文章信息
- 刘长英, 张梦, 魏从进, 许亚珍, 曹博宁, 郑莎, 范伟, 向仲怀, 赵爱春
- Liu Changying, Zhang Meng, Wei Congjin, Xu Yazhen, Cao Boning, Zheng Sha, Fan Wei, Xiang Zhonghuai, Zhao Aichun
- 转桑树MAPK5基因拟南芥在不同逆境胁迫下的抗性分析
- Resistance of Arabidopsis thaliana Transformed with Mulberry MAPK5 under Stress Conditions
- 林业科学, 2017, 53(9): 35-44.
- Scientia Silvae Sinicae, 2017, 53(9): 35-44.
- DOI: 10.11707/j.1001-7488.20170905
-
文章历史
- 收稿日期:2016-09-30
- 修回日期:2017-02-20
-
作者相关文章
促分裂原活化蛋白激酶(mitogen-activated protein kinases,MAPK)在植物响应逆境胁迫中发挥着重要的作用,可将细胞外的信号转入胞内,影响其下游基因的表达,从而调节生物体内各种生理反应,响应外界环境。植物中的MAPK基因最早在紫花苜蓿(Medicago sativa)中被鉴定,此后陆续在拟南芥(Arabidopsis thaliana)、烟草(Nicotiana tabacum)、水稻(Oryza sativa)等植物中被发现(Duerr et al., 1993;Mizoguchi et al., 1996;Zhang et al., 1998;He et al., 2000)。随着分子生物学和基因组测序的快速发展,MAPK基因家族的鉴定和功能研究已在多种单子叶和双子叶植物中报道。前人研究表明,在拟南芥MAPK级联中的AtMPK9和AtMPK12可调控干旱诱导的气孔运动(Fabien et al., 2009;Salam et al., 2013),MPK6在盐胁迫条件下参与调控磷脂酸的积累(Yu et al., 2010),过表达MKK4能够降低转基因拟南芥株系的水分损失率并提高其耐旱性(Tsugama et al., 2012)。拟南芥AtMEKK1、AtMKK2和AtMPK4/MPK6被发现可调控盐胁迫(Teige et al., 2004),主要通过直接磷酸化来激活盐胁迫响应基因(如SOS1、ZAT6、RD29A和RD29B等),从而提高植物的抗盐能力(Yu et al., 2010;Liu et al., 2012)。植物MAPK也能够响应温度胁迫。烟草细胞中的HAMK(heat-activated MAP kinase)可促使HSP70蛋白积累,调控对高温胁迫的适应(Suri et al., 2008)。拟南芥和玉米(Zea mays)中的MAPK能够被冷胁迫诱导(Ichimura et al., 2000; Pan et al., 2011),过表达玉米ZmMPK4提高了转基因烟草对低温胁迫的耐受性(Zhou et al., 2012)。此外,MAPK还参与了植物对氧化胁迫、机械损伤和重金属胁迫等非生物胁迫的响应(Liu et al., 2010;Lumbreras et al., 2010;Zhou et al., 2012)。尽管MAPK已在多种植物中被鉴定,但其在逆境胁迫中的功能主要在草本植物中被解析,在木本植物中还不清楚。
桑树(Morus)光合作用强、生长迅速,还具有对干旱、洪涝、盐碱害和冷害等逆境胁迫良好的抵抗能力,而对桑树的抗逆性机制的研究较少。在前期研究中鉴定了10个桑树MAPK基因,发现其能够被不同胁迫处理诱导表达,表明桑树MAPK在不同的胁迫处理中发挥着一定的作用(Wei et al., 2014)。为了探究MAPK在桑树非生物胁迫响应中的作用机制,本研究对川桑(Morus notabilis)MnMAPK5进行上游启动子活性分析,并探究其在拟南芥中过量表达对植株抗性的影响。
1 材料与方法 1.1 试验材料生态型拟南芥Columbia(西南大学罗克明教授惠赠)用于遗传转化;植物过表达载体pLGNL和pBI121由实验室保存;根癌农杆菌(Agrobacterium tumefaciens)菌株LBA4404(实验室保存)用于拟南芥的遗传转化。
1.2 亚细胞定位载体的构建依据MnMAPK5(GenBank登录号:KF683080) 和EGFP 基因序列信息及pLGNL载体上的多克隆位点,找到的合适的酶切位点有Kpn Ⅰ、BamH Ⅰ、Spe Ⅰ,并设计相应的引物。以川桑叶片的cDNA为模板进行PCR扩增MnMAPK5,经过T克隆验证后将MnMAPK5与EGFP一同连接到pLGNL载体上,并通过冻融法转入根癌农杆菌中。将含阳性质粒的农杆菌接种至YEB液体培养液中,培养至OD=0.8,然后用渗透培养基(MS+10 mmol·L-1 MgC12+ 100 mmol·L-1 AS+0.01% (W/V) MES)重悬菌体至OD600=0.8。使用重悬菌液侵染洋葱(Allium cepa)表皮细胞20 min后于16 h光照/8 h暗培养16~24 h。最后取出洋葱表皮用DAPI染色4 min,压片法制片,于荧光显微镜下观察并拍照。
1.3 MnMAPK5启动子的功能分析从川桑基因组数据库(http://morus.swu.edu.cn/morusdb/)中获取MnMAPK5的上游1 500 bp的序列作为启动子分析序列,使用PlantCARE(http://www.dna.affrc.go.jp/PLACE/)在线软件预测其顺式作用元件。
根据获得的MnMAPK5启动子序列设计引物(上游引物序列:5′-CCAAGCTTCTCAACTGTGTAG-3′;下游引物序列:5′-CGGGATCCTTTCTCCGATGA CTCTTTG-3′),并在上下游引物中分别加上酶切位点Hind Ⅲ和BamH Ⅰ,以川桑基因组为模板进行PCR扩增,采用1%的琼脂糖凝胶分离PCR产物,切下目的条带,使用胶回收试剂盒回收,最后克隆进入pMD19-T载体中并转入DH5α感受态细胞中,筛选阳性克隆并进行测序鉴定。将获得的含MnMAPK5启动子片段的T载体进行双酶切,并利用T4连接酶(Promega)将酶切产物连接到pBI121载体上。
将构建完毕的植物表达载体转入根癌农杆菌LBA4404中,侵染开花期的野生型拟南芥植株。用含有抗生素的MS培养基筛选收获的T0代拟南芥种子,并进行GUS染色和分子检测获得阳性植株。经过连续筛选得到的T3代种子用于后续试验。将种子在MS培养基中培养6天,再将其转入含有100 mmol·L-1 NaCl或30%PEG的MS培养基中处理0,1,3,6,9,12,24 h。将MS培养基中培养6天的拟南芥置于40 ℃和4 ℃环境下0,1,3,6,9,12,24 h。将处理后的材料进行GUS染色,并在解剖显微镜下进行拍照和记录。
1.4 MnMAPK5转基因拟南芥在不同胁迫下的功能为进一步研究MnMAPK5的功能,构建了MnMAPK5过表达的载体。根据报道的川桑MnMAPK5的CDS序列设计引物(表 1),并在上下游引物中分别加上酶切位点Kpn Ⅰ和BamH Ⅰ,以川桑叶片的cDNA为模板进行PCR扩增,目的片段经T克隆验证后连接到pLGNL载体上,并按照1.3中描述的方法获得T3代转基因拟南芥种子用于胁迫处理试验。将春化2天的野生型和转基因拟南芥消毒后均匀铺到含1/2 MS的培养基上进行不同胁迫下的种子萌发试验。将在正常MS培养基中培养5天的拟南芥种子进行胁迫处理2周后,对根长进行统计。将在正常MS中培养1周的幼苗转入营养土中继续培养,培养2周后进行胁迫处理;当野生型和转基因拟南芥的表型呈现明显差异时,拍照、记录,并测定其生理指标,包括丙二醛含量、可溶性糖含量、脯氨酸含量、叶绿素含量、过氧化氢含量、过氧化氢酶活性、过氧化物酶活性等指标。
|
|
参照RNA抽提试剂盒说明书(TaKaRa)提取总RNA,分别取适量RNA进行电泳检测,并用NANODROP 2000检测RNA的浓度和质量。以总RNA为模板,参照反转录试剂盒PrimeScript RT reagent Kit with gDNA Eraser(TaKaRa)说明书合成cDNA第1链,并使用以内参基因设计的引物检测是否有基因组污染。
参照TaKaRa公司的SYBR Premix Ex Taq TM Ⅱ Kits说明书进行实时荧光定量PCR分析,反应体系和运行程序参考作者此前的报道(Wei et al., 2014)。以MnRPL15为内参基因,使用2-ΔCt法计算MnMAPK5相对于内参基因MnRPL15的表达量,其相对表达量定义为2-[Ct(target gene)-Ct(control gene)]。使用Excel进行数据处理,使用SPSS16.0软件进行差异显著性分析。
2 结果与分析 2.1 MnMAPK5的亚细胞定位使用CELLO version 2.5在线软件预测MnMAPK5定位在细胞核和细胞质中;而使用cNLS Mapper和NucPred在线软件预测却发现MnMAPK5的核定位信号很弱。为确定MnMAPK5的亚细胞定位情况,构建了35S-MnMAPK5 ∷EGFP和35S-EGFP过表达载体(图 1A),并将其分别在洋葱表皮细胞中转染。在荧光显微镜下观察发现(图 1B),在转化了35S-MnMAPK5 ∷EGFP的表皮细胞的细胞核和细胞质有荧光信号,与转化了35S-EGFP的表皮细胞相似,表明MnMAPK5行使功能的场所可能在细胞质和细胞核中。
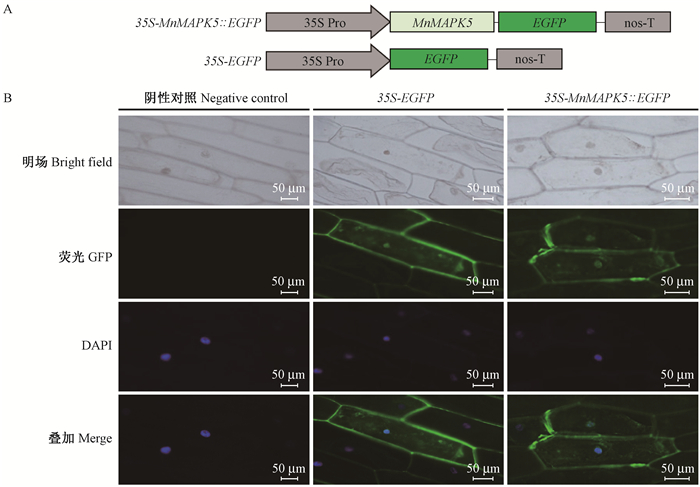
|
图 1 MnMAPK5在洋葱表皮细胞中的亚细胞定位 Fig.1 Subcellular localization of MnMAPK5 in onion epidermal cells A:35S-MnMAPK5∷EGFP和35S-EGFP的载体结构示意图;B:35S-EGFP和35S-MnMAPK5∷EGFP在洋葱表皮细胞的瞬时表达。 A: Structure diagram of the 35S-MnMAPK5∷EGFP and 35S-EGFP vectors; B: Transient expression of 35S-EGFP and 35S-MnMAPK5∷EGFP in onion epidermal cells. |
为研究MnMAPK5启动子的活性,从川桑基因组数据库中获得了MnMAPK5基因上游的的启动子序列,并进行了克隆。使用PlantCARE在线软件预测出MnMAPK5启动子序列中含多种顺式作用元件,主要包括胁迫(包括生物和非生物胁迫)相关元件、植物激素响应相关元件和植物生长发育相关元件3种类型,如ARE、HSE、LTR、MBS、AuxRR-core和CGTCA-motif等(图 2)。
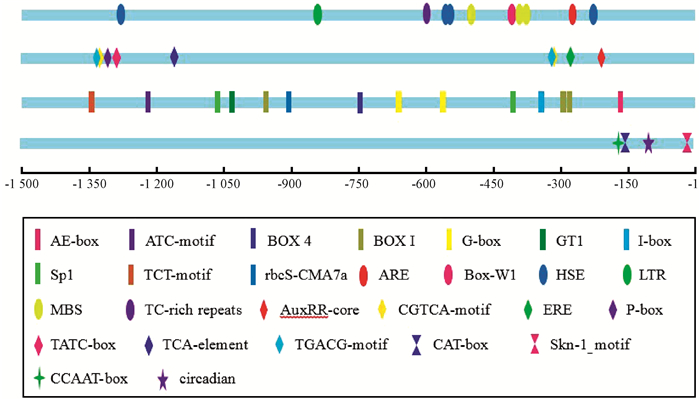
|
图 2 MnMAPK5上游启动子顺式作用元件分析 Fig.2 Analysis of cis-element in the 5′-upstream region of MnMAPK5 长方形表示光响应调控元件,椭圆表示生物和非生物胁迫响应调控元件,菱形表示激素响应调控元件,对三角表示生长发育相关调控元件。 The rectangle repesents light responsive element, the ellipse represents cis-acting element involved in stress responsiveness, the rhombus represents hormone responsive element, and the hourglass represents growth and development related cis-acting element. |
构建了MnMAPK5驱动GUS基因表达的转基因载体,并将其转化到拟南芥中获得T0代的种子,经过Kan抗性筛选和分子鉴定获得了转基因系。为检测MnMAPK5启动子在不同胁迫下的活性,将表达ProMnMAPK5-GUS的T3代转基因拟南芥在100 mmol·L-1 NaCl、40 ℃、4 ℃和30%PEG处理1,3,6,9,12,24 h后进行GUS染色,检测MnMAPK5启动子对各种胁迫的响应模式。如图 3所示,转基因幼苗经过高盐、高温、低温和干旱胁迫后呈现出GUS颜色,且在NaCl处理6 h、40 ℃处理9 h、4 ℃和30%PEG处理3 h后GUS颜色最深,说明MnMAPK5启动子能够响应不同的非生物胁迫。
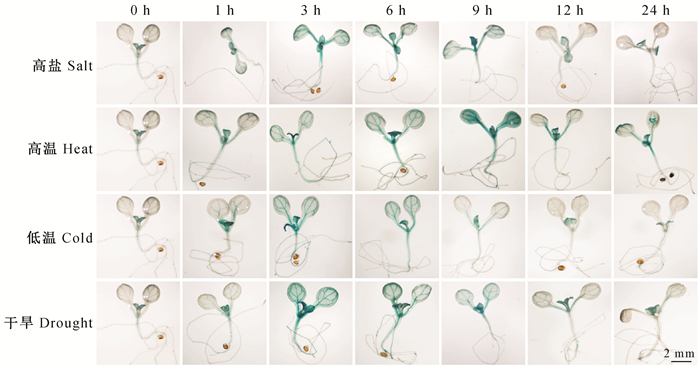
|
图 3 MnMAPK5启动子在拟南芥中驱动的GUS基因在胁迫诱导下的表达 Fig.3 MnMAPK5 promoter drived expression partterns of GUS in Arabidopsis thaliana |
为深入探究MnMAPK5在植物胁迫响应中的作用机制,将MnMAPK5基因转化进入拟南芥中,经卡那霉素筛选、GUS染色和分子检测获得5棵转基因株系,选取3株转基因株系(OE1、OE3和OE5) 研究过表达MnMAPK5对拟南芥抗性的影响(图 4)。在盐胁迫条件下,过表达MnMAPK5抑制了转基因拟南芥的萌发和生长,其叶片黄化程度比野生型拟南芥更严重。由此可见,过表达MnMAPK5降低了转基因拟南芥对盐胁迫的抗性。对NaCl胁迫后的生理指标测定发现,与野生型拟南芥相比,过表达MnMAPK5降低了转基因拟南芥中的H2O2含量、可溶性糖含量和CAT活性,提高了MDA含量和POD活性,脯氨酸含量与野生型拟南芥相比没有显著变化。此外,AtCAT和AtRD22基因在正常情况下表达无差异,经盐胁迫处理后在转基因拟南芥中的表达量显著低于野生型(图 5)。
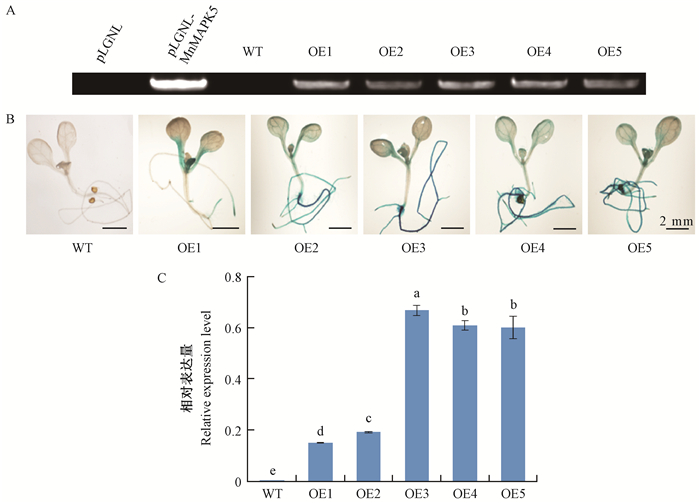
|
图 4 转基因拟南芥阳性检测 Fig.4 Determination of the transgenic Arabidopsis thaliana A:基因组PCR鉴定;B:GUS染色鉴定;C:实时荧光定量-PCR鉴定。WT:野生型拟南芥; OE:超表达株系。 A: PCR analysis using genomic DNA as the template; B: Histochemical GUS staining of transgenic lines; C: Quantitative real-time PCR analysis using cDNA as the template. WT: The wild A.thaliana plants; OE: The overexpressed transgenic plants. |
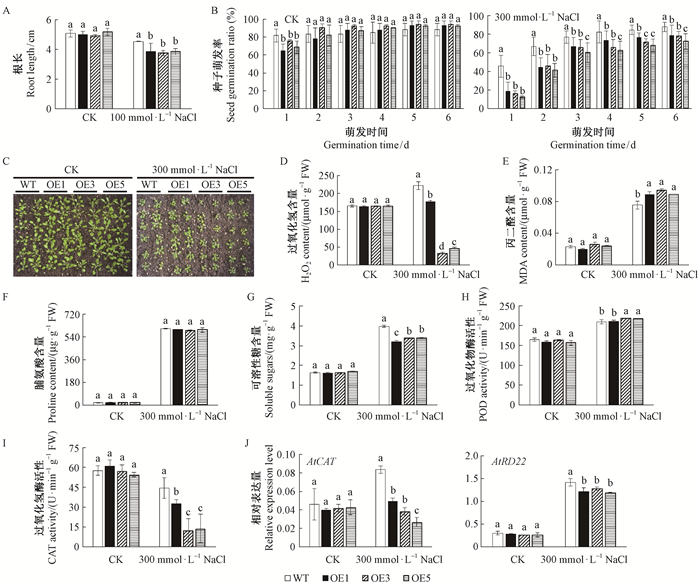
|
图 5 转基因拟南芥的抗盐性分析 Fig.5 Salt tolerance analysis of transgenic Arabidopsis thaliana C:盐胁迫下的生长状况;J:盐胁迫下胁迫相关基因的表达分析。WT:野生型拟南芥;OE:超表达株系。误差线表示标准偏差(SDs)。柱形图上的字母不同表示同一处理下不同株系有显著性差异(P < 0.05)。下同。 C: Growth under salt condition; J: The expression level of stress-related genes under salt stress. WT: The wild A.thaliana plants; OE: The overexpressed transgenic plants. Error bars on each column indicate SDs. Different letters above the bars indicate the significant difference of different lines under the same treatments at P < 0.05. The same below. |
使用PEG6000模拟干旱胁迫处理后发现,野生型拟南芥表现出优于转基因拟南芥的萌发率和生长情况。在营养生长阶段,相较于野生型拟南芥,转基因拟南芥经干旱处理后出现更为严重的萎蔫和干枯死亡现象。该结果表明过表达MnMAPK5提高了转基因拟南芥对干旱的敏感性。通过测定干旱胁迫后植株的生理指标发现,过表达MnMAPK5提高了转基因拟南芥中的H2O2含量、MDA含量,降低了脯氨酸含量、可溶性糖含量、POD活性和CAT活性。此外,AtPOD、AtCAT和AtMYB44基因的表达量在正常情况下无显著差异,经盐胁迫处理后在转基因拟南芥中的表达量显著低于野生型(图 6)。
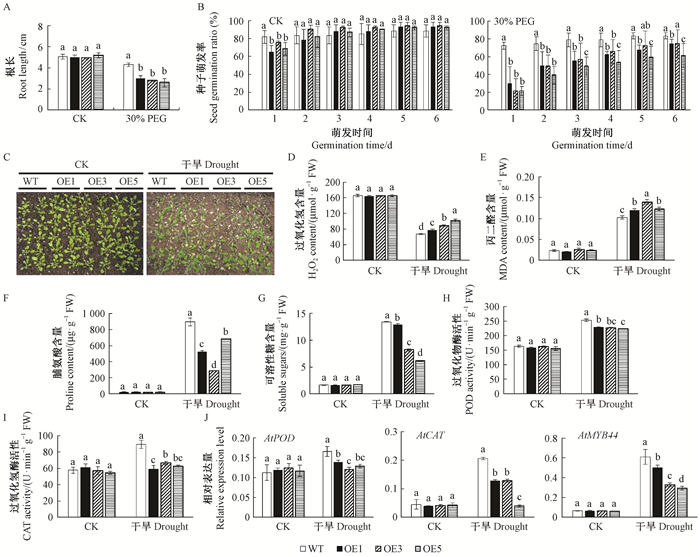
|
图 6 转基因拟南芥的抗旱性分析 Fig.6 Drought tolerance analysis of transgenic Arabidopsis thaliana C:干旱胁迫下的生长状况;J:干旱胁迫下胁迫相关基因的表达分析。 C: Growth under drought condition; J: The expression level of stress-related genes under drought stress. |
由于在4 ℃条件下,拟南芥种子不能在MS培养基中萌发,选择12 ℃作为低温胁迫检测种子的萌发率。12 ℃条件下,幼苗萌发后3天并未出现显著差异。此外,营养生长阶段的拟南芥在4 ℃胁迫处理下,转基因拟南芥出现更严重的萎蔫和死亡现象,叶绿素含量显著降低,表明过表达MnMAPK5提高了转基因拟南芥对冷胁迫的敏感性。通过测定冷胁迫下的生理指标及胁迫相关基因表达发现,过表达MnMAPK5降低了转基因拟南芥中的脯氨酸含量、可溶性糖含量、POD活性和CAT活性,AtPOD、AtCAT和AtRD22等胁迫相关基因的表达量都显著低于野生型拟南芥(图 7)。
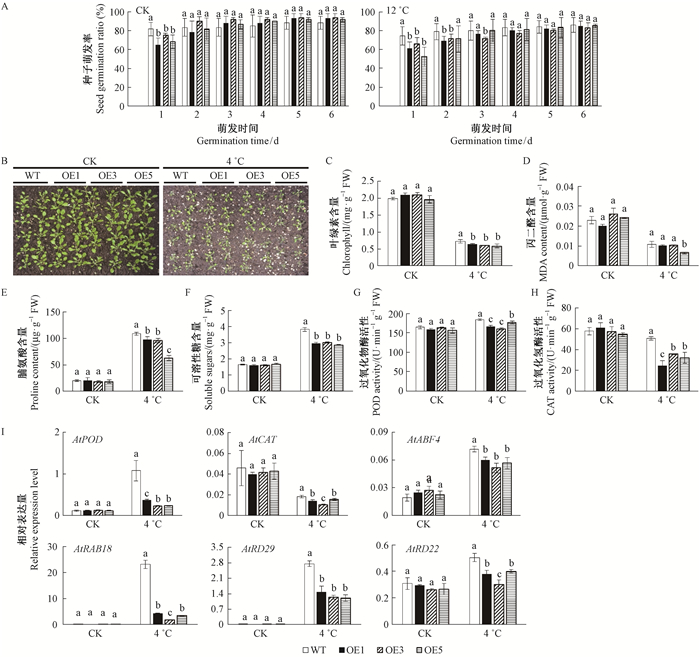
|
图 7 转基因拟南芥的抗冷胁迫分析 Fig.7 Cold tolerance analysis of transgenic Arabidopsis thaliana B:4 ℃下的生长状况;I:4 ℃下胁迫相关基因的表达分析。 B: Growth under 4 ℃ condition; I: The expression level of stress-related genes under 4 ℃ condition. |
氧化环境胁迫下,转基因拟南芥在H2O2处理后其萌发率和长势不如野生型拟南芥。对H2O2处理下的生理指标及胁迫相关基因表达测定发现,过表达MnMAPK5提高了转基因拟南芥的MDA含量,降低了脯氨酸含量和可溶性糖含量,AtPOD、AtCAT和AtABF4的表达量显著低于野生型拟南芥(图 8)。
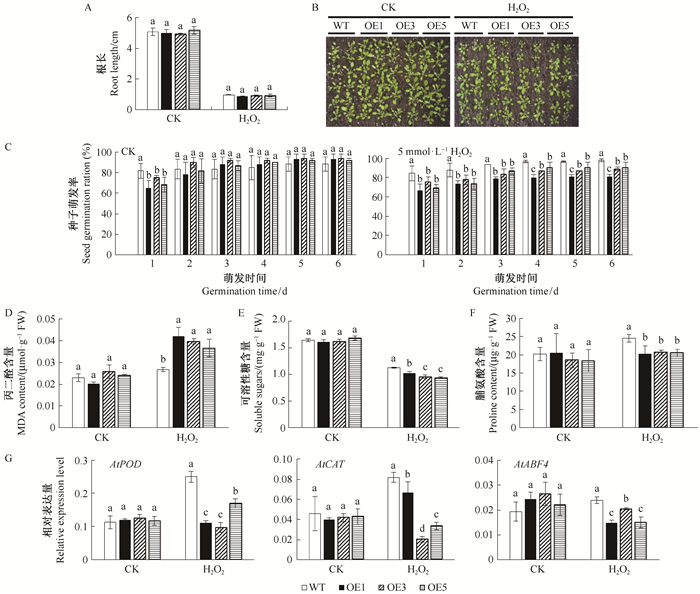
|
图 8 转基因拟南芥的抗H2O2胁迫分析 Fig.8 H2O2 tolerance analysis of transgenic Arabidopsis thaliana B:H2O2胁迫下的生长状况;G:H2O2胁迫下胁迫相关基因的表达分析。 B: Growth under drought condition; G: The expression level of stress-related genes under H2O2stress. |
MAPK作为MAPK级联途径中最下游的蛋白激酶,在植物适应逆境胁迫中发挥了极其重要的作用。根据植物MAPK的蛋白结构域特点和功能差异,可分为A、B、C和D等4个亚家族。目前对植物中MAPK的相关研究主要集中于A家族MAPK(Ichimura et al., 2002),拟南芥中该家族的MPK3和MPK6已被证明是介导植物防卫反应和非生物胁迫响应重要的调节元件(Beckers et al., 2009)。B家族MAPK在生物及非生物胁迫应答中也起着重要的作用。研究表明,过表达烟草NtMPK4可提高植株对ROS的抵抗能力(Kenji et al., 2005),AtMPK4可响应低温、干旱、机械损伤和渗透胁迫(Tomoyuki et al., 2014),过表达ZmSIMK1能够提高植物的抗旱性和系统获得抗病性(Wang et al., 2014)。但对B家族MAPK的功能还有待深入研究。本研究基于前期对川桑MAPK基因家族的分析,对MnMAPK5基因的功能进行进一步研究。通过对MnMAPK5的启动子序列预测发现其含有多种参与胁迫响应的顺式作用元件,MnMAPK5启动子驱动的GUS基因在拟南芥中的表达能够响应多种非生物胁迫处理,暗示MnMAPK5可能在非生物胁迫响应中发挥着一定的作用。
为研究MnMAPK5在植物抗逆境胁迫中的作用,将该基因转入拟南芥中进行了过表达分析。在盐胁迫、干旱、低温和H2O2处理条件下,转基因拟南芥的萌发和生长相比野生型受到了更严重的抑制,表明过表达MnMAPK5提高了转基因植株对非生物胁迫的敏感性,暗示该基因在植物抗逆中可能是一个负调控因子。通过对拟南芥的生理指标分析发现,在正常生长条件下,野生型和转基因拟南芥的MDA含量、脯氨酸含量和POD活性等指标没有显著差异,但在干旱胁迫下,过表达MnMAPK5提高了转基因拟南芥中的MDA含量和H2O2含量,而降低了CAT和POD活性,表明过表达MnMAPK5可能导致了CAT和POD酶活性的降低,无法清除大量的H2O2,进而诱发细胞的膜脂过氧化物作用导致细胞膜的结构被破坏。在低温下,过表达MnMAPK5降低了CAT活性、POD活性、脯氨酸含量和可溶性糖含量,表明植物在遭受冷胁迫时,使得膜系统既可从液晶态变成凝胶态进而发生相变,降低生物膜上的酶的活性(马媛媛等,2012),导致植物不能进行正常的抗性反应,而MnMAPK5促进了这一过程的发生。在盐胁迫下,过表达MnMAPK5提高了转基因植株中的MDA含量和POD活性,降低了CAT活性和可溶性糖含量,但H2O2含量也被降低,推测MnMAPK5可能通过影响其他途径降低植株的抗盐性。在氧化环境胁迫下,过表达MnMAPK5提高了转基因拟南芥的MDA含量,降低了脯氨酸含量和可溶性糖含量,这表明在氧化胁迫下过表达MnMAPK5抑制了脯氨酸和可溶性糖合成,从而降低转基因植株的抗氧化胁迫能力。此外,在各个胁迫环境条件下,胁迫相关基因在转基因植株中的表达量都要低于野生型拟南芥,这表明MnMAPK5可能通过负调控胁迫应答相关基因的表达降低植物的抗逆性。此前的诸多报道显示MAPK在植物抵抗逆境胁迫中扮演着正调控的角色(Suri et al., 2008;Liu et al., 2012;Tsugama et al., 2012;Yu et al., 2010;Zhou et al., 2012),而MnMAPK5具有负调控作用,丰富了对MAPK在抗逆境胁迫中的功能的认识,也为今后通过基因工程改良植物的抗逆性提供了新的视野。
4 结论桑树MnMAPK5基因的编码蛋白定位于细胞质和细胞核中;MnMAPK5启动子活性受到了高温、低温、高盐和干旱胁迫的诱导;过表达MnMAPK5降低了转基因拟南芥对高盐、干旱、低温和H2O2的抵抗能力。
| [] |
马媛媛, 肖霄, 张文娜. 2012. 植物低温逆境胁迫研究综述. 安徽农业科学, 40(12): 7007–7008.
( Ma Y Y, Xiao X, Zhang W N. 2012. Research progress of plants under cold stress. Journal of Anhui Agricultural Sciences, 40(12): 7007–7008. DOI:10.3969/j.issn.0517-6611.2012.12.007 [in Chinese] ) |
| [] | Beckers G J, Jaskiewicz M, Liu Y, et al. 2009. Mitogen-activated protein kinases 3 and 6 are required for full priming of stress responses in Arabidopsis thaliana. Plant Cell, 21(3): 944–953. DOI:10.1105/tpc.108.062158 |
| [] | Duerr B, Gawienowski M, Ropp T, et al. 1993. MsERK1:a mitogen-activated protein kinase from a flowering plant. Plant Cell, 5(1): 87–96. DOI:10.1105/tpc.5.1.87 |
| [] | Fabien J, Charlotte S, Dongjin S, et al. 2009. MAP kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS-mediated ABA signaling. Proceedings of the National Academy of Sciences of the United States of America, 106(48): 20520–20525. DOI:10.1073/pnas.0907205106 |
| [] | He C, Fong S H, Yang D, et al. 2000. BWMK1, a novel MAP kinase induced by fungal infection and mechanical wounding in rice. Molecular Plant-Microbe Interactions, 12(12): 1064–1073. |
| [] | Ichimura K, Shinozaki K, Tena G, et al. 2002. Mitogen-activated protein kinases cascades in plants:a new nomenclature. Trends in Plant Science, 7(7): 301–308. DOI:10.1016/S1360-1385(02)02302-6 |
| [] | Ichimura K, Mizoguchi T, Yoshida R, et al. 2000. Various abiotic stresses rapidly activate Arabidopsis MAP kinases ATMPK4 and ATMPK6. Plant Journal, 24(5): 655–665. DOI:10.1046/j.1365-313x.2000.00913.x |
| [] | Kenji G, Daisuke O, Shinpei K, et al. 2005. A mitogen-activated protein kinase NtMPK4 activated by SIPKK is required for jasmonic acid signaling and involved in ozone tolerance via stomatal movement in tobacco. Plant and Cell Physiology, 46(12): 1902–1914. DOI:10.1093/pcp/pci211 |
| [] | Liu X M, Kim K E, Kim K C, et al. 2010. Cadmium activates Arabidopsis MPK3 and MPK6 via accumulation of reactive oxygen species. Phytochemistry, 71(5/6): 614–618. |
| [] | Liu X M, Xuan C N, Kim K E, et al. 2012. Phosphorylation of the zinc finger transcriptional regulator ZAT6 by MPK6 regulates Arabidopsis seed germination under salt and osmotic stress. Biochemical and Biophysical Research Communications, 430(3): 1054–1059. |
| [] | Lumbreras V, Vilela B, Irar S, et al. 2010. MAPK phosphatase MKP2 mediates disease responses in Arabidopsis and functionally interacts with MPK3 and MPK6. Plant Journal, 63(6): 1017–1030. DOI:10.1111/tpj.2010.63.issue-6 |
| [] | Mizoguchi T, Irie K, Hirayama T, et al. 1996. A gene encoding a MAP kinase kinase kinase is induced simultaneously with genes for a MAP kinase and an S6 kinase by touch, cold and water stress in Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the United States of America, 93(2): 765–779. DOI:10.1073/pnas.93.2.765 |
| [] | Pan J, Zhang M, Kong X, et al. 2011. ZmMPK17, a novel maize group D MAP kinase gene, is involved in multiple stress responses. Planta, 235(4): 661–676. |
| [] | Salam M A, Jammes F, Hossain M A, et al. 2013. Two guard cell-preferential MAPKs, MPK9 and MPK12, regulate YEL signalling in Arabidopsis guard cells. Plant Biology, 15(3): 436–442. DOI:10.1111/plb.2013.15.issue-3 |
| [] | Suri S S, Dhindsa R S. 2008. A heat-activated MAP kinase (HAMK) as a mediator of heat shock response in tobacco cells. Plant Cell and Environment, 31(2): 218–226. |
| [] | Teige M, Scheikl E, Eulgem T, et al. 2004. The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Molecular Cell, 15(1): 141–152. DOI:10.1016/j.molcel.2004.06.023 |
| [] | Tomoyuki F, Daisuke M, Takashi N. 2014. Membrane rigidification functions upstream of the MEKK1-MKK2-MPK4 cascade during cold acclimation in Arabidopsis thaliana. FEBS Letters, 588(11): 2025–2030. DOI:10.1016/j.febslet.2014.04.032 |
| [] | Tsugama D, Liu S, Takano T. 2012. Drought-induced activation and rehydration-induced inactivation of MPK6 in Arabidopsis. Biochemical & Biophysical Research Communications, 426(4): 626–629. |
| [] | Wang L, Liu Y, Cai G, et al. 2014. Ectopic expression of ZmSIMK1 leads to improved drought tolerance and activation of systematic acquired resistance in transgenic tobacco. Journal of Biotechnology, 172(1): 18–29. |
| [] | Wei C, Liu X, Long D, et al. 2014. Molecular cloning and expression analysis of mulberry MAPK gene family. Plant Physiology and Biochemistry, 77: 108–116. DOI:10.1016/j.plaphy.2014.02.002 |
| [] | Yu L, Nie J, Cao C, et al. 2010. Phosphatidic acid mediates salt stress response by regulation of MPK6 in Arabidopsis thaliana. New Phytologist, 188(3): 762–773. DOI:10.1111/j.1469-8137.2010.03422.x |
| [] | Zhang S, Klessig D F. 1998. The tobacco wounding-activated mitogen-activated protein kinase is encoded by SIPK. Proceedings of the National Academy of Sciences of the United States of America, 95(12): 7225–7230. DOI:10.1073/pnas.95.12.7225 |
| [] | Zhou Y, Zhang D, Pan J, et al. 2012. Overexpression of a multiple stress-responsive gene, ZmMPK4, enhances tolerance to low temperature in transgenic tobacco. Plant Physiology and Biochemistry, 58: 174–181. DOI:10.1016/j.plaphy.2012.06.020 |
 2017, Vol. 53
2017, Vol. 53

