaseolus vulgaris)体内的Cd主要与根和叶的细胞壁结合(Leita et al., 1996);萱草(Hemerocallis fulva)地上部分的Cd大部分累积在细胞壁(李红婷等, 2015)。Cd胁迫下,烟草(Nicotina tabacum)幼苗绝大部分Cd-PCs都被转移至液泡储存(Huang et al., 2012);美国商陆(Phytolacca Americana)和水蚀香蒲(Typha angusti-folia)吸收的Cd主要累积在液泡(Fu et al., 2011; Xu et al., 2011),阻止Cd在胞质溶胶中自由循环,降低Cd对细胞质基质及细胞器的毒害。因此,研究Cd在植物体内的亚细胞分布特征,有助于了解植物对Cd的耐受和解毒机制。
秋华柳(Salix variegata)是柳属多年生灌木,生长快,生物量大,在修复Cd污染土壤方面具有广阔的应用前景(贾中民等, 2012)。秋华柳对Cd具有较强的富集能力和生理适应性(贾中民等, 2011;孙晓灿等, 2012),但其耐受解毒机制尚不明确。鉴于此,本文采用水培方式,对秋华柳幼苗进行梯度浓度Cd处理,研究Cd在秋华柳各器官的积累特征,分析秋华柳叶片中Cd的亚细胞分布,从器官和亚细胞水平上探讨秋华柳的耐受解毒机制,以期为Cd污染地区秋华柳在植物修复中的应用提供参考依据。
1 材料与方法 1.1 试验材料2015年3月,在重庆北碚嘉陵江同兴街段河岸(29°41′2″N,106°26′56″E)剪取长15~17 cm,茎径0.7~1 cm的秋华柳插条,带回实验室用超纯水冲洗干净,固定后插入1/2 Hoagland改良营养液中于光照培养箱中培养,每盆5株,每3日更换营养液1次。光照培养箱参数设定:光照/无光照时间16 h/8 h,光照度10 000/0 Lux,温度25 ℃/20 ℃,相对湿度60%。
1.2 试验设计待扦插苗生根发芽长成完整植株后(约为55天,萌条长约40 cm),于2015年8月选取生长基本一致的秋华柳幼苗进行随机分组,以CdCl2·2.5H2O的形式加入Cd。设置5个Cd2+浓度:CK(0 mg·L-1)、T1(2 mg·L-1)、T2(10 mg·L-1)、T3(20 mg·L-1)、T4(50 mg·L-1),每个处理4个重复。分别在处理后0、6、12、18天取秋华柳叶片测量其亚细胞Cd含量,处理后18天测量秋华柳各器官Cd含量。
1.3 试验方法 1.3.1 亚细胞组分的分离测定每次采集秋华柳叶片后,用超纯水冲洗3次,并擦拭干净,放入-80 ℃超低温冰箱保存。采用差速离心法分离秋华柳叶片不同亚细胞组分,分别得到细胞壁、细胞器及细胞质组分(Weigel et al., 1980)。各亚细胞组分经消解定容后,用电感耦合等离子体发射光谱仪(ICP-OES, Thermo Fisher iCAP 6300, UK)测定Cd含量。
1.3.2 各器官Cd含量的测定收取植物全株,用2 mol·L-1 EDTA-Na2浸泡5 min,去除植株表面的Cd2+。将植株分离成根、茎和叶3部分,烘干至恒质量。取根、茎、叶样0.050 0 g,消解定容后用电感耦合等离子体发射光谱仪(ICP-OES, Thermo Fisher iCAP 6300, UK)测定Cd含量。
1.4 数据分析利用SPSS 20.0进行数据统计分析。用单因素方差(One-way ANOVA)及Duncan(Duncan’s multiple range test)多重比较分析不同浓度下各器官Cd含量的差异,用重复度量分析不同处理时间、不同浓度Cd胁迫及二者交互效应对秋华柳叶片中亚细胞Cd含量的影响,采用Origin 8.5作图。
2 结果与分析 2.1 秋华柳各器官对Cd的积累特征由图 1可知,随着Cd处理浓度的增加,秋华柳根、茎、叶中的Cd含量逐渐增加,且各浓度处理组Cd含量均显著高于对照(P<0.05)。不同浓度Cd处理下,秋华柳各器官积累的Cd均为根>茎>叶,其中,根部积累的Cd含量远高于茎和叶。
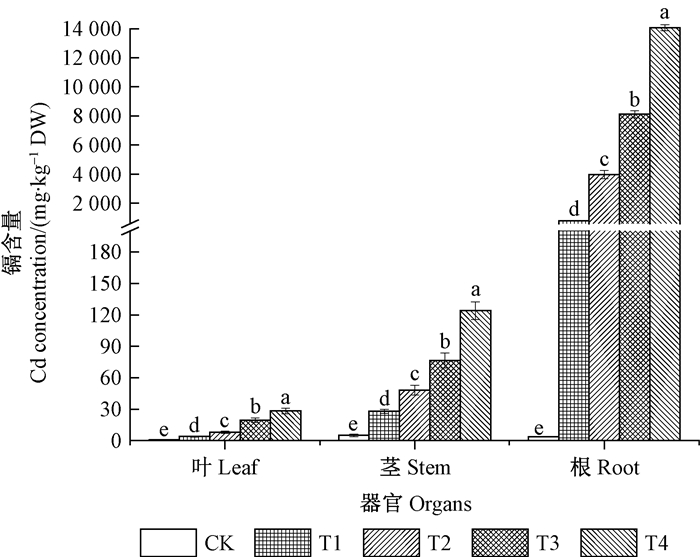
|
图 1 秋华柳各器官的Cd含量 Figure 1 Cd concentration in organs of Salix variegate CK:0 mg·L-1 Cd2+;T1:2 mg·L-1 Cd2+;T2:10 mg·L-1 Cd2+;T3:20 mg·L-1 Cd2+;T4:50 mg·L-1 Cd2+。图中结果表示为平均值±标准误(n=4),不同小写字母表示秋华柳同种器官在不同浓度下差异显著(P<0.05),下同。 Data were shown means ± standard error (n=4). Different letters meant significant difference among treatments in same organs of S.variegate at 0.05 level. The same below. |
表 1为不同处理浓度和时间及其交互作用对秋华柳叶片中亚细胞组分Cd含量的影响。由表 1可知,不同处理浓度和时间及其交互效应均对秋华柳叶片中细胞壁结合的Cd具有极显著性影响(P<0.01)。由图 2可知,相同时间处理下,除了6天时,细胞壁结合的Cd在T2组显著高于对照外,其他时间细胞壁结合的Cd即在T1组显著高于对照。随着处理时间的延长,4个浓度组细胞壁结合的Cd均增加,其中T4组增加最显著,与0天相比,T4组6、12和18天细胞壁的Cd含量分别增加了16.14%、95.93%和99.21%,其中6~12天的增加幅度较大,12~18天的增加幅度较小。18天时,各浓度处理组细胞壁结合的Cd达到最大,与0天相比,增幅分别为6.16%、18.21%、49.47%和72.84%。
|
|
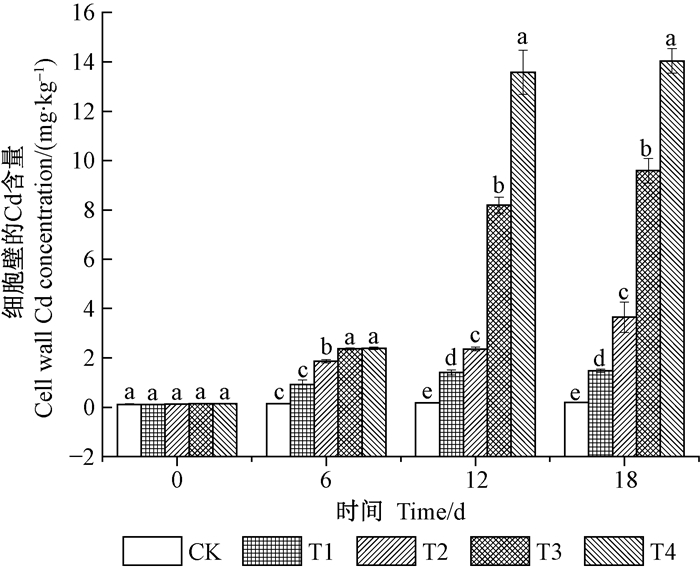
|
图 2 秋华柳叶片中细胞壁的Cd含量 Figure 2 Cell wall Cd concentrationin in leaf of S.variegate |
不同处理浓度和时间及其交互效应均对秋华柳叶片中细胞质的Cd含量具有极显著性影响(P<0.01)(表 1)。由图 3可知,相同时间处理下,细胞质的Cd含量随着处理浓度的增加而增加。6天时,T4组细胞质的Cd含量显著高于对照;12天时,T3组细胞质的Cd含量即显著高于对照;18天时,4个浓度处理组细胞质的Cd含量均显著高于对照组。12天和18天时,T3和T4组的Cd含量明显高于0天和6天。随着处理时间的延长,T4组细胞质中Cd含量的增幅最大,与0天相比,分别增加了2.63%、49.05%和48.58%。同一时间不同Cd浓度或同一Cd浓度不同时间处理时,秋华柳叶片中细胞质的Cd含量及增幅均小于细胞壁。

|
图 3 秋华柳叶片中细胞质的Cd含量 Figure 3 Cytoplasm supernatant Cd concentrationin in leaf of S.variegate |
不同处理浓度和时间及其交互效应均对秋华柳叶片中细胞器的Cd含量具有极显著性影响(P<0.01)(表 1)。相同时间处理下,与CK相比,细胞器的Cd含量在T1组无显著差异,在T2组即显著升高,其中18天时增幅最大,分别增加了1.38%、4.54%、7.62%和13.23%,但增幅远低于细胞壁和细胞质。随着处理时间的延长,与0天相比,各浓度处理组中细胞器的Cd含量均增加,其中T3和T4的增幅最大,但其增幅远低于细胞壁和细胞质(图 4)。

|
图 4 秋华柳叶片中细胞器的Cd含量 Figure 4 Cell organelle Cd concentrationin in leaf of S.variegate |
由图 5可知,Cd处理前(0天时),秋华柳叶片中亚细胞组分的Cd含量比为细胞质>细胞壁>细胞器。在Cd胁迫下,细胞壁组分中的Cd含量迅速增加,呈现出细胞壁>细胞质>细胞器的趋势。同一时间不同Cd处理浓度下,细胞器组分中的Cd含量百分比随着Cd处理浓度的增加而减少,细胞壁和细胞质之和反之,其中细胞质组分中的Cd含量百分比先减少后增加,而细胞壁组分中的Cd含量百分比先增加后减小。在高浓度时,细胞壁组分中Cd含量有所下降,但所占比例仍保持最高。
随着处理时间的延长,秋华柳细胞质组分中的Cd含量百分比上升,而细胞壁组分中的Cd含量百分比下降,但各处理组细胞壁组分中的Cd含量所占比重最大。

|
图 5 秋华柳叶片中亚细胞组分的Cd含量分配比例 Figure 5 Distribution percentages of Cd among different subcellular fractions in leaf of S.variegate |
植物的耐受解毒能力主要与植物对Cd的积累和运输,Cd在细胞中的分配及植物体内Cd的结合形态等因素密切相关(杨居荣等, 1993)。本研究中,秋华柳根、茎、叶中的Cd含量随Cd浓度的增加显著增加,且不同浓度Cd处理下,秋华柳根部积累的Cd含量远高于茎和叶(图 1)。在器官水平上,秋华柳通过根部显著的Cd滞留效应限制其向地上部分转运,从而降低Cd对秋华柳地上部分细胞器的毒害,增强秋华柳对Cd的耐受性。
在细胞水平上,植物的细胞壁是Cd进入细胞内的第一道屏障,也是Cd贮藏的第一个场所(王爱霞等, 2011)。细胞壁由果胶和纤维素组成,其大量的多糖分子(同型半乳糖醛酸聚糖(homogalacturonans, HGA)、鼠李糖半乳糖醛酸聚糖Ⅰ(rhamnogalacturonan, Ⅰ)、鼠李糖半乳糖醛酸聚糖Ⅱ(rhamnogalacturonan, Ⅱ)、木糖聚半乳糖醛酸(xylogalacturonan)等)含有羟基、羧基、醛基和磷酸基等亲金属离子的配位基团(Haynes, 1980),可以与Cd离子结合,使Cd沉淀在细胞壁,阻止Cd进入到植物细胞的原生质,减少其对细胞核心组件细胞器的损害。进入到植物原生质体中的Cd若以自由离子状态存在,会对细胞产生毒害作用,干扰细胞正常代谢(Gallego et al., 2012)。液泡主要参与细胞的水分代谢,同时也是植物细胞代谢废物囤积的场所(陈亚慧等, 2014),在植物原生质体中的Cd离子一般会与植物螯合肽(phytochelatins, PCs)或者金属硫蛋白(metallothioneins, MTs)的巯基结合,形成一种低分子量的复合物(LMW)(Vogeli-Lange et al., 1996; Ortiz et al., 1992),通过依赖于ATP的载体蛋白逆浓度进入液泡。如果原生质体中仍存在自由的Cd离子(Salt et al., 1995),则可能通过Cd2+/H+反运输的方式进入液泡(Salt et al., 1993),与液泡中多种有机酸、蛋白质或糖类结合成稳定的化合物贮存起来(周守标等, 2008),这样液泡将Cd限制在一个代谢不活跃的区域,使Cd离子无法在胞质溶胶中自由循环,降低Cd对细胞质基质及细胞器的损害。有研究表明,植物在胁迫下将大量的Cd储藏于细胞壁,仅有部分进入细胞内(Liu et al., 2007; Vazquez et al., 2007; Wierzbicka et al., 2007; Zhou et al., 2010; Ye et al., 2012),或植物将吸收的Cd大部分储存于液泡内(Lozano et al., 1997; Shi et al., 2005)。本研究的结果表明,秋华柳叶片中亚细胞内同时存在上述2种Cd累积特征,但细胞壁对Cd的结合和固定作用占主导。
本研究中,相同时间处理下,秋华柳叶片中各组分的Cd含量均随着Cd处理浓度的增加而增加,但细胞器组分中Cd含量的增幅远小于细胞壁和细胞质组分的增幅,且细胞器组分中Cd含量的百分比随着Cd处理浓度的增加而减少,这种亚细胞组分的分配方式避免了Cd对秋华柳叶片中功能重要的细胞器的损伤。在T1和T2组时,细胞壁组分中的Cd含量即显著增加,而T3和T4组时,细胞质组分中Cd含量显著增加,且随着时间的延长,秋华柳细胞质组分中的Cd含量百分比上升,而细胞壁组分中的Cd含量百分比下降。这些变化与秋华柳的解毒和耐受机制密切相关。不同浓度或时间胁迫下,秋华柳同时启动细胞壁固定和液泡区室化的解毒机制,随着Cd处理浓度的增加或处理时间的延长,秋华柳叶片中的细胞器也受到了一定的胁迫。细胞器中,高尔基体最为敏感,最容易受到损害,而高尔基体一旦受到伤害会直接损伤新细胞壁的结构(鲁家米2006; Sawidis et al., 1995;刘延盛等, 2007),使其结合和固定Cd的能力有所下降。高Cd浓度可能占用了秋华柳细胞壁上大量的金属结合位点。因此,秋华柳细胞壁结合和固定的Cd有所下降,进入细胞内的Cd增加,液泡表现出显著的区室化来避免Cd的毒害。同一时间不同Cd处理浓度下,随着Cd浓度的增加,细胞质组分中的Cd含量百分比呈先减少后增加的趋势,细胞壁组分中的Cd含量百分比与之相反(图 5),这种现象类似于“闸门”(floodgate)模型(Perriguey et al., 2008),即当Cd浓度低于某一阈值时,随着浓度的增加,进入细胞内的Cd含量比例会降低,一旦超过阈值,进入细胞内的Cd含量比值会急剧增加。虽然高Cd浓度下秋华柳细胞壁组分中的Cd含量百分比有所下降,但所占比例仍保持最高,且不同时间胁迫下,各处理组细胞壁组分中的Cd含量百分比仍为最大,说明秋华柳细胞壁对Cd的结合和固定作用在其亚细胞的解毒中占主导地位。以后可进一步深入研究秋华柳叶片中细胞壁的果胶特异性多聚糖域结构,及与Cd结合的动力学机制,通过基因工程的手段提高细胞壁果胶有效多聚糖域成分,从而增强秋华柳对Cd的耐受性,提高其对Cd污染土壤的植物修复能力。
相同浓度不同时间处理对秋华柳各亚细胞组分中Cd含量的增加有显著性效应,但细胞壁和细胞质增加的幅度远大于细胞器,与相同时间不同浓度处理下秋华柳亚细胞组分中Cd含量的分布一致,都是秋华柳通过细胞壁固定和液泡区室化尽量避免Cd进入细胞器抑制细胞正常代谢的结果。尤其在T4浓度处理下,秋华柳细胞壁和细胞质组分中Cd含量随时间的增幅最大,说明时间和浓度的交互作用对秋华柳亚细胞组分中Cd含量的增加效应大于单一时间或浓度处理作用,在Cd处理浓度和处理时间的双重胁迫下,秋华柳细胞壁的固定作用和液泡的区室化作用更加显著。
4 结论Cd进入到秋华柳体内后,首先根系通过滞留效应限制Cd向地上部分转运,从而减少了Cd对代谢旺盛的叶片的毒害。在细胞水平上,通过细胞壁固定作用和液泡区室化作用将Cd限制在叶片的非代谢部位,其中以细胞壁的固定作用占主导地位,以确保秋华柳的核心细胞器免受Cd毒害。
陈亚慧, 刘晓宇, 王明新, 等. 2014. 蓖麻对镉的耐性、积累及与镉亚细胞分布的关系[J]. 环境科学学报, 34(9): 2440-2446. |
Chen Y H, Liu X Y, Wang M X, et al. 2014. Cadmium tolerance, accumulation and relationship with Cd subcellular distribution in Ricinus communis L[J]. Acta Scientiae Circumstantiae, 34(9): 2440-2446. |
董萌, 赵运林, 库文珍, 等. 2013. 蒌蒿对镉的富集特征及亚细胞分布特点[J]. 植物学报, 48(4): 381-388. |
Dong M, Zhao Y L, Ku W Z, et al. 2013. Cadmium accumulation and subcellular distribution in different organs of Artemisia selengensis[J]. Bulletin of Botany, 48(4): 381-388. |
韩超, 申海玉, 叶嘉, 等. 2014. 模拟镉污染对两个白菜品种生长、镉吸收累积及亚细胞分布的影响[J]. 北方园艺, (10): 9-12. |
Han C, Shen H Y, Ye J, et al. 2014. Effect of simulation of cadmium pollution on growth, cadmium accumulation and its subcellular distribution in two varieties of Brassica pekinensis Rupr[J]. Northern Horticulture, (10): 9-12. |
环境保护部, 国土资源部. 全国土壤污染状况调查公报. (2014-04-18). http://www.mlr.gov.cn/xwdt/jrxw/201404/P020140417573876167417.pdf. (Ministry of Land and Resources, Ministry of Environmental Protection. Survey Bulletin of National Soil Pollution. (2014-04-18). http://www.mlr.gov.cn/xwdt/jrxw/201404/P020140417573876167417.pdf.) |
贾中民, 程华, 魏虹, 等. 2012. 三峡库区岸生植物秋华柳对镉胁迫的光合响应[J]. 林业科学, 48(6): 152-158. DOI:10.11707/j.1001-7488.20120623 |
Jia Z M, Cheng H, Wei H, et al. 2012. Photosynthetic responses of the riparian Salix variegata to cadmium stress in Three Gorges Reservoir Region[J]. Scientia Silvae Sinicae, 48(6): 152-158. |
贾中民, 魏虹, 孙晓灿, 等. 2011. 秋华柳和枫杨幼苗对镉的积累和耐受性[J]. 生态学报, 31(1): 107-114. |
Jia Z M, Wei H, Sun X C, et al. 2011. Accumulation and tolerance of Salix variegate and Pterocarya stenoptera seedings to cadmium[J]. Acta Ecologica Sinica, 31(1): 107-114. |
李红婷, 董然. 2015. 2种萱草对铅、镉的吸收累积及其在亚细胞的分布和化学形态特征[J]. 华南农业大学学报, 36(4): 59-64. DOI:10.7671/j.issn.1001-411X.2015.04.011 |
Li H T, Dong R. 2015. Pb & Cd absorption and accumulation characteristics, subcellular distribution and chemical forms in two types of Hemerocallis plants[J]. Journal of South China Agricultural University, 36(4): 59-64. |
刘延盛, 鲁家米, 周晓阳. 2007. Pb在豌豆幼苗细胞中的超微结构分布与毒性研究[J]. 应用与环境生物学报, 13(5): 647-651. |
Liu Y S, Lu J M, Zhou X Y. 2007. Cellular damages and accumulation of Pbpolluted Pisumsativum L.:implication for phytoremediation[J]. Chinese Journal of Applied and Environmental Biology, 13(5): 647-651. |
鲁家米. 2006. Pb、Cd在刺槐细胞中的分布及毒害机制. 北京: 北京林业大学博士学位论文.
|
Lu J M. 2006. Distribution and toxic mechanisms of Pb, Cd in cells of Robinia pseudoacacia L. Beijing: PhD thesis of Beijing Forestry University. [in Chinese]
|
潘芳慧, 张晓玮, 王友保. 2018. 施肥对吊兰修复镉污染土壤及土壤酶海性的影响[J]. 水土保持学报, 32(2): 346-351. |
Pan F H, Zhang X W, Wang Y B. 2018. Effects of phosphate fertillzer on phytoremediation of Chlorophytum comosum and soil enzyme activity in Cd-contaminated soils[J]. Journal of Soil and Water Conservation, 32(3): 346-351. |
孙晓灿, 魏虹, 谢小红, 等. 2012. 水培条件下秋华柳对重金属Cd的富集特性及光合响应[J]. 环境科学研究, 25(2): 220-225. |
Sun X C, Wei H, Xie X H, et al. 2012. Bioaccumulation and photosynthesis response of Salix variegate to cadmium under hydroponic culture[J]. Reaearch of Environmental Sciences, 25(2): 220-225. |
王爱霞, 方炎明. 2011. 空气重金属元素在悬铃木叶中的亚细胞分布及其区隔化效应[J]. 西北植物学报, 31(3): 479-485. |
Wang A X, Fang Y M. 2011. Subcellular distribution and compartmentalization effects of heavy metal elements from atmosphere in Platanushis panica[J]. Acta Botanica Boreali-Occidentalia Sinica, 31(3): 479-485. |
杨居荣, 鲍子平. 1993. 镉, 铅在植物细胞内的分布及其可溶性结合形态[J]. 中国环境科学, 13(4): 263-268. |
Yang J R, Bao Z P. 1993. The distribution and binding of Cd and Pb in plant cell[J]. China Environmental Science, 13(4): 263-268. |
周守标, 徐礼生, 吴龙华, 等. 2008. 镉和锌在皖景天细胞内的分布及化学形态[J]. 应用生态学报, 19(11): 2515-2520. |
Zhou S B, Xu L S, Wu L H, et al. 2008. Subcellular distribution and chemical forms of Cd and Zn in Sedum jinianum[J]. Chinese Journal of Applied Ecology, 19(11): 2515-2520. |
Fu X P, Dou C M, Chen Y X, et al. 2011. Subcellular distribution and chemical forms of cadmium in Phytolacca Americana L[J]. Journal of Hazardous Materials, 186(1): 103-107. DOI:10.1016/j.jhazmat.2010.10.122 |
Giovanni D C, Silvia F, Antonella F. 2010. Regulatory networks of cadmium stress in plants[J]. Plant Signaling and Behavior, 5(6): 663-667. DOI:10.4161/psb.5.6.11425 |
Gallego S M, Pena L B, Barcia R A, et al. 2012. Unravelling cadmium toxicity and tolerance in plants:insight into regulatory mechanisms[J]. Environmental and Experimental Botany, 83(5): 33-46. |
Hall J L. 2002. Cellular mechanisms for heavy metal detoxification and tolerance[J]. Journal of Experimental Botany, 53(366): 1-11. |
He J Y, Zhu C, Ren Y F, et al. 2008. Uptake, subcellular distribution, and chemical forms of cadmium in wild-type and mutant rice[J]. Pedosphere, 18(3): 371-377. DOI:10.1016/S1002-0160(08)60027-2 |
Huang J, Zhang Y, Peng J S, et al. 2012. Fission yeast HMT1 lowers seed cadmium through phytochelatin-dependent vacuolar sequestration in Arabidopsis[J]. Plant Physiology, 158(4): 1779-1788. DOI:10.1104/pp.111.192872 |
Haynes R J. 1980. Ion exchange properties of roots and ionic interactions within the root apoplasm:their role in ion accumulation by plants[J]. The Botanical Review, 46(1): 75-99. DOI:10.1007/BF02860867 |
Kabata-Pendias A, Pendias H K. 1984. Trace elements in soils and plants. 1nd edition. Boca Raton, USA: Chemical Rubber Company, 951-974.
|
Lai H Y. 2015. Subcellular distribution and chemical forms of cadmium in Impatiens walleriana in relation to its phytoextraction potential[J]. Chemosphere, 138: 370-376. DOI:10.1016/j.chemosphere.2015.06.047 |
Leita L, De Nobili M, Cesco S, et al. 1996. Analysis of intercellular cadmium forms in roots and leaves of bush bean[J]. Journal of Plant Nutrition, 19(3/4): 527-533. |
Liu D, Kottke I, Adam D. 2007. Localization of cadmium in the root cells of Allium cepa by energy dispersive X-ray analysis[J]. Biologia Plantarum, 51(2): 363-366. DOI:10.1007/s10535-007-0075-z |
Lozano-Rodriguez E, Hernandez L E, Bonay P, et al. 1997. Distribution of cadmium in shoot and root tissues of maize and pea plants:Physiological disturbances[J]. Journal of Experimental Botany, 48(306): 123-128. |
Meharg A A. 2006. Integrated tolerance mechanisms:constitutive and adaptive plant responses to elevated metal concentrations in the environment[J]. Plant Cell and Environment, 17(9): 989-993. |
Mohammad A H, Pukclai P, Jaime A T S, et al. 2012. Molecular mechanism of heavy metal toxicity and tolerance in plants:central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation[J]. Journal of Botany. DOI:10.1155/2012/872875 |
Ortiz D F, Kreppel L, Speiser D M, et al. 1992. Heavy metal tolerance in the fission yeast requires an ATP-binding cassette-type vacuolar membrane transporter[J]. The EMBO Journal, 11(10): 3491-3499. |
Perriguey J, Sterckeman T, Morel J L. 2008. Effect of rhizosphere and plant-related factors on the cadmium uptake by maize (Zea mays L.)[J]. Environmental and Experimental Botany, 63(1/3): 333-341. |
Shi G R, Cai Q S, Liu C F, et al. 2010. Silicon alleviates cadmium toxicity in peanut plants in relation to cadmium distribution and stimulation of antioxidative enzymes[J]. Plant Growth Regulation, 61(1): 45-52. DOI:10.1007/s10725-010-9447-z |
Salt D E, Rauser W E. 1995. MgATP-dependent transport of phytochelatins across the tonoplast of oat roots[J]. Plant Physiology, 107(4): 1293-1301. DOI:10.1104/pp.107.4.1293 |
Salt D E, Wagner G J. 1993. Cadmium transport across tonoplast of vesicles from oat roots:evidence for a Cd2+/H+ antiport activity[J]. Journal of Biological Chemistry, 268(17): 12297-12302. |
Sawidis T, Reiss H D. 1995. Effects of heavy metals on pollen tube growth and ultrastructure[J]. Protoplasma, 185(3/4): 113-122. |
Shi X H, Zhang C C, Wang H, et al. 2005. Effect of Si on the distribution of Cd in rice seedlings[J]. Plant and Soil, 272(1/2): 53-60. |
Tong Y P, Kneer R, Zhu Y G. 2004. Vacuolar compartmentalization:a second-generation approach to engineering plants for phytoremediation[J]. Trends in Plant Science, 9(1): 7-9. DOI:10.1016/j.tplants.2003.11.009 |
Valérie P, Laure W, Urs F. 2006. Heavy metals in white lupin:uptake, root-to-shoot transfer and redistribution within the plant[J]. New Phytologist, 171(2): 329-341. DOI:10.1111/nph.2006.171.issue-2 |
Vázquez S, Goldsbrough P, Carpena R O. 2006. Assessing the relative contributions of phytochelatins and the cell wall to cadmium resistance in white lupin[J]. Physiologia Plantarum, 128(3): 487-495. DOI:10.1111/ppl.2006.128.issue-3 |
Vogeli-Lange R, Wagner G J. 1996. Relationship between cadmium, glutathione and cadmium-binding peptides (phytochelatins) in leaves of intact tobacco seedlings[J]. Plant Science, 114(1): 11-18. DOI:10.1016/0168-9452(95)04299-7 |
Vazquez S, Fernandez-Pascual M, Sanchez-Pardo B, et al. 2007. Subcellular compartmentalisation of cadmium in white lupins determined by energy-dispersive X-ray microanalysis[J]. Journal of Plant Physiology, 164(9): 1235-1238. DOI:10.1016/j.jplph.2006.11.011 |
Weigel H J, Jager H J. 1980. Subcellular distribution and chemical form of cadmium in bean plants[J]. Plant Physiology, 65(3): 480-482. DOI:10.1104/pp.65.3.480 |
Wierzbicka M H, Przedpelska E, Ruzik R, et al. 2007. Comparison of the toxicity and distribution of cadmium and lead in plant cells[J]. Protoplasma, 231(1/2): 99-111. |
Xu W F, Shi W M, Yan F, et al. 2011. Mechanisms of cadmium detoxification in cattail (Typha angustifolia L.)[J]. Aquatic Botany, 94(1): 37-43. DOI:10.1016/j.aquabot.2010.11.002 |
Ye J, Yan C L, Liu J C, et al. 2012. Effects of silicon on the distribution of cadmium compartmentation in root tips of Kandelia obovata (S. L.) Yong[J]. Environmental Pollution, 162(5): 369-373. |
Zhu Q H, Huang D Y, Liu S L, et al. 2013. Accumulation and subcellular distribution of cadmiumin ramie (Boehmeria nivea L. Gaud.) planted on elevated soil cadmium contents[J]. Plant Soil and Environment, 59(2): 57-61. DOI:10.17221/PSE |
Zhou Y Q, Huang S Z, Yu S L, et al. 2010. The physiological response and sub-cellular localization of lead and cadmium in Iris pseudacorus L[J]. Ecotoxicology, 19(1): 69-76. DOI:10.1007/s10646-009-0389-z |
 2018, Vol. 54
2018, Vol. 54

