文章信息
- 李威, 杨德光, 牟尧, 杨自超, 王雪蓉, 刘彤彤, 李淑敏
- Li Wei, Yang Deguang, Mu Yao, Yang Zichao, Wang Xuerong, Liu Tongtong, Li Shumin
- 去遮荫后东北红豆杉幼苗和幼树光合特性对比
- Photosynthesis and Chlorophyll Fluorescence Characteristics of Seedlings and Saplings of Taxus cuspidata After Removing Shade
- 林业科学, 2018, 54(2): 179-185.
- Scientia Silvae Sinicae, 2018, 54(2): 179-185.
- DOI: 10.11707/j.1001-7488.20180221
-
文章历史
- 收稿日期:2017-02-05
- 修回日期:2017-12-05
-
作者相关文章
2. 东北林业大学 林木遗传育种国家重点实验室 哈尔滨 150040;
3. 东北农业大学农学院 哈尔滨 150030
2. State Key Laboratory of Tree Genetics and Breeding, Northeast Forestry University Harbin 150040;
3. College of Agricultare, Northeast Agricultural Unirersity Harbin 150030
东北红豆杉(Taxus cuspidata)是第三纪孑遗树种,因资源稀少,被列为我国一级珍稀树种加以保护,是全世界公认的植物界“大熊猫”(刁云飞等,2016; 刘彤等,2009)。东北红豆杉为多年生常绿乔木除了可以作为观赏树种外,从其树皮中提取出的紫杉醇是一种有效的抗癌药物(Kobayashi et al., 1994)。调查研究发现,东北红豆杉幼苗受到自然强光照射后,其叶片会由绿色变成黄色,甚至死亡,因此,幼苗必须采用人工遮荫的方式进行培养,但幼树却可以生长在自然光照下(Iszkulo et al., 2006;Li et al., 2011)。这表明东北红豆杉幼苗和幼树在光合特性方面可能存在差异。
研究证实,叶片暴露于较强的光照下会对叶片的光合系统产生有害影响(Keller et al., 1976; Lassoie et al., 1983; Demmigadams et al., 1992; Mitchell et al., 1995)。植物在长期生存过程中可进化出一系列光合保护机制以应对强光对光合系统的破坏(黄秋娴等,2015),但如果将长期生长在弱光环境中的植物突然暴露在强光下,其能否适应这种强光环境取决于叶片的光合能力和光保护机制(Anderson et al., 1995; Givnish,1988; Lichtenthaler,1981;1985; Sarijeva et al., 2007)。东北红豆杉幼苗喜荫,而幼树可以生长在自然光照下,幼苗和幼树在光保护机制方面是否存在差异,目前还鲜有报道。鉴于此,本研究以生产在遮荫条件下的东北红豆杉幼苗和幼树为试验材料,去遮荫转自然光环境后测定并比较幼苗和幼树的光合特性,揭示人工培养东北红豆杉幼苗在强光下发生光抑制的原因,为东北红豆杉人工栽培提供理论依据。
1 材料与方法 1.1 试验设计试验在东北林业大学实验林场进行。东北红豆杉幼苗选择标准为树高低于0.5 m且DBH<5 cm,均为盆栽种子萌发。幼树选择标准为树高在1~2 m之间且DBH<5 cm。选取长势一致的10株3年生东北红豆杉幼苗(株高约25 cm,种植盆大小为25 cm×20 cm)和10株11年生东北红豆杉幼树(株高约155 cm,种植盆大小为60 cm×55 cm)为试验材料,在试验前均生长在遮荫网下,遮荫网距离地面约2 m,遮荫网内中午最大光强约800 μmol·m-2s-1,光照强度是自然光强的40%。
7月初,将幼苗和幼树去除遮荫,放置在自然无遮挡条件下,使其受自然光照射,中午最大光强约2 000 μmol·m-2s-1。幼苗和幼树充分适应自然光照条件28天后,测定幼苗和幼树叶片的光合特性和叶绿素荧光参数,灌水量以浇透为止,保证土壤水分充分供应。测量时,选取南侧、树冠层中间部位当年生枝叶进行测定。
1.2 光合作用参数测定采用LI-6400便携式光合仪(LI-COR, Lincoln, NE, USA)测定气体交换参数。光合速率-光强响应曲线的绘制(Pn-PFD,Pn-net photosynthetic rate, PFD-photon flux density)采用杨兴洪等(2005)的方法,CO2浓度为350 μmol·mol-1,用低于200 μmol·m-2s-1PFD下数据直线回归方程的斜率求得表观光量子效率(AQY),同时求得光补偿点。采用LI-6400便携式光合仪的可调式CO2供气系统,在1 400 μmol·m-2s-1PFD下,绘制光合速率-CO2响应曲线(Pn-CO2),用低于250 μmol·mol-1 CO2下数据直线回归方程的斜率求得羧化效率(CE),同时求得CO2补偿点。绘制诱导曲线作用数据为3次重复的平均值,数据测定时间均为上午8:00—10:00,3次重复。
在测定叶片光合诱导的前晚,将测定植株用黑色塑料袋遮光,测定当天上午8:00开始测定,设定光强为1 400 μmol·m-2s-1,诱导时间为60 min,每3 min记录1次光合值,3次重复。
1.3 叶绿素荧光参数测定荧光诱导曲线绘制参照杨兴洪等(2005)的方法。采用FMS-2脉冲调制式荧光仪(Hansatech, Norfolk, UK)测定绿素荧光各参数后,绘制荧光诱导曲线。叶片经过充分暗适应(30 min),在弱调制测量光下测定Fo,然后用饱和脉冲光照射0.8 s,测定Fm,打开光化学光,测定稳态Fs,每隔30 s照射1次饱和脉冲光,测定F'm,关闭光化学光,打开远红光,测定F'o,关闭远红光,打开光化学光,开始下一个循环。相关参数计算如下:实际光化学效率(ΦPSⅡ)=1-Fs/F'm,非光化学猝灭系数(NPQ)= (Fm -F'm)/Fm,光化学猝灭系数(qP)= (F'm-Fs)/(F'm-F'o),PSⅡ还原程度以1-qP表示,光化学反应速率(Prate)=实际光化学效率(ΦPSⅡ) ×光强(PFD)。每个曲线所用数据为3次重复的平均值。
1.4 光合色素测定光合及叶绿素荧光参数测定完成后,取测定植株叶片约0.2 g,利用HP扫描仪和ImageJ软件测量叶面积,采用宋洋等(2016)的方法提取色素。在常温、避光环境下,用95%乙醇(8 mL)浸提叶片至无色,用紫外分光光度计UV2800 (Hitachi, Japan)在检测波长665、649和470 nm处测定吸光值。每处理重复5次。
1.5 数据分析采用SPSS12.0软件的One-Way ANOVA功能分析幼苗和幼树叶绿素含量、光合速率和荧光参数的差异。
2 结果与分析 2.1 幼苗和幼树叶片叶绿素含量比较幼树叶片叶绿素a(Chl a)和总叶绿素[Chl(a+b)]含量高于幼苗叶片,幼苗叶片叶绿素b(Chl b)含量高于幼树叶片,但3项指标二者之间均无显著性差异(P>0.05)。幼树叶片类胡萝卜素(Cars)含量显著高于幼苗叶片(P<0.05)。幼苗和幼树叶片的Chl a/b之间无显著差异,但其Chl/Cars之间差异显著(P<0.05),且幼苗的Chl/Cars较高(表 1)。
|
|
Pn-PFD响应曲线(图 1)表明,随着光强增加,幼树和幼苗叶片光合速率也随之增加,但光强达到一定程度后,光合速率不再升高,保持稳定。其中,幼树的光补偿点(LCP)、光饱和点(LSP)及达到饱和光强时的光合速率(Pn)显著高于幼苗(P<0.05),而幼苗叶片具有较高的表观量子效率(表 2)。
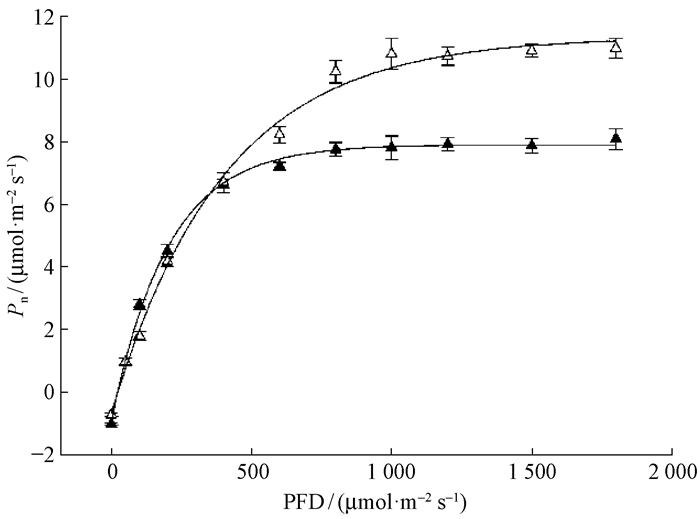
|
图 1 东北红豆杉幼苗和幼树叶片的Pn-PFD响应曲线 Figure 1 Light response of photosynthesis in seedling and sapling leaves of Taxus cuspidata ▲和△分别代表幼苗和幼树叶片。下同。△ and ▲ represent seedling leaves and sapling leaves, respectively. The same below. |
|
|
Pn-CO2响应曲线(图 2)表明,在饱和光强下,随着CO2浓度增加,幼树和幼苗叶片光合速率也随之增加,CO2浓度达到一定程度后,光合速率不再升高,保持稳定。其中,幼树的CO2补偿点(τ)、CO2饱和点(CSP)、羧化效率(CE)以及光强和CO2达到饱和时的光合速率(Amax)显著高于幼苗(P<0.05)(表 2)。
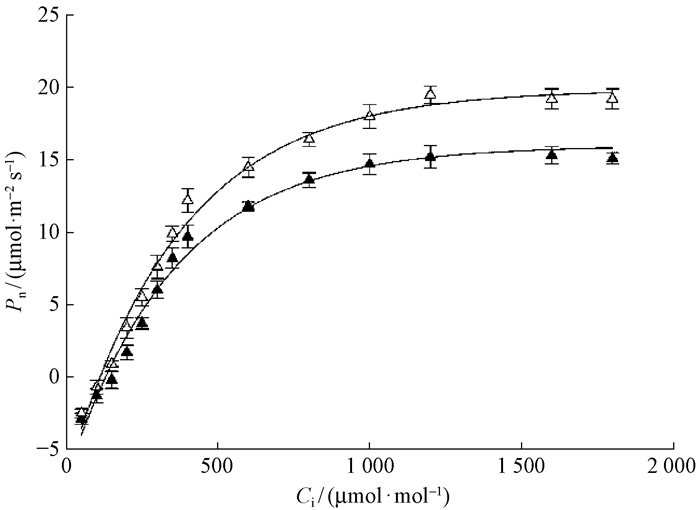
|
图 2 东北红豆杉幼苗和幼树叶片的Pn-CO2响应曲线 Figure 2 CO2 response of photosynthesis in seedling and sapling leaves of T.cuspidata |
对Pn-PFD响应曲线和Pn-CO2响应曲线参数进行对比见表 2,幼苗和幼树叶片的光合特征差异明显,这些参数均达到差异显著或极显著水平。
2.3 幼苗和幼树叶片对光合诱导过程的响应当幼苗和幼树叶片突然受到1 400 μmol·m-2s-1强光照射后,其光合诱导曲线表现出不同的适应过程。如图 3所示,幼苗和幼树叶片的光合速率随诱导时间增加逐渐升高,但幼树叶片的光合诱导过程明显较快,在光合诱导的前25 min,幼树叶片就达到了光合速率最大值12.3 μmol·m-2s-1,而幼苗叶片则需要约50 min才达到光合速率最大值8.3 μmol·m-2s-1。
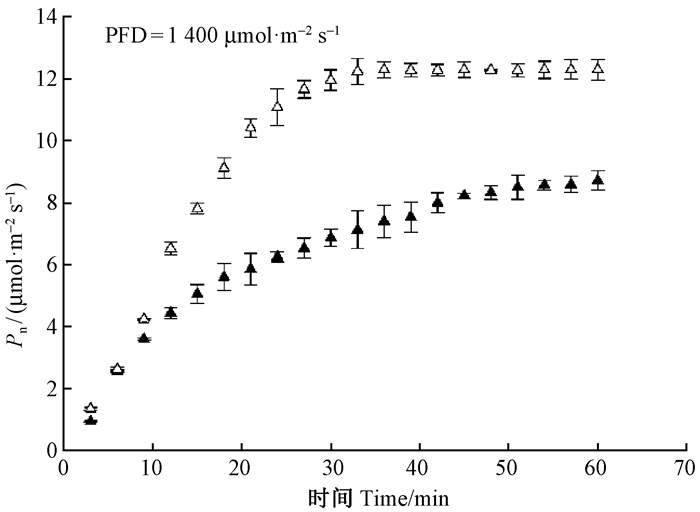
|
图 3 东北红豆杉幼苗和幼树叶片的光合诱导曲线 Figure 3 Time course of the photosynthetic rate in seedling and sapling leaves of T.cuspidata PFD=1 400μmol·m-2s-1. |
从图 4可以看出,幼苗和幼树叶片实际光化学效率(ΦPSⅡ,图 4A)和非光化学猝灭(NPQ,图 4B)诱导曲线有所差别。在PFD为2 050 μmol·m-2s-1的强光照条件下(叶片由黑暗突然转到强光),东北红豆杉幼苗和幼树叶片的ΦPSⅡ和NPQ都随着时间增加而增加,但幼树叶片的ΦPSⅡ和NPQ增加较快。在诱导曲线的终点,幼树和幼苗叶片的ΦPSⅡ和NPQ都达到最大值,但幼树的ΦPSⅡ和NPQ终点值比幼苗叶片大,且差异达到极显著水平(P<0.01),幼苗叶片的ΦPSⅡ和NPQ则在一定时间后趋于稳定。
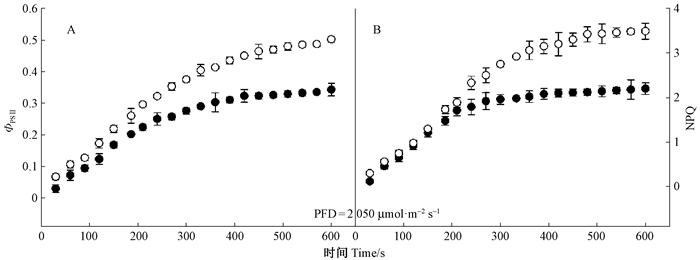
|
图 4 东北红豆杉幼苗和幼树叶片实际光化学效率(ΦPSⅡ)(A)和非光化学淬灭(NPQ)(B)诱导曲线 Figure 4 Time-course of ΦPSⅡ (A) and NPQ (B) in seedling and sapling leaves of Taxus cuspidata in high-light conditions (●)和(○)分别代表幼苗和幼树叶片。下同。(●) and(○)represent seedling leaves and sapling leaves, respectively.The same below. |
从图 5可以看出,幼苗和幼树叶片的实际光化学效率(ΦPSⅡ)随着光强增加而降低,但幼树叶片的ΦPSⅡ在曲线终点高于幼苗叶片,且差异达到极显著水平(P<0.01)。幼树叶片的NPQ随着光强的增加而增加,但幼苗叶片的NPQ在光强相对较低的时候就达到稳定状态,且幼苗叶片的NPQ相对较低。随着光强增加,还原态的PSⅡ也随之增加,但幼苗叶片的大部分反应中心在低光强下就表现出关闭状态,而幼树叶片的还原态PSⅡ相对较低,在曲线终点幼树和幼苗叶片的还原态PSⅡ差异达到极显著水平(P<0.01)。
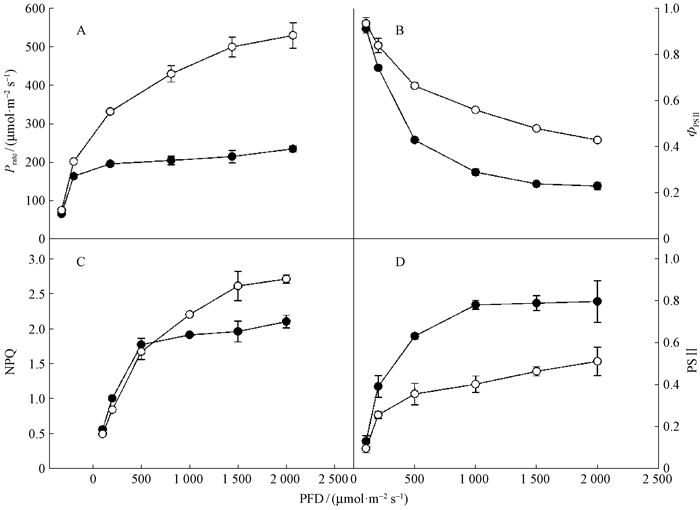
|
图 5 东北红豆杉幼苗和幼树叶片光化学反应速率(Prate)(A)、实际光化学效率(ΦPSⅡ)(B)、非光化学猝灭(NPQ)(C)及PSⅡ还原状态(PSⅡ reduction state)(D)响应曲线 Figure 5 Responses of PSⅡ photochemistry (A), ΦPSⅡ (B), NPQ (C) and the PSⅡ (D) reduction |
叶片的结构和功能受遗传因素控制,然而环境变化也可改变叶片结构和光合特性,使植物适应相应的环境(Anderson,1986)。叶绿素是光能吸收的主要色素,类胡萝卜素包括胡萝卜素和叶黄素,其中β-胡萝卜素能够猝灭三线态叶绿素(3Chl)和单线态氧(1O2),而叶黄素与非光化学淬灭直接相关(Hormaetxe et al., 2005)。因此,类胡萝卜素有猝灭过剩光能的作用,使植物免受光抑制和光破坏。本研究结果显示,与幼树相比,幼苗叶片的总叶绿素含量虽然较低,但是二者之间差异不显著,说明叶绿素含量并不是二者光合速率差异显著的主要原因。东北红豆杉幼苗叶片类胡萝卜素含量显著低于幼树叶片,且幼苗叶片的Chl/Cars显著高于幼树叶片,说明幼苗叶片对过剩光能淬灭能力较低,这可能是东北红豆杉幼苗不适应自然强光环境的主要因素之一,该结果与宋洋等(2016)结果一致。
光补偿点和光饱和点是区分阴生和阳生植物的重要指标,阴生植物一般具有较低的光补偿点和光饱和点(Albert et al., 2009)。本研究结果显示,幼苗叶片的光补偿点和光饱和点相对于幼树叶片低,表明幼苗叶片更适合弱光环境;同时,幼苗叶片在较低的光强下具有较高的AQY,说明幼苗利用弱光的能力较强,这与董志新等(2007)结果相似。Pn-CO2响应曲线的初始阶段受RuBp羧化效率(CE的限制,CE反映Rubisco的活性;第二阶段受RuBp再生限制受同化力ATP、NADPH制约,Amax被看成RuBp最大再生速率,因为其与光合电子传递相偶联又被当成电子最大传递速率(Anderson,1986; Lichtenthaler et al., 1981)。与幼树相比,幼苗叶片的Amax相对较低,这样就降低了其电子传递速率,形成的ATP较少,而Rubisco的活性主要受ATP供应的调节(Sims et al., 1998),使羧化效率降低(表 2),同时RuBP再生速率也相对较低。光饱和点也反映了光合系统对同化力的需求量(Yang et al., 2001),幼苗叶片光和系统对同化力的需求量较少,可能是由于光反应阶段同化力产生较少的结果。因此,幼苗叶片光合速率较低可能是因为相对较低的同化力产生和较低的羧化效率造成的。
叶绿素荧光可以作为光合作用的有效探针(万盼等,2016)。幼苗和幼树的实际光化学效率(ΦPSⅡ)和非光化学猝灭(NPQ)诱导曲线与相应的光合速率诱导过程有关,同时也与依赖于叶黄素循环的热耗散有关(孙山等,2009)。由于幼树叶片在持续强光下的光合速率诱导过程较快,即在较短时间内就能达到光合速率最大值,所以幼树叶片能够相对较快地启动卡尔文循环来消耗由光反应产生的同化力,这使得光反应的作用中心很快打开,使ΦPSⅡ快速升高。植物在强光下都有过剩光能的产生,植物叶片可以通过热耗散机制减轻过剩光能的伤害(李清雪等,2016)。持续的强光照射,使得幼苗和幼树叶片的NPQ升高,但二者在热耗散能力的大小上存在差异,幼树叶片NPQ最大值显著高于幼苗叶片(P<0.01),表明幼苗叶片通过依赖叶黄素循环耗散过剩光能的能力高于幼苗叶片。当幼苗叶片生长在自然强光环境时,由于光合速率相对较低,幼苗叶片会产生较多的过剩光能,而其依赖于叶黄素循环的热耗散又相对较少,所以幼苗叶片中的过剩光能要多于幼树叶片。随着光强增加,幼苗和幼树叶片的实际光化学效率(ΦPSⅡ)都在降低,但幼树叶片降低幅度较小。ΦPSⅡ降低主要是因为光反应中心的关闭或者激发能从其他途径的耗散(杨兴洪等,2005)。幼树叶片的NPQ随着光强增加而升高,达到相对较高的水平,而幼苗叶片的NPQ在较低的光强下就能达到其最大值,同时,幼苗叶片NPQ最大值远低于幼树叶片。还原态的PSⅡ反映了光反应中心的关闭程度,幼苗叶片在较低光强下多数反应中心处于关闭状态,而幼树叶片在强光下仍有多数反应中心处于开发状态。所以,幼苗ΦPSⅡ降低主要是反应中心关闭的结果,幼树ΦPSⅡ降低主要是热耗散增加的结果,因此,幼苗叶片反应中心更适应弱光环境。
4 结论东北红豆杉幼苗叶片具有较低的光合速率、较低的羧化效率和较低的RuBP再生速率,使得幼苗叶片在强光下产生相对较多的过剩能量,同时在强光下其光化学效率和热耗散机制也相对较低,这些是幼苗叶片适应弱光环境的光合特性,表明与幼树叶片相比,幼苗叶片的光合系统还不够完善,同时表明叶片的光合特性可能与树龄有关。如果把幼苗短时间放在自然强光下,会造成幼苗叶片光抑制的产生,而长时间的强光照射,则可能发生光破坏,如何提高幼苗叶片的光合速率,完善其光保护机制,需要进一步探讨。
刁云飞, 金光泽, 田松岩, 等. 2016. 黑龙江省穆棱东北红豆杉林物种组成与群落结构[J]. 林业科学, 52(5): 26-36. (Diao Y F, Jin G Z, Tian S Y, et al. 2016. Species composition and community structure of a Taxus cuspidata forest in Muling nature reserve of Heilongjiang province, China[J]. Scientia Silvae Sinicae, 52(5): 26-36. [in Chinese]) |
董志新, 韩清芳, 贾志宽, 等. 2007. 不同苜蓿(Medicago sativa L.)品种光合速率对光和CO2浓度的响应特征[J]. 生态学报, 27(6): 2272-2278. (Dong Z X, Han Q F, Jia Z K, et al. 2007. Photosynthesis rate in response to light and CO2concentration in different alfalfa varieties[J]. Acta Ecologica Sinica, 27(6): 2272-2278. [in Chinese]) |
黄秋娴, 赵顺, 刘春梅, 等. 2015. 遮荫处理对铁尾矿基质臭柏实生苗快速叶绿素荧光特性的影响[J]. 林业科学, 51(6): 17-26. (Huang Q X, Zhao S, Liu C M, et al. 2015. Effects of shading treatments on chlorophyll fluorescence characteristics of Sabina vulgaris seedlings grown in iron tailings media[J]. Scientia Silvae Sinicae, 51(6): 17-26. [in Chinese]) |
李清雪, 兰岚, 贾志清, 等. 2016. 4种锦鸡儿属植物幼苗叶绿素荧光参数对重复低温胁迫的响应[J]. 林业科学, 52(10): 31-37. (Li Q X, Lan L, Jia Z Q, et al. 2016. Response of chlorophyll fluorescence characteristics of seedlings of four caragana species to repeated low temperature stresses[J]. Scientia Silvae Sinicae, 52(10): 31-37. DOI:10.11707/j.1001-7488.20161004 [in Chinese]) |
刘彤, 胡林林, 郑红, 等. 2009. 天然东北红豆杉土壤种子库研究[J]. 生态学报, 29(4): 1870-1876. (Liu T, Hu L L, Zheng H, et al. 2009. Researches on soil seed bank of Japanese yew[J]. Acta Ecologica Sinica, 29(4): 1870-1876. [in Chinese]) |
宋洋, 廖亮, 刘涛, 等. 2016. 不同遮荫水平下香榧苗期光合作用及氮分配的响应机制[J]. 林业科学, 52(5): 55-63. (Song Y, Liao L, Liu T, et al. 2016. Response of photosynthesis and nitrogen distribution of Torreya grandis 'Merrilli' seedlings in different light regimes[J]. Scientia Silvae Sinicae, 52(5): 55-63. [in Chinese]) |
孙山, 张立涛, 杨兴华, 等. 2009. 板栗幼叶展叶过程的反射光谱和叶绿素荧光动力学[J]. 林业科学, 45(4): 162-166. (Sun S, Zhang L T, Yang X H, et al. 2009. Spectral reflectance and chlorophyll fluorescence kinetics of young leaves at the various stages of leaf expansion in field2grown chestnut plants[J]. Scientia Silvae Sinicae, 45(4): 162-166. DOI:10.11707/j.1001-7488.20090427 [in Chinese]) |
杨兴洪, 邹琦, 赵世杰. 2005. 遮荫和全光下生长的棉花光合作用和叶绿素荧光特征[J]. 植物生态学报, 29(1): 8-15. (Yang X H, Zou Q, Zhao S J, et al. 2005. Photosynthetic characteristics and chlorophyll fluorescence in leaves of cotton plants grown in full light and 40% sunlight[J]. Acta Phytoecologica Sinica, 29(1): 8-15. [in Chinese]) |
万盼, 熊兴政, 黄小辉, 等. 2016. 2种农药胁迫对油桐幼苗叶绿素荧光特性及生长的影响[J]. 林业科学, 52(7): 22-29. (Wan P, Xiong X Z, Huang X H, et al. 2016. Effects of pesticides stress on the chlorophyll fluorescence characteristics and growth of Vernicia fordii seedlings[J]. Scientia Silvae Sinicae, 52(7): 22-29. [in Chinese]) |
Albert N W, Lewis D H, Zhang H B, et al. 2009. Light-induced vegetative anthocyanin pigmentation in Petunia[J]. Journal of Experimental Botany, 60(7): 2191-2202. DOI:10.1093/jxb/erp097 |
Anderson J M, Chow W S, Park Y I. 1995. The grand design of photosynthesis:acclimation of the photosynthetic apparatus to environmental cues[J]. Photosynthesis Research, 46(1): 129-139. |
Anderson J M. 1986. Photoregulation of the composition, function, and structure of thylakoid membranes[J]. Annual Review of Plant Physiology, 37(1): 93-136. DOI:10.1146/annurev.pp.37.060186.000521 |
Demmigadams A B, Lü W W A. 1992. Photoprotection and other responses of plants to high light stress[J]. Annual Review of Plant Biology, 43(1): 599-626. DOI:10.1146/annurev.pp.43.060192.003123 |
Givnish T J. 1988. Adaptation to sun and shade:a whole-plant perspective[J]. Functional Plant Biology, 15(2): 63-92. |
Hormaetxe K, Becerril J M, Fleck I, et al. 2005. Functional role of red (retro)-carotenoids as passive light filters in the leaves of Buxus sempervirens L.:increased protection of photosynthetic tissues?[J]. J Exp Bot, 56(420): 2629-2636. |
Iszkulo G, Boratynski A. 2006. Analysis of the relationship between photosynthetic photon flux density and natural Taxus baccata seedlings occurrence[J]. Acta Oecol, 29(1): 78-84. DOI:10.1016/j.actao.2005.08.001 |
Keller R A, Tregunna E B. 1976. Effects of exposure on water relations and photosynthesis of western hemlock in habitat forms[J]. Canadian Journal of Forest Research, 6(1): 40-48. DOI:10.1139/x76-006 |
Kobayashi J, Ogiwara A, Hosoyama H, et al. 1994. Taxuspines A~C, new taxoids from Japanese yew (Taxus cuspidata) inhibiting drug transport activity of P-glycoprotein in multidrug-resistant cells[J]. Tetrahedron, 50(25): 7401-7416. DOI:10.1016/S0040-4020(01)90470-3 |
Lassoie J P, Dougherty P M, Reich P B, et al. 1983. Ecophysiological investigations of understory eastern redcedar in central Missouri[J]. Ecology, 64(6): 1355-1366. DOI:10.2307/1937490 |
Lichtenthaler H K. 1981. Adaptation of leaves and chloroplasts to high quanta fluence rates[J]. Photosynthesis, 6: 273-287. |
Lichtenthaler H K. 1985. Differences in morphology and chemical composition of leaves grown at different light intensities and qualities//Baker N R, Davies W J, Ong C K. First published. London: University of Cambridge, 201-221.
|
Lichtenthaler H K, Buschmann C, Döll M, et al. 1981. Photosynthetic activity, chloroplast ultrastructure, and leaf characteristics of high-light and low-light plants and of sun and shade leaves[J]. Photosynth Res, 2(2): 115-141. DOI:10.1007/BF00028752 |
Li W, Zhao Y S, Zhou Z Q. 2011. Difference in photoinhibition and photoprotection between seedings and saplings leaves of Taxus cuspidata under high irradiance[J]. African Journal of Microbiology Research, 5(32): 5978-5984. |
Mitchell A K, Arnott J T. 1995. Effects of shade on the morphology and physiology of amabilis fir and western hemlock seedlings[J]. New Forests, 10(1): 79-98. |
Sarijeva G, Knapp M, Lichtenthaler H K, et al. 2007. Differences in photosynthetic activity, chlorophyll and carotenoid levels, and in chlorophyll fluorescence parameters in green sun and shade leaves of Ginkgo and Fagus[J]. Journal of plant physiology, 164(7): 950-955. DOI:10.1016/j.jplph.2006.09.002 |
Sims D A, Seemann J R, Luo Y. 1998. The significance of differences in the mechanisms of photosynthetic acclimation to light, nitrogen, and CO2 for return on investment in leaves[J]. Functional Ecology, 12(2): 185-194. DOI:10.1046/j.1365-2435.1998.00194.x |
Yang X H, Zhou Q, Wang W. 2001. Photoinhibition in shaded cotton leaves after exposing to high light and the time course of its restoration[J]. Acta Botanica Sinica, 43(12): 1255-1259. |
 2018, Vol. 54
2018, Vol. 54

